Abstract
Heme oxygenase-1 (HO-1) and its enzymatic by-product carbon monoxide (CO) have emerged as important regulators of acute and chronic inflammation. Mechanisms underlying their anti-inflammatory effects are only partially understood. In this addendum, we summarize current understanding of the role of the HO-1/CO pathway in regulation of intestinal inflammation with a focus on innate immune function. In particular, we highlight our recent findings that HO-1 and CO ameliorate intestinal inflammation through promotion of bacterial clearance. Our work and that of many others support further investigation of this global homeostatic pathway in the human inflammatory bowel diseases (IBDs).
Introduction
The maintenance of immune homeostasis in the intestine requires a complex interplay between host and environmental factors that regulate immune responses directed against the enteric microbiota. Loss of tolerance to the enteric microbiota is a unifying pathogenic hypothesis in the initiation and perpetuation of chronic intestinal inflammation.Citation1 Our studies were conceived to understand mechanistically the impact of clinically relevant environmental factors on host-microbiota interactions in IBD. An important epidemiologic observation in the human IBDs is that cigarette smoking is protective against development of ulcerative colitis (UC).Citation2 Immunomodulatory components in cigarette smoke that may explain this phenomenon remain poorly defined. Cigarette smoke is a complex mixture of over 5000 known compounds, many of which may exert immunologic effects. Despite this complexity, immunologic and clinical studies to address this issue in IBD have focused on nicotine. However, clinical trial experience in UC patients with nicotine gum and transdermal nicotine has been inconclusive.Citation3,Citation4 Carbon monoxide (CO) is a prominent component of cigarette smoke and has been shown to have anti-inflammatory effects in multiple experimental models of acute inflammation.Citation5-Citation8 The heme oxygenase (HO) group of enzymes, of which HO-1 is inducible in immune cells, mediate heme degradation, generating equimolar amounts of iron, biliverdin, and CO. We and others have demonstrated that the HO-1/CO pathway can regulate intestinal inflammation in experimental models of acute and chronic intestinal inflammation.Citation9-Citation13 Indeed, in our work, anti-inflammatory effects of CO required the induction of HO-1. CO/HO-1 affect multiple immunologic cell types including T cells, B cells, epithelial cells, neutrophils, mast cells, dendritic cells, and macrophages.Citation9,Citation14-Citation19 Our studies have focused on the role of these pathways in macrophage biology. We recently reported that intestinal HO-1 is induced by the enteric microbiota and modulates macrophage bactericidal activity, suggesting its importance in maintaining homeostasis.Citation12 Our work leads to a model where, in the local microenvironment of the intestine, CO and HO-1 are components of an evolutionarily conserved homeostatic pathway modulated in health and disease by cross-talk between the microbiota and the mucosal immune compartment.
HO-1 is Induced by the Microbiota
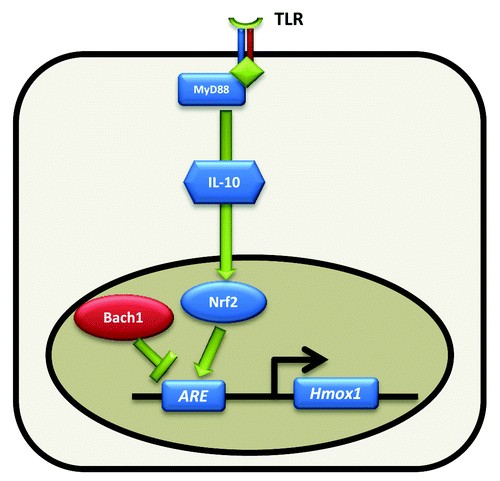
The enteric microbiota induce intestinal HO-1, with a requirement for the homeostatic cytokine IL-10.Citation12 To define this pathway, germ free (GF) wild-type (WT) and colitis prone Il10−/− mice, were colonized with a conventional microbiota. HO-1 was significantly induced post-colonization in WT but not in Il10−/− mice relative to respective GF controls. Induction of HO-1 was mediated by the transcription factor, nuclear factor erythroid 2-related factor 2 (Nrf2) that is activated under cellular stress and inflammation and is largely responsible for activation of heme oxygenase gene (Hmox1) transcription in mice and humans.Citation20 Additionally, Hmox1 expression in bone marrow derived macrophages (BMDM) was induced in a MyD88 and IL-10 dependent fashion upon stimulation with Toll-like receptor (TLR) ligands.Citation11 The protective effect of HO-1 induction on enteric microbiota-driven colitis was further supported by experiments where GF Il10−/− mice were treated with a small molecule HO-1 inducer, cobalt protoporphyrin IX (CoPP), prior to colonization with a conventional microbiota, resulting in attenuation of colitis.Citation12 Hence, although in the absence of IL-10, the microbiota cannot induce Hmox1 expression, pharmacologic induction of Hmox1 can overcome this defect and protect against colitis development. Induction of HO-1 was also observed in GF zebrafish colonized with a conventional microbiota, demonstrating evolutionary conservation of this central homeostatic pathway among vertebrate hosts.Citation12
HO-1/CO Ameliorate Colitis through Altered Cytokine Expression
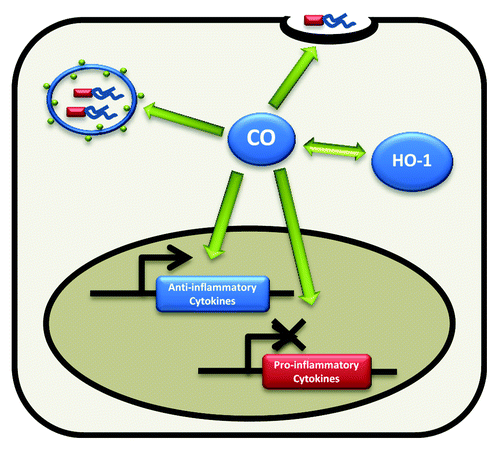
The HO-1/CO pathway can profoundly alter the balance of pro-inflammatory and anti-inflammatory cytokine expression in the intestine.Citation9-Citation11,Citation14 Importantly, CO exposure and HO-1 induction have been shown to ameliorate colitis in numerous experimental models of IBD with different immunopathogeneses, highlighting the importance of this pathway as a homeostatic checkpoint in intestinal immune responses.Citation9-Citation11,Citation13,Citation14,Citation21 Using Il10−/− mice as a model of microbiota dependent, IL-12/23-mediated chronic intestinal inflammation, we demonstrated that exposure to CO ameliorated colitis in an HO-1 dependent manner.Citation14 T cell receptor α deficient (TCRα−/−) mice develop spontaneous, Th2-mediated chronic colitis, driven by IL-4 and IL-1β.Citation22,Citation23 Exposure of mice to CO or treatment with CoPP ameliorated active colitis. Decreased levels of intestinal pro-inflammatory TNF, IL-1β, IL-4 and IL-17 cytokines were seen with increased colonic levels of the anti-inflammatory cytokines IL-10 and IL-22.Citation11 Similarly, CO exposure in 2, 4, 6-trinitrobenzine sulfonic acid (TNBS) and dextran sulfate sodium (DSS) experimental colitis was associated with attenuation of colitis and decreased intestinal TNF expression.Citation9,Citation10 Furthermore, modulation of HO-1 transcriptional regulation alters development of experimental colitis with consequent changes in cytokine expression. Mice deficient in broad complex, tramtrack, bric-a-brac domain (BTB), and cap’n’collar (CNC) homolog 1 (Bach1), a transcriptional repressor of HO-1, are protected against TNBS colitis.Citation24,Citation25 This effect is mediated by macrophages and is associated with HO-1 induction and decreased expression of TNF and interferon-γ.Citation24 Nrf2−/− mice have impaired HO-1 induction and demonstrate increased susceptibility to DSS induced colitis with increased levels of IL-1β, TNF, and IL-6.Citation26
HO-1 and CO Promote Bactericidal Activity in Innate Immune Cells
There is also emerging evidence that pleiotropic effects of the HO-1/CO pathway include significant antimicrobial activities. Defects in innate immune responses to enteric bacteria have been implicated in the pathogenesis of human IBDs through genetic associations,Citation1,Citation27 including the confirmation of synonymous IBD susceptibility single nucleotide polymorphisms (SNPs) in autophagy genes encoding ATG16L1 and IRGM and a SNP in the phagosomal gene NCF4.Citation28-Citation31 The HO-1/CO pathway has been shown to augment responses to systemic bacterial infection through enhanced phagocytosis.Citation32,Citation33 In a mouse model of bacterial sepsis, Hmox1−/− mice demonstrated significantly decreased survival compared with WT controls. Likewise, systemic overexpression of human HO-1 in mice resulted in improved clearance of bacteria and survival.Citation32 In these HO-1 overexpressing mice, there was an increase in intestinal nuclear binding oligomerization domain containing 2 (NOD2) expression as well as increased phagocytosis by peritoneal macrophages, mediated by CO and NOD2. Single nucleotide polymorphisms in the intracellular pattern recognition receptor NOD2 provide the strongest genetic risk for Crohn disease. Moreover, NOD2 has been linked to autophagy signaling and anti-microbial responses.Citation34-Citation36 Additionally, HO-1 has been identified as a regulator of autophagy signaling in LPS exposed macrophages.Citation37 HO-1 activity is also important for bacterial clearance in a Salmonella typhimurium model of peritonitis in mice.Citation38 We demonstrated that CO exposure of colitis-prone Il10−/− mice resulted in improved clearance of intestinal bacteria from extraintestinal sites.Citation12 Additionally, induction of HO-1 by CoPP led to increased bacterial clearance from the intestinal mucosa and systemic sites in an enteric bacterial infectious colitis model with S. typhimurium in WT mice. Given that macrophages are critical for Salmonella eradication,Citation39 we further examined the effect of the HO-1/CO pathway in augmenting macrophage responses to enteric bacteria. Knock-down of HO-1 expression in BMDMs using Hmox1 small interfering RNA resulted in decreased killing of Escherichia coli, S. typhimurium, and Enterococcus faecalis. Augmented bactericidal activity was also demonstrated in BMDMs exposed to CO. Possible mechanisms for enhanced bactericidal activity include HO-1 augmentation of autophagy via TLR signaling as well as increased phagolysosomal formation/activation.Citation12,Citation37,Citation40
Hence, the HO-1/CO pathway regulates intestinal immune homeostasis through pleiotropic mechanisms. In intestinal macrophages, the HO-1/CO pathway is induced by the enteric microbiota (). TLR stimulation leads to MyD88 and IL-10 mediated induction of Hmox1, via Nrf2 transcriptional control. Subsequently, increased CO production leads to augmented phagocytosis, promotion of bacterial killing, and increased anti-inflammatory and decreased pro-inflammatory cytokine production (). However, whether HO-1 has effects on microbicidal activity and cytokine expression that are independent of CO are unaddressed possibilities. Indeed, another byproduct of heme metabolism by HO, biliverdin (metabolized in the liver to bilirubin), has been shown to directly inhibit inflammatory cytokine production in macrophages.Citation41
CO, HO-1, and the Enteric Microbiota
Recent work has demonstrated that alterations in the enteric microbiota, e.g., dysbiosis, plays a role in the development of colitis.Citation42 Environmental factors such as cigarette smoking have been associated with changes in the microbiota in human IBD.Citation43 We recently found that exposure to CO in mice can alter the microbiota and induce bacterial species that express HO homologs involved in iron acquisition (N. Maharshak et al., manuscript submitted). The proximity of the mucosal immune compartment, including the epithelium, to the enteric microbiota leads to signaling cross-talk that can potentially be harnessed therapeutically. The existence of enteric bacterial HO homologs leads us to speculate that the enteric microbiota may actually provide a significant source of CO in the gut, involved in the maintenance of homeostasis. These findings also raise the possibility that enteric bacterial HO may be utilized as a therapeutic strategy in IBD. Our unpublished results suggest that bacterial HO homologs can modulate innate immune responses, possibly through elaboration of CO (N. Maharshak et al., manuscript submitted). Indeed, another group has reported that genetically engineered enteric bacteria that express mammalian HO-1 have therapeutic potential in a mucosal injury model.Citation44
Future Directions
CO and HO-1 define a central homeostatic pathway in the intestine, affecting critical innate immune processes, such as cytokine expression and macrophage bactericidal activity, linked to the genetics and immunopathology of the human IBDs. Consequently, these investigations underscore a therapeutic potential of the HO-1/CO pathway for human IBDs. Progress has been made in translating these findings into future therapies through development of methodologies to finely control CO release to target organs of interest such as CO-releasing small molecules which may eventually merit testing in humans.Citation45,Citation46 We are currently exploring approaches to deliver CO locally to the intestine via enteric bacteria that express HO. Likewise, therapeutic strategies to induce intestinal HO-1 could be a powerful approach to correct fundamental defects and ameliorate inflammation in human IBD. A more complete understanding of the HO-1 regulatory pathway will further elucidate novel approaches and compounds to treat human disease.
| Abbreviations: | ||
| CO | = | carbon monoxide |
| CoPP | = | cobalt protoporphyrin |
| GF | = | germ free |
| HO-1 | = | heme oxygenase 1 |
| IL | = | interleukin |
| LPS | = | lipopolysaccharide |
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Acknowledgments
We thank all contributors to the original article. This work was supported in part by National Institutes of Health grants R01 DK54452, Gastroenterology Research Training grant T32 DK007737, National Research Service Award F32 DK083186, and a Crohn’s and Colitis Foundation of America Research Fellowship Award.
References
- Abraham C, Medzhitov R. Interactions between the host innate immune system and microbes in inflammatory bowel disease. Gastroenterology 2011; 140:1729 - 37; http://dx.doi.org/10.1053/j.gastro.2011.02.012; PMID: 21530739
- Cope GF, Heatley RV, Kelleher J, Lee PN. Cigarette smoking and inflammatory bowel disease: a review. Hum Toxicol 1987; 6:189 - 93; http://dx.doi.org/10.1177/096032718700600303; PMID: 3298000
- Pullan RD, Rhodes J, Ganesh S, Mani V, Morris JS, Williams GT, Newcombe RG, Russell MA, Feyerabend C, Thomas GA, et al. Transdermal nicotine for active ulcerative colitis. N Engl J Med 1994; 330:811 - 5; http://dx.doi.org/10.1056/NEJM199403243301202; PMID: 8114833
- Lashner BA, Hanauer SB, Silverstein MD. Testing nicotine gum for ulcerative colitis patients. Experience with single-patient trials. Dig Dis Sci 1990; 35:827 - 32; http://dx.doi.org/10.1007/BF01536795; PMID: 2194767
- Otterbein LE, Lee PJ, Chin BY, Petrache I, Camhi SL, Alam J, Choi AM. Protective effects of heme oxygenase-1 in acute lung injury. Chest 1999; 116:Suppl 61S - 3S; http://dx.doi.org/10.1378/chest.116.suppl_1.61S-a; PMID: 10424595
- Soares MP, Lin Y, Anrather J, Csizmadia E, Takigami K, Sato K, Grey ST, Colvin RB, Choi AM, Poss KD, et al. Expression of heme oxygenase-1 can determine cardiac xenograft survival. Nat Med 1998; 4:1073 - 7; http://dx.doi.org/10.1038/2063; PMID: 9734404
- Ryter SW, Choi AM. Cytoprotective and anti-inflammatory actions of carbon monoxide in organ injury and sepsis models. Novartis Found Symp 2007; 280:165 - 75, discussion 175-81; http://dx.doi.org/10.1002/9780470059593.ch12; PMID: 17380794
- Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell RA, Choi AM. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med 2000; 6:422 - 8; http://dx.doi.org/10.1038/74680; PMID: 10742149
- Takagi T, Naito Y, Mizushima K, Akagiri S, Suzuki T, Hirata I, Omatsu T, Handa O, Kokura S, Ichikawa H, et al. Inhalation of carbon monoxide ameliorates TNBS-induced colitis in mice through the inhibition of TNF-α expression. Dig Dis Sci 2010; 55:2797 - 804; http://dx.doi.org/10.1007/s10620-009-1112-x; PMID: 20094779
- Takagi T, Naito Y, Uchiyama K, Suzuki T, Hirata I, Mizushima K, Tsuboi H, Hayashi N, Handa O, Ishikawa T, et al. Carbon monoxide liberated from carbon monoxide-releasing molecule exerts an anti-inflammatory effect on dextran sulfate sodium-induced colitis in mice. Dig Dis Sci 2011; 56:1663 - 71; http://dx.doi.org/10.1007/s10620-010-1484-y; PMID: 21086163
- Sheikh SZ, Hegazi RA, Kobayashi T, Onyiah JC, Russo SM, Matsuoka K, Sepulveda AR, Li F, Otterbein LE, Plevy SE. An anti-inflammatory role for carbon monoxide and heme oxygenase-1 in chronic Th2-mediated murine colitis. J Immunol 2011; 186:5506 - 13; http://dx.doi.org/10.4049/jimmunol.1002433; PMID: 21444764
- Onyiah JC, Sheikh SZ, Maharshak N, Steinbach EC, Russo SM, Kobayashi T, Mackey LC, Hansen JJ, Moeser AJ, Rawls JF, et al. Carbon monoxide and heme oxygenase-1 prevent intestinal inflammation in mice by promoting bacterial clearance. Gastroenterology 2013; 144:789 - 98; http://dx.doi.org/10.1053/j.gastro.2012.12.025; PMID: 23266559
- Zuckerbraun BS, Otterbein LE, Boyle P, Jaffe R, Upperman J, Zamora R, Ford HR. Carbon monoxide protects against the development of experimental necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 2005; 289:G607 - 13; http://dx.doi.org/10.1152/ajpgi.00055.2005; PMID: 15890710
- Hegazi RA, Rao KN, Mayle A, Sepulveda AR, Otterbein LE, Plevy SE. Carbon monoxide ameliorates chronic murine colitis through a heme oxygenase 1-dependent pathway. J Exp Med 2005; 202:1703 - 13; http://dx.doi.org/10.1084/jem.20051047; PMID: 16365149
- Kapturczak MH, Wasserfall C, Brusko T, Campbell-Thompson M, Ellis TM, Atkinson MA, Agarwal A. Heme oxygenase-1 modulates early inflammatory responses: evidence from the heme oxygenase-1-deficient mouse. Am J Pathol 2004; 165:1045 - 53; http://dx.doi.org/10.1016/S0002-9440(10)63365-2; PMID: 15331427
- Brusko TM, Wasserfall CH, Agarwal A, Kapturczak MH, Atkinson MA. An integral role for heme oxygenase-1 and carbon monoxide in maintaining peripheral tolerance by CD4+CD25+ regulatory T cells. J Immunol 2005; 174:5181 - 6; PMID: 15843512
- Megías J, Busserolles J, Alcaraz MJ. The carbon monoxide-releasing molecule CORM-2 inhibits the inflammatory response induced by cytokines in Caco-2 cells. Br J Pharmacol 2007; 150:977 - 86; http://dx.doi.org/10.1038/sj.bjp.0707184; PMID: 17339836
- Takamiya R, Murakami M, Kajimura M, Goda N, Makino N, Takamiya Y, Yamaguchi T, Ishimura Y, Hozumi N, Suematsu M. Stabilization of mast cells by heme oxygenase-1: an anti-inflammatory role. Am J Physiol Heart Circ Physiol 2002; 283:H861 - 70; PMID: 12181112
- Brandsma CA, Hylkema MN, van der Strate BW, Slebos DJ, Luinge MA, Geerlings M, Timens W, Postma DS, Kerstjens HA. Heme oxygenase-1 prevents smoke induced B-cell infiltrates: a role for regulatory T cells?. Respir Res 2008; 9:17; http://dx.doi.org/10.1186/1465-9921-9-17; PMID: 18252008
- Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, Cook JL. Nrf2, a Cap’n’Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem 1999; 274:26071 - 8; http://dx.doi.org/10.1074/jbc.274.37.26071; PMID: 10473555
- Jurjus AR, Khoury NN, Reimund JM. Animal models of inflammatory bowel disease. J Pharmacol Toxicol Methods 2004; 50:81 - 92; http://dx.doi.org/10.1016/j.vascn.2003.12.002; PMID: 15385082
- Mombaerts P, Mizoguchi E, Grusby MJ, Glimcher LH, Bhan AK, Tonegawa S. Spontaneous development of inflammatory bowel disease in T cell receptor mutant mice. Cell 1993; 75:274 - 82; http://dx.doi.org/10.1016/0092-8674(93)80069-Q; PMID: 8104709
- Mizoguchi A, Mizoguchi E, Chiba C, Spiekermann GM, Tonegawa S, Nagler-Anderson C, Bhan AK. Cytokine imbalance and autoantibody production in T cell receptor-alpha mutant mice with inflammatory bowel disease. J Exp Med 1996; 183:847 - 56; http://dx.doi.org/10.1084/jem.183.3.847; PMID: 8642289
- Harusato A, Naito Y, Takagi T, Uchiyama K, Mizushima K, Hirai Y, Higashimura Y, Katada K, Handa O, Ishikawa T, et al. BTB and CNC homolog 1 (Bach1) deficiency ameliorates TNBS colitis in mice: role of M2 macrophages and heme oxygenase-1. Inflamm Bowel Dis 2013; 19:740 - 53; http://dx.doi.org/10.1097/MIB.0b013e3182802968; PMID: 23446334
- Oyake T, Itoh K, Motohashi H, Hayashi N, Hoshino H, Nishizawa M, Yamamoto M, Igarashi K. Bach proteins belong to a novel family of BTB-basic leucine zipper transcription factors that interact with MafK and regulate transcription through the NF-E2 site. Mol Cell Biol 1996; 16:6083 - 95; PMID: 8887638
- Khor TO, Huang MT, Kwon KH, Chan JY, Reddy BS, Kong AN. Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitis. Cancer Res 2006; 66:11580 - 4; http://dx.doi.org/10.1158/0008-5472.CAN-06-3562; PMID: 17178849
- Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA, et al, International IBD Genetics Consortium (IIBDGC). Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012; 491:119 - 24; http://dx.doi.org/10.1038/nature11582; PMID: 23128233
- Rioux JD, Xavier RJ, Taylor KD, Silverberg MS, Goyette P, Huett A, Green T, Kuballa P, Barmada MM, Datta LW, et al. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat Genet 2007; 39:596 - 604; http://dx.doi.org/10.1038/ng2032; PMID: 17435756
- Hampe J, Franke A, Rosenstiel P, Till A, Teuber M, Huse K, Albrecht M, Mayr G, De La Vega FM, Briggs J, et al. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat Genet 2007; 39:207 - 11; http://dx.doi.org/10.1038/ng1954; PMID: 17200669
- Franke A, McGovern DP, Barrett JC, Wang K, Radford-Smith GL, Ahmad T, Lees CW, Balschun T, Lee J, Roberts R, et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat Genet 2010; 42:1118 - 25; http://dx.doi.org/10.1038/ng.717; PMID: 21102463
- McGovern DP, Gardet A, Törkvist L, Goyette P, Essers J, Taylor KD, Neale BM, Ong RT, Lagacé C, Li C, et al, NIDDK IBD Genetics Consortium. Genome-wide association identifies multiple ulcerative colitis susceptibility loci. Nat Genet 2010; 42:332 - 7; http://dx.doi.org/10.1038/ng.549; PMID: 20228799
- Chung SW, Liu X, Macias AA, Baron RM, Perrella MA. Heme oxygenase-1-derived carbon monoxide enhances the host defense response to microbial sepsis in mice. J Clin Invest 2008; 118:239 - 47; http://dx.doi.org/10.1172/JCI32730; PMID: 18060048
- Otterbein LE, May A, Chin BY. Carbon monoxide increases macrophage bacterial clearance through Toll-like receptor (TLR)4 expression. Cell Mol Biol (Noisy-le-grand) 2005; 51:433 - 40; PMID: 16309564
- Hugot JP, Chamaillard M, Zouali H, Lesage S, Cézard JP, Belaiche J, Almer S, Tysk C, O’Morain CA, Gassull M, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 2001; 411:599 - 603; http://dx.doi.org/10.1038/35079107; PMID: 11385576
- Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 2001; 411:603 - 6; http://dx.doi.org/10.1038/35079114; PMID: 11385577
- Homer CR, Richmond AL, Rebert NA, Achkar JP, McDonald C. ATG16L1 and NOD2 interact in an autophagy-dependent antibacterial pathway implicated in Crohn’s disease pathogenesis. Gastroenterology 2010; 139:1630 - 41, e1-2; http://dx.doi.org/10.1053/j.gastro.2010.07.006; PMID: 20637199
- Waltz P, Carchman EH, Young AC, Rao J, Rosengart MR, Kaczorowski D, Zuckerbraun BS. Lipopolysaccaride induces autophagic signaling in macrophages via a TLR4, heme oxygenase-1 dependent pathway. Autophagy 2011; 7:315 - 20; http://dx.doi.org/10.4161/auto.7.3.14044; PMID: 21307647
- Zaki MH, Fujii S, Okamoto T, Islam S, Khan S, Ahmed KA, Sawa T, Akaike T. Cytoprotective function of heme oxygenase 1 induced by a nitrated cyclic nucleotide formed during murine salmonellosis. J Immunol 2009; 182:3746 - 56; http://dx.doi.org/10.4049/jimmunol.0803363; PMID: 19265153
- Mittrücker HW, Kaufmann SH. Immune response to infection with Salmonella typhimurium in mice. J Leukoc Biol 2000; 67:457 - 63; PMID: 10770276
- Sanjuan MA, Dillon CP, Tait SW, Moshiach S, Dorsey F, Connell S, Komatsu M, Tanaka K, Cleveland JL, Withoff S, et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature 2007; 450:1253 - 7; http://dx.doi.org/10.1038/nature06421; PMID: 18097414
- Wang WW, Smith DL, Zucker SD. Bilirubin inhibits iNOS expression and NO production in response to endotoxin in rats. Hepatology 2004; 40:424 - 33; http://dx.doi.org/10.1002/hep.20334; PMID: 15368447
- Maharshak N, Packey CD, Ellermann M, Manick S, Siddle JP, Huh EY, Plevy S, Sartor RB, Carroll IM. Altered enteric microbiota ecology in interleukin 10-deficient mice during development and progression of intestinal inflammation. Gut Microbes 2013; 4:316 - 24; http://dx.doi.org/10.4161/gmic.25486; PMID: 23822920
- Li E, Hamm CM, Gulati AS, Sartor RB, Chen H, Wu X, Zhang T, Rohlf FJ, Zhu W, Gu C, et al. Inflammatory bowel diseases phenotype, C. difficile and NOD2 genotype are associated with shifts in human ileum associated microbial composition. PLoS One 2012; 7:e26284; http://dx.doi.org/10.1371/journal.pone.0026284; PMID: 22719818
- Pang QF, Ji Y, Bermúdez-Humarán LG, Zhou QM, Hu G, Zeng Y. Protective effects of a heme oxygenase-1-secreting Lactococcus lactis on mucosal injury induced by hemorrhagic shock in rats. J Surg Res 2009; 153:39 - 45; http://dx.doi.org/10.1016/j.jss.2008.03.042; PMID: 18694575
- Motterlini R, Otterbein LE. The therapeutic potential of carbon monoxide. Nat Rev Drug Discov 2010; 9:728 - 43; http://dx.doi.org/10.1038/nrd3228; PMID: 20811383
- Motterlini R, Clark JE, Foresti R, Sarathchandra P, Mann BE, Green CJ. Carbon monoxide-releasing molecules: characterization of biochemical and vascular activities. Circ Res 2002; 90:E17 - 24; http://dx.doi.org/10.1161/hh0202.104530; PMID: 11834719