Abstract
Tolerance established by host-commensal interactions regulates host immunity at both local mucosal and systemic levels. The intestinal commensal strain Bacteroides fragilis elicits immune tolerance, at least in part, via the expression capsular polysaccharide A (PSA). How such niche-specific commensal microbial elements regulate extra-intestinal immune responses, as in the brain, remains largely unknown. We have recently shown that oral treatment with PSA suppresses neuro-inflammation elicited during experimental autoimmune encephalomyelitis (EAE), an animal model for multiple sclerosis. This protection is dependent upon the expansion of immune-regulatory CD4 T cells (Treg) expressing CD39, an ectonucleotidase. Here, we further show that CD39 modulation of purinergic signals enhances migratory phenotypes of both total CD4 T cells and Foxp3+ CD4 Tregs at central nervous system (CNS) lymphoid-draining sites in EAE in vivo and promotes their migration in vitro. These changes are noted during PSA treatment, which leads to heightened accumulation of CD39+ CD4 Tregs in the CNS. Deficiency of CD39 abrogates accumulation of Treg during EAE, and is accompanied by elevated Th1/Th17 signals in the CNS and in gut-associated lymphoid tissues. Our results demonstrate that immune-modulatory commensal bacterial products impact the migratory patterns of CD4 Treg during CNS autoimmunity via the regulation of CD39. These observations provide clues as to how intestinal commensal microbiome is able to modulate Treg functions and impact host immunity in the distal site.
Introduction
Host-commensal bacterial interactions contribute to the maintenance of health. Humans are colonized with a complex microbial ecosystem that incorporates a huge quantity of commensal bacteria from myriads of phylogenetic divisions. Among the colonized body niches, the lower gastrointestinal tract harbors the greatest quantity of commensals, most of which fall under the genus Bacteroides and the phylum Firmicutes.Citation1,Citation2 Historically, the impact of intestinal microbiota on host metabolism, mucosal barrier integrity, and defense against infection has been studied. Recent findings have revealed the effects of intestinal microbiota on host immunity at both local and systemic sites.Citation1,Citation3 Commensal microbes and their products regulate immunity at homeostatic and inflammatory conditions.Citation4 Notably, alteration of intestinal microbiota affects autoimmunity targeting the distal site of central nervous system (CNS). In experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis, re-colonization of germ-free mice with Segmented Filamentous Bacteria (SFB) restores the severity of EAE whereas antibiotic modulation of normal mice intestinal microbiota decreases EAE severity.Citation5,Citation6 Furthermore, probiotic treatment with an intestinal symbiont strain Bacteroides fragilis or oral administration of its capsular polysaccharide PSA can effectively induce immune tolerance and suppress EAE development.Citation7-Citation9
Although it appears that commensal microbiota influence extra-intestinal autoimmunity, it remains unclear how such signals are transmitted during host-intestinal microbiota interactions. While microbiota are restricted from contact with host tissues by mucosal barriers, anti-microbial peptides and defensive humoral responses, e.g., non-specific IgA, there also exist diverse mechanisms that allow for extra-intestinal immune effects of intestinal commensal microbiota. First, the diffusion of commensal-derived microbial-associated molecular patterns (MAMPs) and microbial metabolites into the circulation, even at low amount, is able to affect the process of hematopoiesis.Citation10,Citation11 Second, CD103+ dendritic cells or CX3CR1hi mononuclear phagocytes capture and transport luminal commensal bacteria and antigens to mesenteric lymph nodes (MLNs) where priming of lymphocytes occurs.Citation12,Citation13 It is unknown whether lumen-sensing antigen presenting cells (APCs) can traffic beyond the intestine-to-MLN route and guide immune maturation at distal inductive sites. Third, lymphocytes developed at intestinal mucosal sites can traffic into peripheral sites without the mediation of APCs. Endoscopic photo-conversion has identified the efflux of a broad range of immune subsets from the colon to proximal and distant lymphoid organs at both steady and autoimmune conditions.Citation14
How immune cells traffic between inflammatory sites and draining lymph nodes (DLNs) determines clinical outcomes of autoimmune diseases. In the case of the EAE model, which is traditionally viewed as a CD4 T cell-driven condition, how CD4 T cell subsets traffic between the CNS and cervical lymph nodes (CLNs) is particularly important. Regulatory subsets among CD4 T cells can counter-act inflammatory immune subsets and ameliorate autoimmunity. Both Foxp3+ (conventional) and non-Foxp3 regulatory CD4 T cells (Treg) have been characterized. As the suppressive ability of CD4 Tregs can be contact-dependent, or more effective in close paracrine type interactions with effector cells, it is clear that migration to sites of injury optimize the functions of Tregs.Citation15 The intestine, or more broadly, the gut-associated lymphoid tissues (GALT) plays a crucial role in shaping T cell phenotype and migration. There are indications that a bulk of Tregs home to GALT in early life.Citation16 Exposure to intestinal commensal microbial products could potentially trigger the migratory capacity of functional Tregs as a part of the peripheral education.
To this end, treatment of EAE with B. fragilis PSA via the oral route presents a unique opportunity to investigate how commensal products might influence the homing patterns of leukocytes at distant locations. Our previous and current studies have shown that protective functions of PSA involve the expansion of regulatory CD4 T cell subsets.Citation7,Citation9 Here, we further define which cell-associated signals define CNS homing patterns of Tregs. In this manuscript addendum, we find that CD39, an established Treg marker that has been shown to denote regulatory capacity mediated by generation of extracellular adenosine,Citation17 can also modulate migratory capacity in both total CD4 T cells and Foxp3+ CD4 Tregs. Ablation of CD39 signaling results in exacerbated EAE and is correlated with decreased levels of Tregs homing at into the inflamed CNS.
Results
CD39 defines an enhanced expression of migratory markers in total CD4 T cells and Foxp3+ CD4 Tregs
Bacteroides fragilis is a Gram-negative commensal anaerobe prevalent in the GI tracts of mammals, which has been characterized as a prominent symbiont due to its repression of mucosal inflammation.Citation18,Citation19B. fragilis relies on its capsular PSA as a symbiosis factor to actively tune the host immunity in favor of its colonization.Citation19,Citation20 Purified PSA ameliorates host inflammation at both mucosal sites in the case of inflammatory bowel disease (IBD) and neural sites in the case of EAE.Citation7,Citation19 Herein, we continue investigating the mechanisms of CD4 Treg-mediated EAE protection by PSA. Wild-type C57BL/6 (B6) mice were prophylactically treated with PSA or PBS as the vehicle control every 3 d during optimal active EAE. The treatment commenced 6 d before and ended 9 d after EAE induction. As revealed by the clinical curves followed up to day 25 (), PSA delayed the onset and reduced the severity of EAE development, consistent with our previous results. Immune responses in the CNS require efferent and afferent pathways. It has been shown that the transit of antigens from the CNS to the CNS-draining CLNs is required for the induction of encephalitogenic immune responses.Citation21,Citation22 Hence, phenotypes and functions of lymphocytes within CLNs may determine EAE outcomes. We analyzed total CD4 T cells within CLNs from PSA- or PBS-treated mice at day 18 EAE (defined as the onset and/or peak stage). Using CD39 and Foxp3 as markers, we demonstrated a dramatic increase of CD39+ fraction among CD4 T cells in PSA-treated mice. More precisely, both CD39+ CD4 T cells and CD39+Foxp3+ CD4 Tregs were upregulated by PSA ().
Figure 1. CD39 defines enhanced expression of migratory markers in total CD4 T cells and Foxp3+ CD4 Tregs. Wild-type C57BL/6 mice were treated with 100 μg of purified Bacteroides fragilis PSA or PBS control by oral gavage every 3 d. Treatment was initiated 6 d before the induction of optimal active EAE and terminated 9 d after disease induction. (A) EAE clinic scores were monitored till day 25. Depicted are the combined results of two independent experiments (n = 8, per group). *, P < 0.05; **, P < 0.01 (Mann-Whitney U-test). (B) Cervical lymph nodes (CLNs) were harvested from mice of day 18 EAE. Expression of CD39 vs. Foxp3 on CD4 T cells was analyzed by flow cytometry. Representative flow plots for indicated groups are shown. (C) CD39+ CD4 T cells have elevated migratory signatures as compared with CD39- CD4 T cells. CD39+ and CD39- CD4 T cells sourced as in (B) were flow profiled for the expression of each indicated chemokine receptor. Left, representative histograms for individual marker. Red line, CD39+CD4 T cells; blue line, CD39-CD4 T cells; gray shade, isotype. Right, MFI value for individual marker was quantified and compared. (D) CD39+ Foxp3+CD4 Tregs have elevated migratory signatures as compared with CD39- Foxp3+CD4 Tregs. Similar as in (C), CD39+ and CD39- Foxp3+CD4 Tregs subsets were analyzed for the expression of each indicated chemokine receptor. Left, representative histograms. Red line, CD39+Foxp3+ CD4 Tregs; blue line, CD39-Foxp3+ CD4 Tregs; gray shade, isotype. Right, MFI quantification and comparison. P value was calculated by two-tailed Student’s t test in (C) and (D) (n = 4–5 for each group).
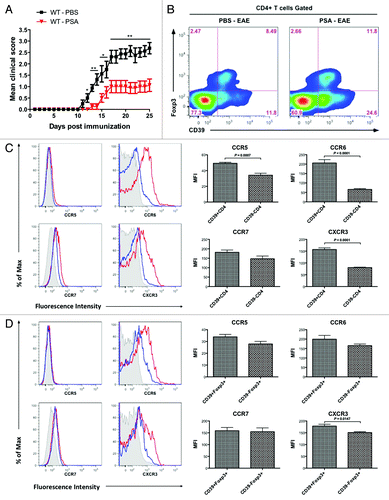
We have shown that PSA elicitation of CD39 signaling defined enhanced levels of anti-inflammatory cytokines and surface markers in regulatory type CD4 T cells irrespective of Foxp3 presence. We hereby tested whether CD39 also defines the homing capacity of CD4 T cell subsets. CLN-sourced CD4 T cells from peak stage EAE mice were gated into CD39+ and CD39- subsets and phenotyped for the levels of chemokine receptors CCR5, CCR6, CCR7, and CXCR3 (). CD39+ CD4 T cells expressed significantly higher levels of CCR5, CCR6, and CXCR3, but not CCR7, than do CD39- CD4 T cells. To study whether CD39 confers intrinsic migratory phenotypes, CLN Foxp3+ CD4 Tregs sourced from the same setting were gated based on CD39 expression and analyzed for the same array of chemokine receptors (). CD39+Foxp3+ CD4 Tregs were noted for a significant higher level of CXCR3, a relatively higher level of CCR5 and CCR6 but a similar level of CCR7 when compared with CD39-Foxp3+ CD4 Tregs. Collectively, CD39 defines enhanced migratory phenotypes in CD4 T cells not as a collateral effect of Foxp3.
Oral treatment with Bacteroides fragilis PSA upregulates CD39+ CD4 T cells and CD39+Foxp3+ CD4 Tregs in the CNS during murine EAE
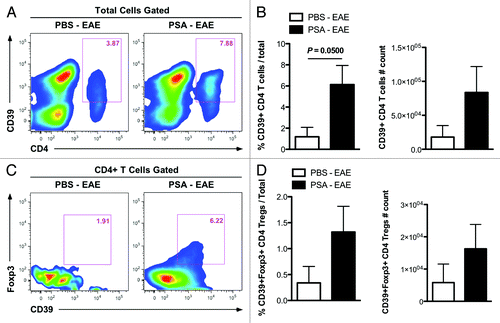
As PSA-stimulated CD39+ CD4 T cells and CD39+Foxp3+ CD4 Tregs are phenotypically more migratory, we set to examine whether oral treatment of EAE mice with PSA results in the elevated presence of these CD39+ CD4 T cell subsets in the CNS. Wild-type B6 mice were orally treated with PSA or PBS control and induced with EAE as in . CNS-infiltrating leukocytes isolated from peak disease stage mice were analyzed by flow cytometry. Indeed, there were increased proportions and numbers of CD39+ CD4 T cells among total leukocytes in the CNS of PSA-treated mice as opposed to the control group (). Moreover, among CNS-infiltrating CD4 T cells, the percentage and total quantity of CD39+Foxp3+ subset among CNS-infiltrating CD4 T cells were enhanced by PSA treatment during EAE (). These observations, in concert with the results of our forthcoming study that PSA expanded CD39+ CD4 T cells in MLNs and CLNs of EAE mice (data not shown), suggest that PSA administration at intestinal mucosae triggers systemic immune-modulation at GALTs and inflamed sites of EAE mice.
Figure 2. Oral treatment with Bacteroides fragilis PSA upregulates accumulation of CD39+ CD4 T cells and CD39+Foxp3+ CD4 Tregs in the CNS during murine EAE. Wild-type C57BL/6 mice were orally treated with PSA or PBS control and induced with optimal active EAE as in . At day 18 post EAE induction, CNS-infiltrating immune cells were isolated and analyzed by flow cytometry. (A) Frequencies of CD39+ CD4 T cells among total cells were compared. Representative flow plots are shown. (B) Frequencies and absolute numbers of CD39+ CD4 T cells in (A) were quantified (n = 4, per group). P value was calculated by two-tailed Student’s t test. (C) Frequencies of CD39+Foxp3+ CD4 Tregs among total CD4 T cells were compared. Representative flow plots are shown. (D) Frequencies and absolute numbers of CD39+Foxp3+ CD4 Tregs within total cells were quantified (n = 4, per group).
PSA-sensitized CD39+ CD4 T cells display high migratory capacity toward CNS extracts of EAE mice in vitro
The concomitant expansion of CD39+ CD4 T cell subsets at CLNs and the CNS in vivo during PSA amelioration of EAE could be explained by the high migratory capacity of CD39+ T cells or the de novo induction of this population at both sites. To study whether CD39 defines higher CNS-tropic migratory activities in CD4 T cells, in vitro migration assay was adopted. CLN leukocytes sourced from PSA- or PBS-treated EAE-induced WT B6 mice were tested for chemotactic migration toward CNS homogenates of naïve or EAE mice (). Flow cytometric analysis revealed a higher frequency of CD39+ subset within total CD4 T cells that migrated to the CNS homogenates from EAE mice as compared with those of naïve mice. This effect was observed for CLN leukocytes from both naïve and EAE sources. Furthermore, when comparing PSA- and PBS-treated group, we found a base level increase of CD39+ CD4 T cells that migrated to CNS homogenates derived at both conditions. These findings indicate that CD39+ CD4 T cell subsets have higher migratory proclivities toward inflamed CNS tissues, and that PSA sensitization strengthens their migratory functions. As reported by other groups and us, there is an increased expression of chemokines and adhesion molecules in the CNS of EAE mice.Citation22 While this facilitates the recruitment of pro-inflammatory immune subsets into the CNS, regulatory subsets may use the same address code to target and suppress CNS inflammation. Our results suggest that CD39 could be a key marker to define migrating effector CD4 Tregs.
Figure 3. PSA-sensitized CD39+ CD4 T cells display increased migratory capacity toward CNS extracts of EAE mice in vitro. The chemotactic migration of CD39+ vs. CD39- CD4 T cells to CNS extracts of naïve or EAE mice was analyzed by transwell migration assay. (A) CLN leukocytes from PSA- or PBS-treated EAE-induced C57BL/6 mice were added to the insert well, and the lower chambers were supplied with naïve or EAE murine CNS extracts. Twenty-four hr after incubation, cells migrated into the lower chambers were analyzed by flow cytometry. Frequencies of CD39+ and CD39- subsets within CD4 T cells were compared for all conditions. Representative flow plots are shown. (B) CLN leukocytes from PSA- or PBS-treated EAE-induced 2D2 mice (MOG35–55 TCR Tg mice) were added to the insert well, and lower chambers were conditioned as in (A). The same analysis was performed 24 h after incubation, and representative flow plots are shown.
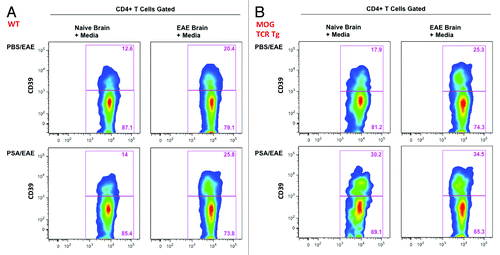
Myelin oligodendrocyte glycoprotein (MOG) is a CNS auto-antigen used for the induction of active EAE. The T cell receptors (TCR) of 2D2-TCR transgenic mice are specific for the MOG 35–55 epitope. CLN leukocytes sourced from PSA- or PBS-treated EAE-induced 2D2 mice were tested for chemotactic migration in the same setting as (). Identical migratory patterns were revealed by flow cytometric analysis of CD39+ subset among CD4 T cells. Remarkably, all groups in exhibited a base level increase of migratory CD39+ CD4 T cells as compared with the corresponding groups in . This implicates that TCR specificity for myelin antigen may further enhance the migration of regulatory CD39 Tregs into the CNS.
CD39 signaling is required for the reciprocal control of total leukocytes and CD4 Tregs accumulation in the CNS during PSA prevention of EAE
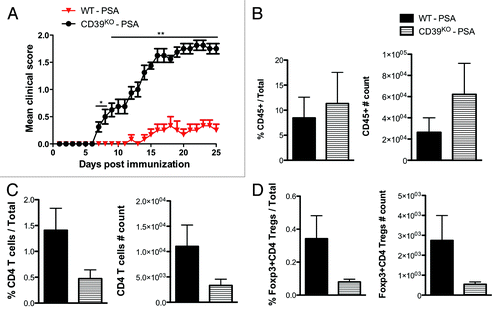
As PSA elicitation of CD39 signals ameliorated EAE and promoted the migration of CD4 T cell subsets, we aim to investigate the requirement for CD39 signals in PSA-mediated immune suppression and migratory effects. WT and CD39KO mice on B6 background were prophylactically treated with PSA or PBS control as in and induced with sub-optimal active EAE (). Sub-optimal EAE was used to highlight clinic differences. Clinical curve showed that PSA protection of EAE observed in WT mice was abrogated in CD39KO mice. This outcome was not due to base level differences as non-treated WT and CD39KO mice developed comparable EAE (data not shown). PSA-treated CD39KO mice displayed higher frequency and absolute number of CD45+ leukocytes among total CNS infiltrating cells than did PSA-treated WT mice (), consistent with our previous findings in histological staining of CNS tissues from these two groups. Meanwhile, it was noted that PSA-treated CD39KO mice displayed reduced frequency and absolute number of CD4 T cells () and Foxp3+ CD4 Tregs () among total CNS infiltrating cells than did PSA-treated WT mice. Therefore, CD39 signaling is required for the PSA-mediated immune regulation during EAE, which is associated with its ability to control the reciprocal entry of total leukocytes and CD4 T cell subsets into the CNS. The diminished infiltration of CD4 T cell subsets could be linked to a deficiency of CNS-tropic CD39+ CD4 T cells and CD39+Foxp3+ CD4 Tregs.
Figure 4. CD39 signaling is required for the reciprocal control of total leukocytes and CD4 Tregs accumulation in the CNS during PSA prevention of EAE. Wild-type and CD39KO C57BL/6 mice were orally treated with 100 μg of PSA every 3 d from day -6 to day +9 of the induction of sub-optimal active EAE. (A) EAE clinic scores were monitored till day 25. Depicted are the combined results of two independent experiments (n = 8, per group). *, P < 0.05; **, P < 0.01 (Mann-Whitney U-test). (B-D), CNS-infiltrating immune cells were harvested from mice of day 18 sub-optimal EAE and analyzed by flow cytometry. (B) Frequencies and absolute numbers of CD45+ leukocytes were quantified (n = 4, per group). (C) Frequencies and absolute numbers of CD4 T cells (n = 4, per group). (D) Frequencies and absolute numbers of Foxp3+ CD4 Tregs (n = 4, per group).
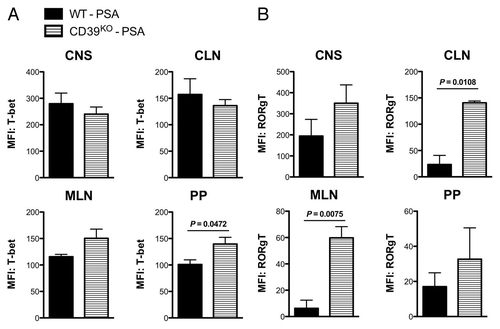
Deficiency of CD39 signaling correlates with elevated Th1/Th17 markers in the CNS and gut mucosal sites during PSA prevention of EAE
As PSA stimulates CD39+ CD4 T cell subsets at the tissue sites proximal and distal to intestine during EAE, we finally aim to examine whether CD39 signaling is required for the regulation of CD4 T cell-associated pro-inflammatory signals at corresponding sites. Th1 and Th17 cells are main CD4 helper T cell subsets that drive the pathogenesis of MS and EAE. Adoptive transfer of Th1 or Th17 cells can lead to different patterns of passive EAE.Citation23,Citation24 T-box expressed in T cells (T-bet) and retinoid-related orphan receptors (ROR) γt are the signature transcriptional factors for Th1 and Th17, respectively. We measured the CD4 T cell-associated intracellular expression of T-bet and RORγt by flow cytometry in WT and CD39KO mice subjected to the same regimen as in . Intestine mucosae-drainage sites (Peyer’s patches, MLNs) and distal tissue sites (CLNs, CNS) are surveyed. In comparison with PSA-treated WT mice, PSA-treated CD39KO mice showed higher T-bet expression within CD4 T cells at MLNs and PP, but not at CLNs or in the CNS when compared with PSA-treated WT mice (). On the other hand, CD4 T cells from PSA-treated CD39KO mice displayed a systemic higher RORγt expression across these tissues than did those from PSA-treated WT mice, with the most evident differences in MLNs and CLNs (). This is consistent with results in parallel studies that PSA-mediated expansion of CD39+ CD4 T cells occurred specifically in MLNs and CLNs of EAE mice and that CD39 was required for the suppression of IL-17A rather than IFNγ responses. In conclusion, PSA-mediated CD39 signals that define the migratory activities of regulatory CD4 T cell subsets are indispensable for the regulation of pro-inflammatory responses at systemic sites and in the CNS.
Figure 5. Deficiency of CD39 signaling correlates with elevated Th1/Th17 markers in the CNS and gut mucosal sites during PSA prevention of EAE. Wild-type and CD39KO C57BL/6 mice were treated and induced with sub-optimal EAE as in . At the day 18 of disease, total leukocytes were isolated from the CNS, cervical lymph nodes (CLNs), mesenteric lymph nodes (MLNs) and Peyer’s patches and flow cytometry was performed. MFI levels of (A) T-bet (Th1 transcription factor) and (B) RORγt (Th17 transcription factor) within CD4 T cells were quantified for the indicated tissue sites. For both (A) and (B), n = 4 per group. P value was calculated by two-tailed Student’s t test.
Discussion
While it has been established that the intestinal commensal microbiome influences host immunity, it is not fully understood how this interaction impacts the migration of immune cells. Circulatory TLR ligands can drive the emigration of monocytes from bone marrow.Citation11 How commensal-derived MAMPs can access primary lymphoid organs is yet largely unknown. On the other hand, it is easier to speculate that commensals may impact the migration of immune cells at secondary lymphoid organs, especially at GALTs, which are permeated by commensal products. Previous research indicates that the common commensal metabolites, short chain fatty acids (SCFAs), can induce neutrophil chemotaxis through the GPR43 receptor in vitro.Citation25 Here we show that B. fragilis PSA, a commensal-derived TLR2 ligand, can induce the chemotaxis of CD4 T cell subsets likely through CD39 modulation of purinergic signals in vivo. We have demonstrated that PSA specifically favors the expansion of CD39+ CD4 T cells at MLNs and CLNs (data not shown).
The GI tract is loaded with a wide range of foreign antigens. It has been suggested that encounter with commensal microbiota is a key step in the peripheral education of host immune system, particularly the reactivity of Tregs.Citation26,Citation27 While the current study cannot conclude where migratory CD39+ CD4 T subsets originate and how CD39 coordinates stimulatory or chemotactic cell signals, our data may imply that GALT serve as critical inductive sites for these Tregs. We are able to suggest herein that the peripheral education by commensal products may also imprint the homing patterns of Tregs, another hallmark of Treg maturation.
CD39 is a cell surface member of the ecto-nucleoside triphosphate diphosphohydrolase (E-NTPDase) family. In collaboration with the ecto-5′-nucleotidase CD73, it converts the pro-inflammatory extraceullar ATP into the anti-inflammatory extracellular adenosine.Citation28 ATP hydrolyzing activity is present at the surface of many cell types. In accordance, the presence of CD39 mediates immune-inhibitory functions in multiple immune subsets.Citation17,Citation29-Citation35 Our work and others show that within CD4 T cells, CD39 is a marker that delineates regulatory subsets independent of Foxp3.Citation36,Citation37
It can be inferred that CD39+ CD4 T cells may comprise diverse regulatory subsets, including the canonical Foxp3+ CD4 Tregs, IL-10-secreting T regulatory type 1 (Tr1) cells and TGF-β-secreting T helper 3 (Th3) cells. The profile of chemokine receptors on CD39+ CD4 T cells suggests this postulation. As Foxp3+ CD4 Tregs express CCR5 and CCR6 and Tr1 cells express CCR5 and CXCR3, CD39+ CD4 T cells are coincidentally noted to express significantly higher levels of CCR5, CCR6, and CXCR3.Citation15 The migratory phenotypes of Tregs are associated with their inflammation-seeking activities, as inflamed tissue sites often have exacerbated levels of chemokines.Citation38 We found that the CNS of EAE mice had a significantly enhanced transcription of CXCL10 (ligand for CXCR3) and CCL5 (ligand for CCR5) in the CNS of diseased mice (data not shown). This might explain the expanded CD39+ regulatory CD4 T cell subsets in the CNS as a result of migration in response to CNS inflammation.
There is recent interest in how ectonucleotidase expression by immune cells impacts their functionality, migration, and chemotaxis. CD39 modulates the hematopoietic stem cell recruitment during partial hepatectomy.Citation39 CD39 also modulates responses to chemotactic stimuli via extracellular nucleotides in diverse myeloid-origin immune cells.Citation40-Citation42 Within the immune system, spatial and temporal expression of CD39 and other ectonucleotidases closely regulates phosphohydrolytic activities and the scavenging of extracellular nucleotides to generate adenosine.Citation43 This not only maintains the functional integrity of purinergic P2-receptors but also orchestrates adenosinergic signaling responses regulating leukocytic adhesion, migration, and chemotaxis. These mechanisms are crucial in the immune and vascular homeostasis during inflammation.
Th17 and Treg cells play opposite roles in autoimmune diseases, yet the developmental pathways of Th17 and Treg cells are closely related.Citation44 Futher, Th17 and Treg cells have similar migratory properties and share CCR6 as a key migratory marker.Citation15 Remarkably, there is plasticity between Th17 and Treg cells. FoxP3+RORγt+ CD4 T cells have been identified in mouse and human that can suppress effector cells while secreting IL-17.Citation45,Citation46 In mice, these CD4 T cells are found in the intestinal lamina propria. Environmental cues, especially commensal microbiome, may therefore guide the terminal differentiation of this transient subset into Th17 or Tregs cells. Indeed, the intestinal site is important for the development of both cell types. While B. fragilis and PSA can induce intestinal Foxp3+ Tregs, segmented filamentous bacteria and their antigens are found to induce Th17 cells.Citation47-Citation49 Interestingly, CD39 has been associated with both CD4 Tregs and IL-17-secreting memory CD4 T cells.Citation50 It appears that CD39 imparts plasticity to Th17 and Treg cells. Based on our findings, we further speculate CD39 as a responsive element to intestinal commensals that can tune the mucosal and systemic balance of Th17 and Tregs.
Methods
Mice and treatment
C57BL/6 mice were obtained from the Jackson Laboratories. CD39KO mice were generated as described by Enjyoji et al.Citation51 and obtained from Dr Simon C Robson (Harvard University). Mice were treated with 100 μg of purified B.fragilis PSA or PBS control by oral gavage every 3 d. Treatment begins 6 d before EAE induction and terminates 9 d after disease induction. All animal care and procedures were in accordance with protocols approved by the Institutional Animal Care and Use Committee of Dartmouth College.
EAE induction
Active optimal or sub-optimal EAE was induced with sub-cutaneous (s.c.) injection of 200 or 160 μg MOG35–55 peptide (Peptides International) respectively in 150 μl of Complete Freund’s Adjuvant (Sigma-Aldrich). On day 0 and 2 post-immunization, intraperitoneal (i.p.) injection of 400 or 300 ng of Bordetella pertussis toxin (PT; List Biological Laboratories) was respectively performed for optimal or sub-optimal EAE induction. EAE severity was scored daily using the standard scale.
Flow cytometric analysis
Singe cell preparations were isolated from indicated tissue sites and flow stained using conventional methods. Fluorochrome-conjugated mAbs specific to individual markers were purchased from eBioscience, BD Biosciences, and Biolegend. Intracellular stain was performed for transcriptional factors T-bet and RORγt. Stained cells were scanned by MACSQuant Analyzers (Miltenyi).
In vitro migration assay
A migration assay was performed in 24-well plates (Costar) that carry transwell-permeable supports with an 8 μm polycarbonate membrane for leukocytes. CNS homogenate from naïve or EAE-induced C57BL/6 mice were coursed through 40μm filter and supplied to the lower chambers. Cells were incubated for 24 h at 37 °C and 5% CO2 atmosphere. Migratory cells were analyzed by flow cytometry.
Statistical analysis
Two-tailed Student’s t test was used to show statistical differences of fluorescence intensity or cell frequencies and numbers in FACS. Mann-Whitney U-test was applied to day-to-day comparison of clinic scores. Data are shown as means ± SEM P values <0.05, <0.01 and <0.001 were indicated.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Acknowledgments
We thank Dr Randolph J Noelle (Dartmouth College; King’s College London, UK) for research guidance. We thank DartLab (Dartmouth College) members Dr Jacqueline Y Smith, Dr. Daniel W Mielcarz, John DeLong, Gary A Ward, Alan J Bergeron, and Nathan Martin for technical support of flow cytometry and critical guidance of research. We thank Kathryn A Bennett (Dartmouth College) for animal care. We thank Dr Azizul Haque (Dartmouth College) for critical review. This work was supported by National Multiple Sclerosis Society grant RG 4662A2/1 and National Institute of Allergy and Infectious Diseases grants (1R41 AI110170-01 and 1R56 AI098282-01A1). S.C.R. was supported by National Institute of Health grants (P01 HL087203 and U19 AI090959) and Helmsley Charitable Trust.
Author Contributions
Y.W. and S.B-H. performed experiments and did the research. Y.W. designed research, prepared the figures and wrote the manuscript. K.M.T. and J.O-R. contributed research design and data. M.C. and E.J.K. contributed experiments. D.L.K. graciously provided PSA and critical comments on the research. S.C.R. contributed key mouse strain and critical comments on the manuscript. L.H.K. supervised the research and reviewed the manuscript.
References
- Wang Y, Kasper LH. The role of microbiome in central nervous system disorders. Brain Behav Immun 2014; 38:1 - 12; http://dx.doi.org/10.1016/j.bbi.2013.12.015; PMID: 24370461
- Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol 2012; 10:735 - 42; http://dx.doi.org/10.1038/nrmicro2876; PMID: 23000955
- Belkaid Y, Naik S. Compartmentalized and systemic control of tissue immunity by commensals. Nat Immunol 2013; 14:646 - 53; http://dx.doi.org/10.1038/ni.2604; PMID: 23778791
- Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell 2014; 157:121 - 41; http://dx.doi.org/10.1016/j.cell.2014.03.011; PMID: 24679531
- Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A 2011; 108:Suppl 1 4615 - 22; http://dx.doi.org/10.1073/pnas.1000082107; PMID: 20660719
- Ochoa-Repáraz J, Mielcarz DW, Ditrio LE, Burroughs AR, Foureau DM, Haque-Begum S, Kasper LH. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J Immunol 2009; 183:6041 - 50; http://dx.doi.org/10.4049/jimmunol.0900747; PMID: 19841183
- Ochoa-Repáraz J, Mielcarz DW, Wang Y, Begum-Haque S, Dasgupta S, Kasper DL, Kasper LH. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol 2010; 3:487 - 95; http://dx.doi.org/10.1038/mi.2010.29; PMID: 20531465
- Ochoa-Repáraz J, Mielcarz DW, Haque-Begum S, Kasper LH. Induction of a regulatory B cell population in experimental allergic encephalomyelitis by alteration of the gut commensal microflora. Gut Microbes 2010; 1:103 - 8; http://dx.doi.org/10.4161/gmic.1.2.11515; PMID: 21326918
- Ochoa-Repáraz J, Mielcarz DW, Ditrio LE, Burroughs AR, Begum-Haque S, Dasgupta S, Kasper DL, Kasper LH. Central nervous system demyelinating disease protection by the human commensal Bacteroides fragilis depends on polysaccharide A expression. J Immunol 2010; 185:4101 - 8; http://dx.doi.org/10.4049/jimmunol.1001443; PMID: 20817872
- Khosravi A, Yáñez A, Price JG, Chow A, Merad M, Goodridge HS, Mazmanian SK. Gut microbiota promote hematopoiesis to control bacterial infection. Cell Host Microbe 2014; 15:374 - 81; http://dx.doi.org/10.1016/j.chom.2014.02.006; PMID: 24629343
- Shi C, Jia T, Mendez-Ferrer S, Hohl TM, Serbina NV, Lipuma L, Leiner I, Li MO, Frenette PS, Pamer EG. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity 2011; 34:590 - 601; http://dx.doi.org/10.1016/j.immuni.2011.02.016; PMID: 21458307
- Diehl GE, Longman RS, Zhang JX, Breart B, Galan C, Cuesta A, Schwab SR, Littman DR. Microbiota restricts trafficking of bacteria to mesenteric lymph nodes by CX(3)CR1(hi) cells. Nature 2013; 494:116 - 20; http://dx.doi.org/10.1038/nature11809; PMID: 23334413
- Schulz O, Jaensson E, Persson EK, Liu X, Worbs T, Agace WW, Pabst O. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med 2009; 206:3101 - 14; http://dx.doi.org/10.1084/jem.20091925; PMID: 20008524
- Morton AM, Sefik E, Upadhyay R, Weissleder R, Benoist C, Mathis D. Endoscopic photoconversion reveals unexpectedly broad leukocyte trafficking to and from the gut. Proc Natl Acad Sci U S A 2014; 111:6696 - 701; http://dx.doi.org/10.1073/pnas.1405634111; PMID: 24753589
- Ding Y, Xu J, Bromberg JS. Regulatory T cell migration during an immune response. Trends Immunol 2012; 33:174 - 80; http://dx.doi.org/10.1016/j.it.2012.01.002; PMID: 22305714
- Grindebacke H, Stenstad H, Quiding-Järbrink M, Waldenström J, Adlerberth I, Wold AE, Rudin A. Dynamic development of homing receptor expression and memory cell differentiation of infant CD4+CD25high regulatory T cells. J Immunol 2009; 183:4360 - 70; http://dx.doi.org/10.4049/jimmunol.0901091; PMID: 19734224
- Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med 2007; 204:1257 - 65; http://dx.doi.org/10.1084/jem.20062512; PMID: 17502665
- Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol 2009; 9:313 - 23; http://dx.doi.org/10.1038/nri2515; PMID: 19343057
- Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 2008; 453:620 - 5; http://dx.doi.org/10.1038/nature07008; PMID: 18509436
- Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, Mazmanian SK. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 2011; 332:974 - 7; http://dx.doi.org/10.1126/science.1206095; PMID: 21512004
- van Zwam M, Huizinga R, Heijmans N, van Meurs M, Wierenga-Wolf AF, Melief MJ, Hintzen RQ, ’t Hart BA, Amor S, Boven LA, et al. Surgical excision of CNS-draining lymph nodes reduces relapse severity in chronic-relapsing experimental autoimmune encephalomyelitis. J Pathol 2009; 217:543 - 51; http://dx.doi.org/10.1002/path.2476; PMID: 19023878
- Weller RO, Engelhardt B, Phillips MJ. Lymphocyte targeting of the central nervous system: a review of afferent and efferent CNS-immune pathways. Brain Pathol 1996; 6:275 - 88; http://dx.doi.org/10.1111/j.1750-3639.1996.tb00855.x; PMID: 8864284
- O’Connor RA, Prendergast CT, Sabatos CA, Lau CW, Leech MD, Wraith DC, Anderton SM. Cutting edge: Th1 cells facilitate the entry of Th17 cells to the central nervous system during experimental autoimmune encephalomyelitis. J Immunol 2008; 181:3750 - 4; http://dx.doi.org/10.4049/jimmunol.181.6.3750; PMID: 18768826
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med 2005; 201:233 - 40; http://dx.doi.org/10.1084/jem.20041257; PMID: 15657292
- Vinolo MA, Ferguson GJ, Kulkarni S, Damoulakis G, Anderson K, Bohlooly-Y M, Stephens L, Hawkins PT, Curi R. SCFAs induce mouse neutrophil chemotaxis through the GPR43 receptor. PLoS One 2011; 6:e21205; http://dx.doi.org/10.1371/journal.pone.0021205; PMID: 21698257
- Nutsch KM, Hsieh CS. T cell tolerance and immunity to commensal bacteria. Curr Opin Immunol 2012; 24:385 - 91; http://dx.doi.org/10.1016/j.coi.2012.04.009; PMID: 22613090
- Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio CW, Santacruz N, Peterson DA, Stappenbeck TS, Hsieh CS. Peripheral education of the immune system by colonic commensal microbiota. Nature 2011; 478:250 - 4; http://dx.doi.org/10.1038/nature10434; PMID: 21937990
- Robson SC, Sévigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: Structure function relationships and pathophysiological significance. Purinergic Signal 2006; 2:409 - 30; http://dx.doi.org/10.1007/s11302-006-9003-5; PMID: 18404480
- Longhi MS, Moss A, Bai A, Wu Y, Huang H, Cheifetz A, Quintana FJ, Robson SC. Characterization of human CD39+ Th17 cells with suppressor activity and modulation in inflammatory bowel disease. PLoS One 2014; 9:e87956; http://dx.doi.org/10.1371/journal.pone.0087956; PMID: 24505337
- Yoshida O, Kimura S, Jackson EK, Robson SC, Geller DA, Murase N, Thomson AW. CD39 expression by hepatic myeloid dendritic cells attenuates inflammation in liver transplant ischemia-reperfusion injury in mice. Hepatology 2013; 58:2163 - 75; http://dx.doi.org/10.1002/hep.26593; PMID: 23813862
- Nowak-Machen M, Schmelzle M, Hanidziar D, Junger W, Exley M, Otterbein L, Wu Y, Csizmadia E, Doherty G, Sitkovsky M, et al. Pulmonary natural killer T cells play an essential role in mediating hyperoxic acute lung injury. Am J Respir Cell Mol Biol 2013; 48:601 - 9; http://dx.doi.org/10.1165/rcmb.2012-0180OC; PMID: 23349052
- Mascanfroni ID, Yeste A, Vieira SM, Burns EJ, Patel B, Sloma I, Wu Y, Mayo L, Ben-Hamo R, Efroni S, et al. IL-27 acts on DCs to suppress the T cell response and autoimmunity by inducing expression of the immunoregulatory molecule CD39. Nat Immunol 2013; 14:1054 - 63; http://dx.doi.org/10.1038/ni.2695; PMID: 23995234
- Fernández P, Perez-Aso M, Smith G, Wilder T, Trzaska S, Chiriboga L, Franks A Jr., Robson SC, Cronstein BN, Chan ES. Extracellular generation of adenosine by the ectonucleotidases CD39 and CD73 promotes dermal fibrosis. Am J Pathol 2013; 183:1740 - 6; http://dx.doi.org/10.1016/j.ajpath.2013.08.024; PMID: 24266925
- Cohen HB, Briggs KT, Marino JP, Ravid K, Robson SC, Mosser DM. TLR stimulation initiates a CD39-based autoregulatory mechanism that limits macrophage inflammatory responses. Blood 2013; 122:1935 - 45; http://dx.doi.org/10.1182/blood-2013-04-496216; PMID: 23908469
- Wang YM, McRae JL, Robson SC, Cowan PJ, Zhang GY, Hu M, Polhill T, Wang Y, Zheng G, Wang Y, et al. Regulatory T cells participate in CD39-mediated protection from renal injury. Eur J Immunol 2012; 42:2441 - 51; http://dx.doi.org/10.1002/eji.201242434; PMID: 22684996
- Kochetkova I, Thornburg T, Callis G, Pascual DW. Segregated regulatory CD39+CD4+ T cell function: TGF-β-producing Foxp3- and IL-10-producing Foxp3+ cells are interdependent for protection against collagen-induced arthritis. J Immunol 2011; 187:4654 - 66; http://dx.doi.org/10.4049/jimmunol.1100530; PMID: 21967895
- Kochetkova I, Golden S, Holderness K, Callis G, Pascual DW. IL-35 stimulation of CD39+ regulatory T cells confers protection against collagen II-induced arthritis via the production of IL-10. J Immunol 2010; 184:7144 - 53; http://dx.doi.org/10.4049/jimmunol.0902739; PMID: 20483737
- Huehn J, Hamann A. Homing to suppress: address codes for Treg migration. Trends Immunol 2005; 26:632 - 6; http://dx.doi.org/10.1016/j.it.2005.10.001; PMID: 16243583
- Schmelzle M, Duhme C, Junger W, Salhanick SD, Chen Y, Wu Y, Toxavidis V, Csizmadia E, Han L, Bian S, et al. CD39 modulates hematopoietic stem cell recruitment and promotes liver regeneration in mice and humans after partial hepatectomy. Ann Surg 2013; 257:693 - 701; http://dx.doi.org/10.1097/SLA.0b013e31826c3ec2; PMID: 23474584
- Idzko M, K Ayata C, Müller T, Dürk T, Grimm M, Baudiß K, Vieira RP, Cicko S, Boehlke C, Zech A, et al. Attenuated allergic airway inflammation in Cd39 null mice. Allergy 2013; 68:472 - 80; http://dx.doi.org/10.1111/all.12119; PMID: 23452076
- Corriden R, Chen Y, Inoue Y, Beldi G, Robson SC, Insel PA, Junger WG. Ecto-nucleoside triphosphate diphosphohydrolase 1 (E-NTPDase1/CD39) regulates neutrophil chemotaxis by hydrolyzing released ATP to adenosine. J Biol Chem 2008; 283:28480 - 6; http://dx.doi.org/10.1074/jbc.M800039200; PMID: 18713747
- Goepfert C, Sundberg C, Sévigny J, Enjyoji K, Hoshi T, Csizmadia E, Robson S. Disordered cellular migration and angiogenesis in cd39-null mice. Circulation 2001; 104:3109 - 15; http://dx.doi.org/10.1161/hc5001.100663; PMID: 11748109
- Eltzschig HK, Sitkovsky MV, Robson SC. Purinergic signaling during inflammation. N Engl J Med 2012; 367:2322 - 33; http://dx.doi.org/10.1056/NEJMra1205750; PMID: 23234515
- Noack M, Miossec P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun Rev 2014; 13:668 - 77; http://dx.doi.org/10.1016/j.autrev.2013.12.004; PMID: 24418308
- Voo KS, Wang YH, Santori FR, Boggiano C, Wang YH, Arima K, Bover L, Hanabuchi S, Khalili J, Marinova E, et al. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci U S A 2009; 106:4793 - 8; http://dx.doi.org/10.1073/pnas.0900408106; PMID: 19273860
- Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature 2008; 453:236 - 40; http://dx.doi.org/10.1038/nature06878; PMID: 18368049
- Goto Y, Panea C, Nakato G, Cebula A, Lee C, Diez MG, Laufer TM, Ignatowicz L, Ivanov II. Segmented filamentous bacteria antigens presented by intestinal dendritic cells drive mucosal Th17 cell differentiation. Immunity 2014; 40:594 - 607; http://dx.doi.org/10.1016/j.immuni.2014.03.005; PMID: 24684957
- Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 2009; 139:485 - 98; http://dx.doi.org/10.1016/j.cell.2009.09.033; PMID: 19836068
- Ivanov II, Frutos RdeL, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe 2008; 4:337 - 49; http://dx.doi.org/10.1016/j.chom.2008.09.009; PMID: 18854238
- Zhou Q, Yan J, Putheti P, Wu Y, Sun X, Toxavidis V, Tigges J, Kassam N, Enjyoji K, Robson SC, et al. Isolated CD39 expression on CD4+ T cells denotes both regulatory and memory populations. Am J Transplant 2009; 9:2303 - 11; http://dx.doi.org/10.1111/j.1600-6143.2009.02777.x; PMID: 19656134
- Enjyoji K, Sévigny J, Lin Y, Frenette PS, Christie PD, Esch JS 2nd, Imai M, Edelberg JM, Rayburn H, Lech M, et al. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat Med 1999; 5:1010 - 7; http://dx.doi.org/10.1038/12447; PMID: 10470077
