Abstract
A key role for segmented filamentous bacteria (SFB) has recently been demonstrated in several mouse models of autoimmune diseases, including autoimmune arthritis and multiple sclerosis. The mechanism governing the activation of systemic autoreactive T cell responses by such commensals in the gut, however, remained elusive. In this addendum, we discuss recent results addressing the local regulation of autoreactive T cell sensitivity by these unique bacteria. We found that the presence of SFB in the gut microbiota was sufficient to promote a local inflammatory microenvironment altering the T cell-intrinsic desensitization process normally occurring in response to chronic self-antigen stimulation. In the absence of this key tolerance checkpoint, sustained chronic T cell proliferation, IFNγ production, and B cell activation eventually led to the development of enhanced pathologies in a Th1-driven T cell-transfer model of autoimmune arthritis.
Introduction
Commensal bacteria bear an essential role in host metabolism and in defense against invading pathogens.Citation1,Citation2 In addition to conferring direct colonization resistance by outcompeting pathogens for local niches, commensals have been shown to be key in fostering the maturation of host mucosal-associated immune responses.Citation2-Citation6 However, disturbed balance between host and microbiota—dysbiosis—can lead to gut-flora-induced chronic inflammation with both local and systemic consequences on the outcome of various autoimmune diseases.Citation2,Citation7
Segmented filamentous bacteria (SFB) or “Candidatus Arthromitus,” a unique species of filamentous bacteria found tightly attached to the epithelium in the gut of numerous vertebrates,Citation8,Citation9 provide us with a prototypical example of the dual role of the commensal flora.Citation10 While key in promoting the full maturation of the gut-associated adaptive immune system, ranging from Th1 and Th17 differentiation to IgA production as well as proliferation and accumulation of gut-residing T cells,Citation4,Citation5,Citation11,Citation12 it has also recently been highlighted for its triggering role in murine models of colitis,Citation13 multiple sclerosis,Citation14 and autoimmune arthritis.Citation15 The exact mechanism(s) involved, however, remain poorly understood. Given the broad impact of SFB on polyclonal T cell responses, we came to ask, in a T-cell transfer model of autoimmune arthritis, the question of whether at least part of this could be explained by an impact of the gut flora at the level of TCR signaling and T-cell activation.Citation16
SFB Broadly Stimulate Autoreactive CD4+ T Cell Responses in the Gut
This study started with the fortuitous observation that the gut commensal flora, and in particular its colonization by SFB, had a key impact on the severity of autoimmune pathologies occurring in a T-cell transfer model of autoimmune arthritis initially designed in our laboratory as an in vivo model to study CD4+ T cell responses to a known self-antigen.Citation17,Citation18 Originally, all our colonies where housed at Taconic Farms, Inc, in a SPF barrier run under a murine pathogen free status (MPF). In that case, around 80% of T cell-deficient Cd3e−/− mice expressing a transgene coding for pigeon cytochrome C (mPCC,Cd3e−/−) would start developing symptoms of arthritis 4–5 wk after a single infusion of 106 PCC-specific CD4+ T cells purified from a 5C.C7 TCR transgenic mouse.Citation18 Upon rederivation to a much less diversified flora and subsequent housing under a much stricter Restricted Flora status (RF)—devoid of SFB ()—the severity of the arthritis pathologies dropped dramatically, to a point where it became barely detectable (). Similar to previous observations made in the KxB/N model of autoimmune arthritis,Citation15 we could further pin-point this to the specific absence of SFB in the RF flora. Given the unique nature of this T-cell transfer model of arthritis, we sought to elucidate the role of the gut flora, and SFB in particular, on the early and later events driving self-reactive T cell responses.
Figure 1. SFB colonization of host gut microbiota drives autoimmune manifestation in a T-cell transfer model of autoimmune arthritis. (A) PCR for 16S rRNA of Segmented Filamentous Bacteria (SFB) or total bacteria (EUB) in ileum (left) and cecum content (right) of 6-wk-old mPCC,Cd3e−/− mice housed in a Restricted Flora (RF) or Murine Pathogen Free (MPF) barrier in Taconic. Control samples isolated from a 6-wk-old MPF-housed C57Bl/6NTac mouse are also displayed. DNA isolation and subsequent PCR were performed as previously described.Citation16 (B-C) RF- or MPF-housed mPCC,Cd3e−/− mice were injected on day 0 with 1 × 106 naive 5C.C7, Rag2−/− T cells. (B) Representative pictures of RF- (lower) or MPF-housed hosts (upper): lower left leg (left) and digits (right) 63 d after T-cell transfer. (C) Representative picture of lymphoid organs harvested from RF- (lower) or MPF-housed hosts (upper) 20 d post T-cell transfer.
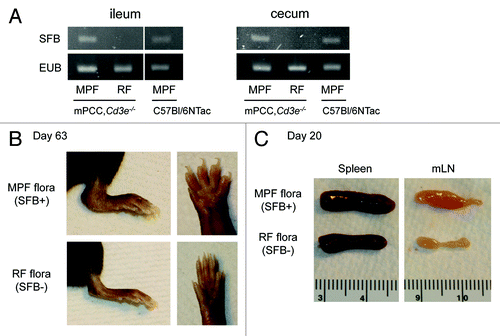
The first surprising result came when we realized that there were no detectable differences in early Th17 differentiation in this model, in line with the fact that blocking IL-17A never recapitulated the protective effect observed in other models of autoimmune arthritis.Citation15,Citation19 Indeed, IFNγ-production by the T cell appeared required for the development of the arthritis pathologies in our model, a key difference with the KxB/N model of autoimmune arthritis.Citation15 A modest but reproducible defect of Th1 differentiation could be observed in the absence of SFB. Such impact of SFB on Th1 differentiation had already been described in other autoimmune models,Citation14,Citation15 yet not investigated further given their Th17-driven nature.
The second key observation was the small splenomegaly and, most of all, sustained lymphadenopathy of mesenteric lymph nodes (mLN) that could be observed in SFB+ MPF-housed hosts as early as 14 d after T cell transfer (). In vivo EdU labeling experiments further demonstrated a sustained chronic proliferation—and subsequent accumulation—of transferred autoreactive T cells in mLN and Lamina Propria of SFB-harboring hosts.
The impact of SFB on pathogenic Th1 differentiation, as well as proliferation and accumulation in gut-associated lymphoid tissues in this model, thus widened the range of potential impact that SFB can have on autoreactive T cell responses, extending it far beyond the sole generation of pathogenic Th17 cells. More importantly, this suggested that SFB-derived signals might actually amplify rather than simply skew pathogenic T cell responses, an hypothesis supported by the recent data regarding SFB’s impact on peripheral polyclonal T cell populations.Citation4,Citation5,Citation10
SFB Regulate Autoreactive CD4+ T Cell Sensitivity in the Gut
Among the observations listed above, one—the uncontrolled chronic T cell proliferation—was of particular interest for us. Previous data obtained in this experimental model indicated that the main constraint on chronic T-cell activation and proliferation was the result of a T-cell-intrinsic desensitization process,Citation17,Citation20 occurring primarily at the level of the TCR proximal signaling machinery—specifically the Zap70-mediated phosphorylation of LAT.Citation21-Citation23 Distal effects on NFAT and AP-1 are also seen, but these would be expected from an amplification of the proximal blockade. Initially referred to as adaptive tolerance,Citation17 it has also been referred to as a T-cell tuning mechanism, in reference to the “tunable activation threshold” model proposed by Paul and Grossmann.Citation24,Citation25 Similar to exhaustion in CD8+ T cells,Citation26 albeit slightly different at the molecular level,Citation27 it has been defined as a process by which a T cell adapts its activation threshold to match the level of a chronic antigenic stimulation.
T-cell activation threshold is best estimated by precisely measuring the EC50 of a T cell population to its cognate antigen in vitro. Performing such experiments, using either cell proliferation or overnight CD69 expression as read-outs of T cell activation, we could easily demonstrate that, although their sensitivity was never restored to the level of naïve T cells, CD4+ T cells isolated from mLN of SFB-harboring hosts as early as 7 d after T-cell transfer displayed a 3-fold reduced T-cell activation threshold as compared with fully tolerant T cells isolated from mLN of SFB-free hosts. Thus the sole presence of SFB in the gut of mPCC,Cd3e−/− hosts, seemed to prevent transferred T cells from adapting their activation threshold to the level of chronic self antigen stimulation.
Such a regulation of autoreactive T cells at the level of T cell activation bears evident consequences in term of their proliferation and cytokine production in response to self-antigen in situ. Ex vivo restimulation with cognate antigen demonstrated a consistent difference in IL-2 production potential between the two sets of T cells. Most importantly, no difference could be detected upon PMA/Ionomycin restimulation, which bypasses the ZAP70-LAT node of TCR signaling, showing that NFAT/AP-1/NFκB elements distal to the TCR were in fact mostly unaffected in both cell populations. This key comparison between both types of stimulation of the tolerant T cells proved key in validating an impact of SFB-derived signals at the level of the proximal TCR signaling machinery, as hypothesized for an alteration of T-cell tuning.Citation22,Citation23 T cell transfer experiments further demonstrated this process to be highly dynamic as already fully tolerant T cells could regain some proliferative and cytokine production potential upon recirculation in mLN of SFB-harboring hosts.
The case of effector cytokines, and most importantly IFNγ in this model, proved more complex to analyze as their production is also dependent on prior T cell differentiation events. Comparing T cells that had resided for two weeks in SFB-harboring hosts, vs. SFB-free hosts, using cognate antigen-mediated restimulation, as should be the case in situ, a close to 6-fold increase in the frequency of IFNγ-producing T cells could be observed in the presence of SFB. While some differences could still be observed following PMA/Ionomycin restimulation, they never exceeded a 2-fold increase in IFNγ-production potential. Thus, while some effect of SFB on distal differentiation events could be seen, the regulation of TCR proximal events by SFB-derived signals was clearly a strong component in enhanced IFNγ-production potential of autoreactive T cells in arthritic prone individuals. This was further amplified by their sustained proliferation and accumulation in gut-associated lymphoid tissues of these hosts, another consequence of altered T cell tuning.
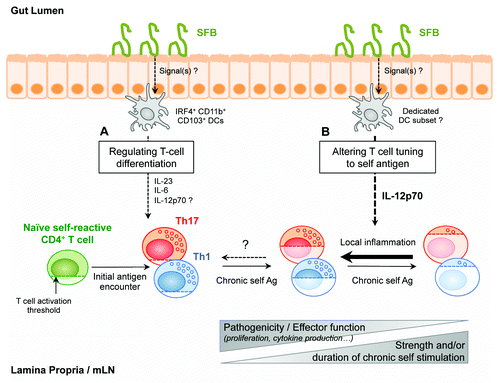
Overall, the results discussed above led us to the conclusion that commensal microbes can amplify systemic Th1-driven autoimmunity in two ways: (1) by promoting Th1 differentiation and (2) by maintaining pathogenic Th1 cells responsiveness—the result of a local alteration in a T-cell intrinsic tolerance mechanism, T cell tuning, normally occurring in vivo in response to chronic stimulation by self-antigens ().
Figure 2. Schematic summarizing the two facets of SFB-mediated amplification of autoreactive CD4+ T cells responses in the gut. (A) SFB can directly enhance pathogenic Th1-, and promote Th17-, differentiation in gut-associated lymphoid tissues. SFB-dependent differentiation of polyclonal Th17 at the steady-state has been shown to depend on IRF4-dependent CD11b+ CD103+ Lamina Propria DCs, which produce IL-6 and IL-23. IL-12p70 production by this particular DC subset has not been detected so far. (B) SFB can additionally locally alter T cell tuning to endogenous ligands. Chronic self-antigen recognition by autoreactive T cells normally leads to progressive desensitization—tuning—of these cells, associated with increased T cell activation thresholds and reduced cytokine secretion potentials. SFB-induced local inflammatory microenvironment, and notably IL-12p70, can partially restore responsiveness and pathogenic potential of these autoreactive T cells in gut-associated lymphoid tissues.
SFB-Induced Inflammation: Local Impact with Systemic Consequences
Following up on observations that IL-6 and IL-12p40 levels were elevated in the serum of SFB-harboring mice, we then performed in vivo neutralizing experiments to investigate the signals that mediate SFB’s impact on the local reactivation of autoreactive T cell in the gut-draining lymphoid tissues. Blocking IL-12p40 for just 7 days was sufficient to restore optimal T cell tuning in SFB-harboring hosts, suggesting that T cell tuning can be locally and dynamically regulated by flora-derived inflammatory signals. Blocking IL-6 or IL-23p19, however, had no effect, pointing toward a unique role for IL-12p70 in this model. Whether the unique attachment of SFB to the ileal mucosa contributes to the induction of a down-stream inflammatory cascade is still a matter of debate.Citation10 So far, most of the studies regarding SFB-derived molecular signals have focused on its non-redundant role in promoting mucosal Th17 differentiation. In the steady-state, IRF4-dependent CD103+CD11b+ DCs have been put forward as key producers of IL-6 and IL-23 in the gut, acting as mediators between the gut microbiota and the differentiating T cells.Citation28-Citation30 Although production of IL-12p70 by this DC subset could not be detected in these studies, such potential has already been detected in intestinal CD103+ DCs during colitis.Citation31 It is also interesting to see that in other contexts—tissues and/or pathologies—other cytokines have been demonstrated as key in regulating overall T cell activation locally. The example of IL-1 in the skinCitation6 or IL-23 and IL-15 in the gut, in models of colitisCitation32 and celiac disease respectively,Citation33 might indeed provide us with a more complex picture of cytokine-mediated regulation of T cell fitness in tissues in the steady-state and during inflammation.
Interestingly, and as observed for the maturation of polyclonal T cell responses in the gutCitation4,Citation5 and in the skin,Citation6 the impact of SFB on autoreactive T cells appeared mostly local, as no major differences in T-cell tuning were seen outside of gut-draining lymphoid organs and only minor differences seen as far away as the spleen later in the response. This is in striking opposition to the systemic autoimmune manifestations that ensue. Earlier studies in this model had shown that T-B interactions, and notably CD40/CD40L and IL-21-mediated signaling, were essential in the disease processCitation18 and led to the appearance of detectable high affinity autoantibodies 4 wk after T cell transfer. Indeed, SFB-harboring mPCC,Rag2−/− hosts—devoid of both T and B cells—were immune to the autoimmune manifestation normally observed in their Cd3e−/− counterparts, despite similar reactivation of transferred autoreactive T cells in their mLN. Interestingly, most aspects of the B cell response, from the generation of autoreactive Tfh to the formation of germinal centers and the differentiation of plasma cells (unpublished observation), were similarly localized to the mLN in SFB-harboring hosts. The first true signs of a systemic autoimmune response appeared to be the recirculating autoantibodies that could be detected in the serum of these hosts no earlier than 21 d after the initial T cell activation events. At these later time points, we cannot exclude a partial migration of autoreactive T and B responses to the spleen, as observed in the KxB/N model of arthritis.Citation15,Citation34 However, further studies would be needed to address their overall contribution to the disease process.
T Cell-Mediated Regulation of SFB’s Impact in the Steady State?
By contrast with our observations under lymphopenic conditions, it is interesting to note that the infusion of a similar number of 5CC7 T cells in T cell replete mPCC,Cd3e+/+ hosts never triggered any overt autoimmune manifestation, regardless of the presence or not of SFB in the microbiota. This is in line with previous observations, which showed, in immunocompetent hosts, that SFB colonization of the gut flora is not associated with overt pathologies in the gut.Citation10 Effects of SFB on autoreactive T cells in T-replete hosts were clearly evident at the most proximal point of SFB encounter—in the Peyer’s patches, with a close to 7-fold increased number of accumulated 5C.C7 T cells by day 10, associated with a 2-fold increase in proliferation. However, these differences were fully constrained in the mLNs, and overall differentiation of such cells appeared fully blunted.
This raises interesting questions about the cellular mechanisms acting in trans, which are called in to regulate such autoreactive responses in T-replete hosts. The tight control exerted by T cell-induced IgA on SFB colonization over time most probably serves to limit long-term chronic inflammation.Citation35 In our hands, SFB became barely detectable after 9–12 wk of age in T cell-replete mPCC,Cd3e+/+ hosts while stable levels of SFB could be detected for more than 3 mo in the ileum and cecum of their T cell-deficient mPCC,Cd3e−/− counterparts. Additionally, Tregs and IL-10-secreting CD4+ T cells are also enriched in the gut of SFB-monocolonized miceCitation4 and could play a key regulatory role, complementing intraclonal T cell-competition mechanisms.Citation36 Nonetheless, the simple fact that cell intrinsic breakdown of autoreactive T cell activation containment could be detected in SFB-harboring T cell-replete hosts suggests that if the second layer of trans-regulatory processes is somehow disrupted, the loss of cell intrinsic control might be expected to drive pathology in this host as well, a good example of the multifactorial nature of autoimmune diseases.
Conclusions
At the level of the intricate relationship between autoimmune diseases and the gut microbiota, this study clearly demonstrates that the impact of the gut microbiota on autoimmune diseases is not limited to the sole induction of pathogenic Th17. In that sense, this model is a key addition to several other arthritis models that have been found to be microbiota-dependent. Whether other members of the commensal flora can have a similar impact and whether SFB acts alone in this setting are still open questions. We did not perform monocolonization experiments in our system, and thus it is entirely possible that SFB acts here indirectly, as observed in models of colitisCitation13 or type 1 diabetes.Citation37
The most interesting result from this study, however, is the unique regulation of a T-cell intrinsic tolerance mechanism achieved by SFB-derived inflammatory signals. Recent observations suggest that commensal bacteria can also enhance anti-tumor T cell responses following chemotherapy.Citation38 It would be of great interest to see if similar mechanisms help restore T-cell responses against tumor or chronic viruses, two situations in which progressive loss of T cell responsiveness have been observed.Citation39-Citation41 A better understanding of the underlying mechanisms at play here could thus prove key in finding new targets to restore function in tuned and/or exhausted T cells.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Acknowledgments
The author would like to thank RH Schwartz, DA Gross, and M Lalfer for helpful comments on the manuscript and figures. The original work was supported by the Intramural Research Program of the NIAID at the National Institutes of Health, Bethesda, MD, USA. The author also acknowledges funding support from Agence Nationale de la Recherche (ANR-11-JSV3-0005).
References
- Buffie CG, Pamer EG. Microbiota-mediated colonization resistance against intestinal pathogens. Nat Rev Immunol 2013; 13:790 - 801; http://dx.doi.org/10.1038/nri3535; PMID: 24096337
- Kamada N, Seo S-U, Chen GY, Núñez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol 2013; 13:321 - 35; http://dx.doi.org/10.1038/nri3430; PMID: 23618829
- Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 2005; 122:107 - 18; http://dx.doi.org/10.1016/j.cell.2005.05.007; PMID: 16009137
- Gaboriau-Routhiau V, Rakotobe S, Lécuyer E, Mulder I, Lan A, Bridonneau C, Rochet V, Pisi A, De Paepe M, Brandi G, et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity 2009; 31:677 - 89; http://dx.doi.org/10.1016/j.immuni.2009.08.020; PMID: 19833089
- Chung H, Pamp SJ, Hill JA, Surana NK, Edelman SM, Troy EB, Reading NC, Villablanca EJ, Wang S, Mora JR, et al. Gut immune maturation depends on colonization with a host-specific microbiota. Cell 2012; 149:1578 - 93; http://dx.doi.org/10.1016/j.cell.2012.04.037; PMID: 22726443
- Naik S, Bouladoux N, Wilhelm C, Molloy MJ, Salcedo R, Kastenmuller W, Deming C, Quinones M, Koo L, Conlan S, et al. Compartmentalized control of skin immunity by resident commensals. Science 2012; 337:1115 - 9; http://dx.doi.org/10.1126/science.1225152; PMID: 22837383
- Cerf-Bensussan N, Gaboriau-Routhiau V. The immune system and the gut microbiota: friends or foes?. Nat Rev Immunol 2010; 10:735 - 44; http://dx.doi.org/10.1038/nri2850; PMID: 20865020
- Klaasen HL, Koopman JP, Van den Brink ME, Bakker MH, Poelma FG, Beynen AC. Intestinal, segmented, filamentous bacteria in a wide range of vertebrate species. Lab Anim 1993; 27:141 - 50; http://dx.doi.org/10.1258/002367793780810441; PMID: 8501895
- Yin Y, Wang Y, Zhu L, Liu W, Liao N, Jiang M, Zhu B, Yu HD, Xiang C, Wang X. Comparative analysis of the distribution of segmented filamentous bacteria in humans, mice and chickens. ISME J 2013; 7:615 - 21; http://dx.doi.org/10.1038/ismej.2012.128; PMID: 23151642
- Schnupf P, Gaboriau-Routhiau V, Cerf-Bensussan N. Host interactions with Segmented Filamentous Bacteria: an unusual trade-off that drives the post-natal maturation of the gut immune system. Semin Immunol 2013; 25:342 - 51; http://dx.doi.org/10.1016/j.smim.2013.09.001; PMID: 24184014
- Talham GL, Jiang HQ, Bos NA, Cebra JJ. Segmented filamentous bacteria are potent stimuli of a physiologically normal state of the murine gut mucosal immune system. Infect Immun 1999; 67:1992 - 2000; PMID: 10085047
- Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 2009; 139:485 - 98; http://dx.doi.org/10.1016/j.cell.2009.09.033; PMID: 19836068
- Stepankova R, Powrie F, Kofronova O, Kozakova H, Hudcovic T, Hrncir T, Uhlig H, Read S, Rehakova Z, Benada O, et al. Segmented filamentous bacteria in a defined bacterial cocktail induce intestinal inflammation in SCID mice reconstituted with CD45RBhigh CD4+ T cells. Inflamm Bowel Dis 2007; 13:1202 - 11; http://dx.doi.org/10.1002/ibd.20221; PMID: 17607724
- Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A 2011; 108:Suppl 1 4615 - 22; http://dx.doi.org/10.1073/pnas.1000082107; PMID: 20660719
- Wu H-J, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, Littman DR, Benoist C, Mathis D. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity 2010; 32:815 - 27; http://dx.doi.org/10.1016/j.immuni.2010.06.001; PMID: 20620945
- Chappert P, Bouladoux N, Naik S, Schwartz RH. Specific gut commensal flora locally alters T cell tuning to endogenous ligands. Immunity 2013; 38:1198 - 210; http://dx.doi.org/10.1016/j.immuni.2013.06.005; PMID: 23809163
- Tanchot C, Barber DL, Chiodetti L, Schwartz RH. Adaptive tolerance of CD4+ T cells in vivo: multiple thresholds in response to a constant level of antigen presentation. J Immunol 2001; 167:2030 - 9; PMID: 11489985
- Singh NJ, Chen C, Schwartz RH. The impact of T cell intrinsic antigen adaptation on peripheral immune tolerance. PLoS Biol 2006; 4:e340; http://dx.doi.org/10.1371/journal.pbio.0040340; PMID: 17048986
- Lubberts E, Koenders MI, Oppers-Walgreen B, van den Bersselaar L, Coenen-de Roo CJJ, Joosten LAB, van den Berg WB. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum 2004; 50:650 - 9; http://dx.doi.org/10.1002/art.20001; PMID: 14872510
- Inobe M, Schwartz RH. CTLA-4 engagement acts as a brake on CD4+ T cell proliferation and cytokine production but is not required for tuning T cell reactivity in adaptive tolerance. J Immunol 2004; 173:7239 - 48; PMID: 15585846
- Singh NJ, Schwartz RH. The strength of persistent antigenic stimulation modulates adaptive tolerance in peripheral CD4+ T cells. J Exp Med 2003; 198:1107 - 17; http://dx.doi.org/10.1084/jem.20030913; PMID: 14530379
- Chiodetti L, Choi S, Barber DL, Schwartz RH. Adaptive tolerance and clonal anergy are distinct biochemical states. J Immunol 2006; 176:2279 - 91; PMID: 16455984
- Choi S, Schwartz RH. Impairment of immunological synapse formation in adaptively tolerant T cells. J Immunol 2011; 187:805 - 16; http://dx.doi.org/10.4049/jimmunol.1003314; PMID: 21685322
- Grossman Z, Paul WE. Adaptive cellular interactions in the immune system: the tunable activation threshold and the significance of subthreshold responses. Proc Natl Acad Sci U S A 1992; 89:10365 - 9; http://dx.doi.org/10.1073/pnas.89.21.10365; PMID: 1438221
- Grossman Z, Paul WE. Autoreactivity, dynamic tuning and selectivity. Curr Opin Immunol 2001; 13:687 - 98; http://dx.doi.org/10.1016/S0952-7915(01)00280-1; PMID: 11677091
- Wherry EJ. T cell exhaustion. Nat Immunol 2011; 12:492 - 9; http://dx.doi.org/10.1038/ni.2035; PMID: 21739672
- Schwartz RH. T cell anergy. Annu Rev Immunol 2003; 21:305 - 34; http://dx.doi.org/10.1146/annurev.immunol.21.120601.141110; PMID: 12471050
- Kinnebrew MA, Buffie CG, Diehl GE, Zenewicz LA, Leiner I, Hohl TM, Flavell RA, Littman DR, Pamer EG. Interleukin 23 production by intestinal CD103(+)CD11b(+) dendritic cells in response to bacterial flagellin enhances mucosal innate immune defense. Immunity 2012; 36:276 - 87; http://dx.doi.org/10.1016/j.immuni.2011.12.011; PMID: 22306017
- Schlitzer A, McGovern N, Teo P, Zelante T, Atarashi K, Low D, Ho AWS, See P, Shin A, Wasan PS, et al. IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity 2013; 38:970 - 83; http://dx.doi.org/10.1016/j.immuni.2013.04.011; PMID: 23706669
- Persson EK, Uronen-Hansson H, Semmrich M, Rivollier A, Hägerbrand K, Marsal J, Gudjonsson S, Håkansson U, Reizis B, Kotarsky K, et al. IRF4 transcription-factor-dependent CD103(+)CD11b(+) dendritic cells drive mucosal T helper 17 cell differentiation. Immunity 2013; 38:958 - 69; http://dx.doi.org/10.1016/j.immuni.2013.03.009; PMID: 23664832
- Laffont S, Siddiqui KRR, Powrie F. Intestinal inflammation abrogates the tolerogenic properties of MLN CD103+ dendritic cells. Eur J Immunol 2010; 40:1877 - 83; http://dx.doi.org/10.1002/eji.200939957; PMID: 20432234
- Ahern PP, Schiering C, Buonocore S, McGeachy MJ, Cua DJ, Maloy KJ, Powrie F. Interleukin-23 drives intestinal inflammation through direct activity on T cells. Immunity 2010; 33:279 - 88; http://dx.doi.org/10.1016/j.immuni.2010.08.010; PMID: 20732640
- DePaolo RW, Abadie V, Tang F, Fehlner-Peach H, Hall JA, Wang W, Marietta EV, Kasarda DD, Waldmann TA, Murray JA, et al. Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens. Nature 2011; 471:220 - 4; http://dx.doi.org/10.1038/nature09849; PMID: 21307853
- Maccioni M, Zeder-Lutz G, Huang H, Ebel C, Gerber P, Hergueux J, Marchal P, Duchatelle V, Degott C, van Regenmortel M, et al. Arthritogenic monoclonal antibodies from K/BxN mice. J Exp Med 2002; 195:1071 - 7; http://dx.doi.org/10.1084/jem.20011941; PMID: 11956298
- Suzuki K, Meek B, Doi Y, Muramatsu M, Chiba T, Honjo T, Fagarasan S. Aberrant expansion of segmented filamentous bacteria in IgA-deficient gut. Proc Natl Acad Sci U S A 2004; 101:1981 - 6; http://dx.doi.org/10.1073/pnas.0307317101; PMID: 14766966
- Singh NJ, Bando JK, Schwartz RH. Subsets of nonclonal neighboring CD4+ T cells specifically regulate the frequency of individual antigen-reactive T cells. Immunity 2012; 37:735 - 46; http://dx.doi.org/10.1016/j.immuni.2012.08.008; PMID: 23021952
- Sofi MH, Gudi R, Karumuthil-Melethil S, Perez N, Johnson BM, Vasu C. pH of Drinking Water Influences the Composition of Gut Microbiome and Type 1 Diabetes Incidence. Diabetes 2014; 63:632 - 44; http://dx.doi.org/10.2337/db13-0981; PMID: 24194504
- Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillère R, Hannani D, Enot DP, Pfirschke C, Engblom C, Pittet MJ, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013; 342:971 - 6; http://dx.doi.org/10.1126/science.1240537; PMID: 24264990
- Oxenius A, Zinkernagel RM, Hengartner H. Comparison of activation versus induction of unresponsiveness of virus-specific CD4+ and CD8+ T cells upon acute versus persistent viral infection. Immunity 1998; 9:449 - 57; http://dx.doi.org/10.1016/S1074-7613(00)80628-7; PMID: 9806631
- Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med 1998; 188:2205 - 13; http://dx.doi.org/10.1084/jem.188.12.2205; PMID: 9858507
- Staveley-O’Carroll K, Sotomayor E, Montgomery J, Borrello I, Hwang L, Fein S, Pardoll D, Levitsky H. Induction of antigen-specific T cell anergy: An early event in the course of tumor progression. Proc Natl Acad Sci U S A 1998; 95:1178 - 83; http://dx.doi.org/10.1073/pnas.95.3.1178; PMID: 9448305
