Abstract
Background: MF59-adjuvanted influenza vaccines have superior immunogenicity in older adults compared with non-adjuvanted vaccines. We assessed whether changing formulation (i.e., increasing H3N2 antigen or decreasing the quantity of adjuvant) of the licensed, MF59-adjuvanted trivalent influenza subunit vaccine Fluad® (Novartis Vaccines and Diagnostics) improves the risk-benefit profile in vaccinees aged ≥65 years.
Results: A significant dose-response relationship was observed between antibody levels and MF59 dose; full dose formulations elicited the strongest immune responses, meeting immunogenicity licensure criteria by Day 8. Doubling H3N2 antigen content did not increase the response to this antigen. Increased frequency of circulating CD4+ T-cells specific for vaccine antigens were detected by Day 8; magnitude and functional profile of the CD4+ T-cell response was comparable across the different vaccination groups. Mild to moderate solicited local reactions were more common with vaccines formulated with higher doses of MF59®, but there were no MF59- or antigen dose-related increase in the frequency of solicited systemic reactions or unsolicited adverse events and serious adverse events.
Methods: We report on 357 subjects who received one of eight intramuscular vaccine formulations. Hemagglutination-inhibiting antibodies were assayed on Days 1, 8 and 22; magnitude and functional profile of CD4+ T-cell responses to vaccine antigens were assessed in subsets. Solicited adverse reactions were reported via diary cards for seven days after vaccination and spontaneous adverse events were monitored throughout the study.
Conclusion: This study confirms that the current formulation is the optimal one for MF59-adjuvanted influenza vaccine for use in older adults.
Introduction
Influenza infection is a major cause of morbidity and mortality among older adults, who are particularly prone to influenza-related complications.Citation1,Citation2 In England and Wales, an estimated 11,300 influenza-related hospitalizations and 9,900 influenza-related deaths among adults aged ≥65 years occur every year.Citation3 In the US, 90% of the 36,000 influenza-associated circulatory—and respiratory—related deaths that occurred annually during the 1990s were among older adults.Citation4
The efficacy of influenza vaccination in young adults is estimated to be 70–90%, however, serological protection rates in older adults are considerably lower,Citation5-Citation9 with a clinical efficacy range of only 17–53%.Citation5,Citation10 The impaired ability of older adults to mount an adequate immune response to influenza vaccine has been attributed to immunosenescence,Citation2,Citation11 an age-related decline in innate and adaptive immune function resulting in suboptimal immune responses.Citation12 Hence, seasonal influenza vaccines with enhanced immunogenicity and consequent greater clinical efficacy in older adults are needed to reduce the burden of influenza-related disease in this increasingly large section of the population. Several strategies have been explored to improve the efficacy of influenza vaccination in older adults, including antigen dose escalation,Citation13-Citation15 the use of adjuvanted trivalent influenza vaccines (ATIV),Citation16,Citation17 virosomal subunit vaccines,Citation18,Citation19 intradermal (ID) vaccine delivery,Citation20 the addition of a second B strain in quadrivalent influenza vaccines,Citation21 and priming recipients with live-attenuated virus before trivalent influenza vaccine (TIV) administration.Citation22,Citation23
High-dose TIV administration (i.e., 60 µg hemagglutinin per strain) has been shown to increase antibody responses in older adults.Citation13-Citation15 However, dose-related increases in reactogenicity are evident,Citation13-Citation15 and increasing the antigen dose would stretch manufacturing resources and limit the available doses of vaccines each year. As an alternative approach, potent adjuvants such as MF59® (Novartis Vaccines and Diagnostics), an oil-in-water emulsion, have been developed to improve the performance of vaccines.Citation24 Previous clinical studies have demonstrated that MF59-adjuvanted influenza vaccines induced higher and broader antibody responses than non-adjuvanted vaccines, especially in subjects with low prevaccination antibody titers, including older adults.Citation16 The seasonal MF59-adjuvanted influenza vaccine, Fluad® (Novartis Vaccines and Diagnostics) has been approved and used in older adults in Europe since 1997. Over 50 million doses that have been distributed worldwide, with no apparent safety concerns.Citation25
In this report, we present the results of a randomized clinical trial, conducted in adults aged ≥65 years in three European countries (Poland, Belgium and Germany), to investigate whether the current Fluad formulation could be further improved by doubling the 15 µg of influenza H3N2 antigen in the normal formulation (H3N2st) to 30 µg (H3N2hi), and\or reducing the dose of MF59® from full dose of the marketed vaccine Fluad (100%) to 50%, 25% or 0% of the MF59® dose in the marketed vaccine Fluad (MF590, MF5925, MF5950 and MF59100) to give eight different vaccine formulations. The H3N2 strain was selected for antigen finding because H3N2 viruses tend to largely affect the elderly population. We assessed safety and tolerability, and antibody responses against vaccine strains assessed by hemagglutination inhibition (HI), together with magnitude and functional profiles of CD4+ T-lymphocyte responses to vaccine antigens in subsets of participants from each group.
Results
Baseline and demographics
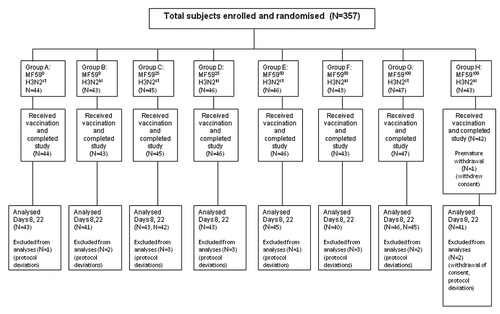
Subject disposition is illustrated in . All enrolled study participants completed the study on Day 22, except for one subject who withdrew consent after Day 1. A total of 95.8% (342/357) and 95.2% (340/357) of subjects were included in the per protocol set (PPS) analyses at Days 8 and 22, respectively. The baseline demographics of the enrolled study population are illustrated in . Vaccination groups were similar with respect to age, sex, weight and height. Across all groups, 40–68% of subjects were male with a mean age of approximately 69 years, and 73–81% of participants had previously received influenza vaccine.
Table 1. Study population demographics
Immunogenicity
Antibody responses to the influenza strains homologous to study vaccine influenza antigens A/Brisbane/59/2007 (H1N1), A/Uruguay 2007 (H3N2) and B/Florida/4/2006 by HI assay are illustrated in .
Table 2. Geometric mean titres (GMTs), GMT ratios, and corresponding 95% CIs at Days 1, 8, and 22.
No group with a reduced MF59 content was noninferior to the full MF59-adjuvanted vaccine groups for any of the three TIV antigen strains. In fact, MF59100 formulations were superior to the MF590 MF5925, and MF5950 groups for H1N1, and to the MF590 groups for H3N2hi and B strains at Days 8 and 22 (upper CI of ratio < 1.00). In addition, none of the vaccine groups with H3N2hi was found to be superior to the reference H3N2st group ().
As illustrated in , a 30 µg H3N2 vaccine antigen dose had no significant effect on the H3N2 antibody response as compared with 15 µg, when measured at Days 8 (p = 0.13; GMR, 0.81) or 22 [p = 0.16; GMR, 0.82 (95% CI, 0.63–1.08)] after vaccination. Furthermore, the increase in H3N2 antigen dose did not affect the antibody response to the H1N1 and B vaccine strains (). No significant interactions were observed between MF59 dose and H3N2 dose, indicating that the difference between H3N2st and H3N2hi can be considered constant over the different MF59 doses.
Figure 2. Effect of high vs. standard H3N2 vaccine antigen dose on homologous H3N2-specific antibody responses (A), and on H1N1 and B (B) strains across all MF59 doses. Haemagglutination inhibition antibody titers (GMT, 95% CI) prevaccination and at Days 8 and 22.GMT, geometric mean titer.
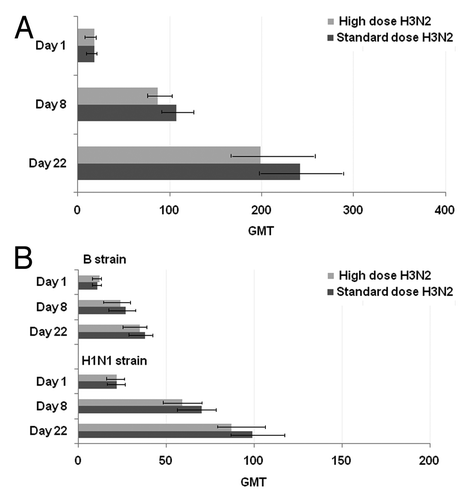
Antibody responses for increasing MF59 content across combined high and standard H3N2 vaccine doses, and H1N1 and B strains are illustrated in Increasing MF59 content elicited higher levels of antibodies at Day 8 and 22 against all influenza strains. Positive slopes (95% CI) between MF59 adjuvant content and Day 22 log10-antibodies were observed for all three strains [H3N2: 1.04 (95% CI, 0.82–1.26), p < 0.0001; H1N1: 0.87 (95% CI, 0.69–1.04); p < 0.0001; B strain: 0.47 (95% CI, 0.33–0.61), p < 0.0001].
Figure 3. Effect of various MF59 doses on the combined (high and standard) H3N2, H1N1 and B strains. Haemagglutination inhibition antibody titers (GMT, 95% CI) at Days 8 (A) and 22 (B) (prevaccination GMTs for strains across MF59 doses were in range 17–21 (H3N2), 21–23 (H1N1) and 11–12 (B strain). GMT, geometric mean titer.
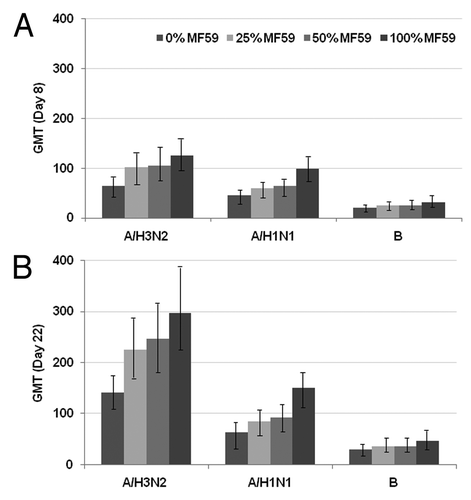
The MF59100 formulation was associated with 138%, 108% and 59% increases in Day 22 antibody responses to H1N1, H3N2 and B strains, respectively, compared with MF590. Compared with MF590 vaccine, lower doses of MF59-adjuvanted vaccine also elicited increases in Day 22 GMTs as follows: 45% increase for H1N1 (p = 0.03), 72% for H3N2 (p < 0.01) and 22% for B (p = 0.19) for MF5950 dose, and 35% for H1N1 (p = 0.09), 59% for H3N2 (p < 0.05) and 25% for B (p = 0.16) for MF5925 dose. When comparing MF59100 with MF5950, additional increases of 64% (H1N1, p < 0.01), 20% (H3N2, p = 0.33) and 30% (B strain, p = 0.07) were observed for Day 22 antibody responses.
Evaluation of GMRs, seroprotection and seroconversion rates according to CHMP criteria across vaccine groups and virus strains are presented in . For the H3N2 strain, all three CHMP criteria were met by all vaccine groups on Days 8 and 22. For the H1N1 strain, strong immune responses were evident for MF5950 and MF59100 vaccine and all criteria were met at Days 8 and 22 after vaccination; MF5925 formulations met two out of three criteria at Day 8 and all criteria at Day 22, and MF590 groups fulfilled one and two criteria at Days 8 and 22, respectively. For the B strain, only the adjuvanted formulations met any of the criteria at Day 8; two out of three criteria were met by MF59100 groups; and one criterion was met by the MF5950 groups. At day 22, all criteria were met by MF5950 and MF59100 groups, two criteria were met by MF5925 groups and only one criterion was met by MF590 groups.
Table 3. Immunogenicity evaluation according to CHMP criteria against A/H3N2 strains, H1N1, and B strain.
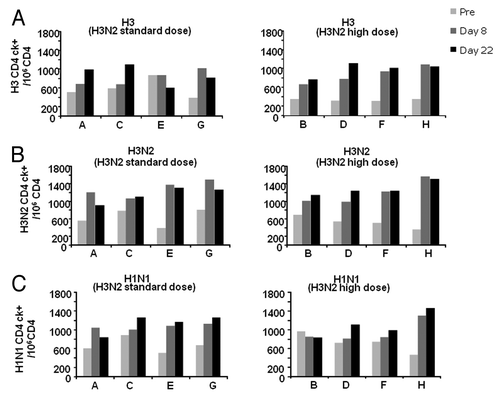
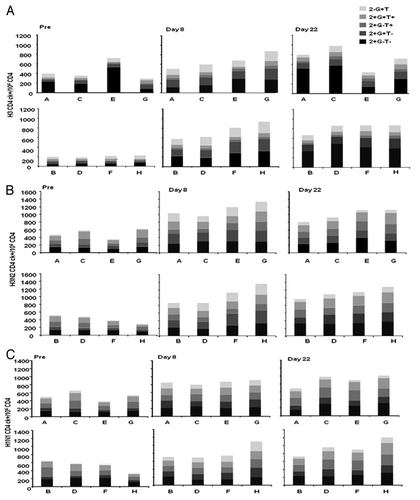
At baseline, CD4+ T lymphocytes specific for vaccine antigens (H1N1 and H3N2 subunits and the pool of peptides spanning H3) were detected, even if at variable frequency, in all groups. At Day 8, increased frequency of H3N2- and H1N1- specific CD4+ T lymphocytes were observed in all vaccination groups, with no further increase at Day 22 (). H3N2- and H3-specific CD4+ T lymphocytes expanded at comparable frequency in response to all vaccine formulations ( and B). H1N1-specific CD4+ T cells expanded at comparable frequency in all groups who received vaccines containing H3N2st, irrespective of the dose of MF59®. A more pronounced expansion of H1N1-specific CD4+ T cells over baseline was observed in subjects who received the vaccine containing H3N2hi and MF59100. However, it must be noted that this group displayed the lowest baseline frequency of H1N1-specific CD4+ T cells (). Analyses of the functional profile of antigen-specific CD4+ T cells showed that at all visits and independently of the administered vaccine, the response to the pool of H3 peptides was dominated by CD4+ T cells producing IL-2, or IL-2 and IFNγ (). Conversely, stimulation with the H3N2 and H1N1 antigens induced a higher frequency of double and triple cytokine-producing CD4+ T cells (IL-2, IL-2 and TNFα, IL-2 and IFNγ, IL-2 and IFNγ and TNFα being equally represented; ).
Safety and tolerability
Rates of solicited local reactions increased with increasing doses of MF59®, whereas the frequency of solicited systemic reactions was similar across the vaccine groups, irrespective of the MF59® dose (). There was no detectable trend for either local or systemic reactions with increasing dose of H3N2 antigen. The majority of solicited reactions were transient and mild to moderate in severity. Overall incidence rates for solicited local reactions with H3N2st and H3N2hi were 45% and 44% in the MF59® groups, 58% and 61% in the MF5925 groups, 63% and 51% in the MF5950 groups and 70% and 65% in the MF59100 groups, respectively. The overall incidence rates for solicited systemic reactions with H3N2st and H3N2hi were 30% and 30% in the MF590 groups, 22% and 28% in the MF5925 groups, 26% and 42% in the MF5950 groups, and 36% and 30% in the MF59100 groups, respectively.
Table 4. Percentage of subjects experiencing mild (severe) solicited local and systemic adverse reactions occurring within one week of vaccination.
Mild to moderate pain at the injection site was the most commonly reported individual solicited local reaction across all vaccination groups (14–40%), followed by erythema (19–38%). Results from Poisson regression analyses showed an increased frequency of pain and induration in the MF59100 groups and an increased frequency of swelling in MF5950 and MF59100 groups, compared with the MF590 groups. Although pain scores assessed by visual analog scale increased with increasing dose of MF59, the pain scores in all groups were very low on the 100-point scale. The mean and maximum pain scores ranged from 1.07 to 3.08 and 3.44 to 11 in the MF590 groups to MF59100 groups, respectively. Few severe local reactions occurred: one subject in the MF59100 H3N2st group experienced severe (>100 mm) erythema, induration and swelling, and one subject in the MF59100 H3N2hi group reported severe pain.
The frequency of subjects experiencing individual solicited systemic reactions was generally similar for all vaccination groups irrespective of MF59® dose. Poisson regression analyses showed no differences between groups for systemic reactions. The most commonly reported systemic reactions were headache and fatigue (range, 7–23%). Fever (≥38°C) following vaccination was rare, experienced by two (<1%) subjects from the entire study population; no severe fever (≥40°C) occurred in any vaccination group. Few reports of severe systemic reactions were reported and included myalgia (four subjects; 0–2% across groups); malaise (three subjects, 0–2% across groups); headache and arthralgia (two subjects, 0–2% across groups); and nausea, fatigue and sweating (one subject, 0–2% across groups) (). There were no differences across groups for the frequency of severe solicited systemic reactions.
For spontaneously reported AEs, no dose-related pattern was observed for MF59® and/or H3N2 antigen dose. The overall frequency of spontaneously reported AEs with H3N2st and H3N2hi were 25% (n = 11) and 16% (n = 7) in the MF590 groups, 24% (n = 11) and 22% (n = 10) in the MF5925 groups, 24% (n = 11) and 23% (n = 10) in the MF5950 groups and 26% (n = 12) and 19% (n = 8) in the MF59100 groups, respectively; 4–11% (n = 2–5) of these AEs across groups were considered at least possible related to study vaccine. The most commonly reported AEs across vaccine groups by system organ class were infections and infestation and general disorder and administration site conditions. Infections and infestations were reported in 16% (n = 7) and 7% (n = 3) in the MF590 groups, 13% (n = 6) and 9% (n = 4) in the MF5925 groups, 4% (n = 2) and 12% (n = 5) in the MF5950 groups and 9% (n = 4) and 5% (n = 2) in the MF59100 groups, with H3N2st and H3N2hi, respectively. Administration site conditions were reported in 9% (n = 4) and 7% (n = 3) in the MF590 groups, 9% (n = 4) and 4% (n = 2) in the MF5925 groups, 7% (n = 3) and 5% (n = 2) in the MF5950 groups and 11% (n = 5) and 5% (n = 2) in the MF59100 groups, with H3N2st and H3N2hi, respectively. Possibly related adverse events, as judged by the investigator, included general disorder and administration site conditions (n = 3: MF590 H3N2st, n = 1: MF590 H3N2hi, n = 4: MF5925 H3N2st, n = 1: MF5925 H3N2hi, n = 3: MF5950 H3N2st, n = 1: MF5950 H3N2hi, n = 3: MF59100 H3N2st, n = 2: MF59100 H3N2hi), nervous system disorders (n = 1 in groups MF590 H3N2st, MF5925 H3N2st, MF5950 H3N2st, MF5950 H3N2hi), infections and infestations (n = 1 in groups MF5925 H3N2hi, MF5950 H3N2hi and MF59100 H3N2st), skin and subcutaneous disorder (n = 1 in groups MF590 H3N2st, MF5925 H3N2st, MF59100 H3N2st), musculoskeletal, connective tissue and bone disorders (n = 1 MF590 H3N2hi, MF5925 H3N2st), gastrointestinal disorders (n = 1: MF590 H3N2st) and blood and lymphatic disorders (n = 1: MF590 H3N2hi).
Serious adverse events (SAEs) were experienced by one subject in the MF590 H3N2st groups (venous thrombosis) and in one subject in the MF590 H3N2hi group (luxation of hip prosthesis). Neither condition was considered to be vaccine related. No premature study withdrawals or deaths due to AEs occurred in any vaccination group.
Discussion
Highly immunogenic seasonal influenza vaccines with enhanced clinical efficacy are needed for individuals aged ≥65 years, who are at increased risk for influenza infection and related complications.Citation1,Citation2 High-dose TIV administration has been shown to effectively increase antibody responses in older adults,Citation13,Citation26 and the MF59® adjuvant has been shown to offer increased antibody responses to homologous strains and long-lasting cross-reactive immune responses that are not reported with non-adjuvanted vaccines.Citation16,Citation27 To investigate whether the current formulation of the licensed MF59-adjuvanted TIV in elderly adults (Fluad) could be further improved, the present study was conducted to assess several doses of MF59® (0%, 25%, 50% or 100% of the standard Fluad dose) and H3N2 antigen (standard 15 μg dose vs. high 30 μg dose) with regard to immunogenicity and safety/tolerability profile, in healthy adults aged ≥ 65 years.
The results of the present study confirm that MF59-adjuvanted vaccines are more immunogenic than non-adjuvanted vaccines in older adults. Moreover, it was shown that the addition of MF59® led to earlier onset (Day 8) of antibody responses associated with protection against influenza. This rapid increase in the antibody response is a clear sign of a potent booster response induced by the adjuvanted vaccine of a pre-existing immunological memory primed by previous influenza vaccinations or previous encounters with the influenza virus. These data also underline the potential benefit offered by MF59-adjuvanted influenza vaccines over conventional vaccines in rapidly conferring protection even in the case of late vaccination at the beginning of the influenza season. In agreement with another MF59® dose-ranging clinical study conducted using a H5N1 influenza vaccine,Citation28 we observed a dose-response trend for immunogenicity with increasing MF59® dose for all influenza strains.
Several previous clinical trials demonstrated that MF59® results in heightened homologous antibody response against vaccine strain antigen and promotes the production of cross-reactive antibodies able to provide heterologous protection against influenza disease.Citation16,Citation27,Citation29,Citation30 Importantly, the use of MF59® will allow for reduced antigen content, ensuring dose-sparing and therefore, increased manufacturing capacity and the widest possible availability of vaccine supply, factors critical to meeting public health needs in the context of a large scale pandemic vaccination campaign.
This study shows that the use of MF59® adjuvant had a greater impact on immunogenicity than increasing antigen. Doubling the H3N2 antigen content did not impact the immunologic response to the matched antigen or other strains in any of the vaccine formulations. Previous studies have reported increased H3N2-specific antibody responses as a result of non-adjuvanted, high-dose H3N2 vaccination.Citation13-Citation15,Citation31 However, the majority of these studies compared a 60-μg dose with the standard 15-μg dose. It has also been suggested that increasing the antigen levels to above >10 or >15 μg of HA dose only induces a plateau in antibody responses,Citation32 and previous studies failed to show an antigen dose response following administration of MF59Citation28 or aluminumCitation33 adjuvanted vaccines. Data from the present study provided no evidence for increasing the H3N2 antigen dose from that currently found in Fluad.
In line with previous studies, it was shown that the antibody response to the B strain was lower compared with the A/H1N1 and A/H3N2 strains contained in the vaccine.Citation30,Citation34 A recent study compared microneutralization (MN) and HI immune responses and demonstrated that the peak HI titers to the B strain were relatively low but that the peak MN titers were similar to those achieved against the A subtypes.Citation35 These findings indicate that the vaccine is eliciting an adequate immune response to the B strain and that that the lower HI titer response to the B strain is related to test sensitivity.
As expected in older individuals who are not immunologically naive to influenza, at baseline CD4+ T cells specific for H3N2 and H1N1 were detectable, at variable frequencies, in all groups. Following vaccination, a measurable expansion of vaccine specific CD4+ T cells was observed already at Day 8 post-vaccination in all groups independently of the vaccine formulation administered. The expansion of H1N1-specific CD4+ T cells was more pronounced in the MF59100 H3N2hi group. However, it is possible that this increase could be due to the fact that this group displayed the lowest baseline frequency of H1N1-specific CD4+ T cells. This, together with the finding that the expansion of antigen-specific T cells was of limited entity in the groups that had the highest frequency at baseline, is in agreement with the knowledge that the expansion of CD4+ T cells reaches a plateau that does not increase further and which is dictated by their number at baseline.Citation36,Citation37 The cytokine profile of antigen-specific CD4+ T cells was comparable in all vaccination groups, confirming that MF59® does not influence the quality of the CD4+ T cell response to influenza vaccines.Citation38,Citation39 Our data clearly show that the enhanced antibody response induced by MF59-adjuvanted vaccines was not paralleled by a more pronounced expansion of vaccine antigen-specific CD4+ T cells. This may appear to be in contrast with previous findings by us demonstrating that MF59-adjuvanted formulations are required for the expansion of H5N1-specific CD4 T cells that precedes and predicts the rise and maintenance of virus-neutralizing antibody titers.
This apparent discrepancy can be explained by the fact that the older subjects enrolled in this study were not immunologically naive to the vaccine antigens. This was confirmed by the high frequency of H1N1- and H3N2-specific CD4+ T cells detectable at baseline, and by the rapid increase of their frequency already one week postvaccination. It should be noted that CD4+ T-cell epitopes, unlike B-cell epitopes, tend to be more conserved across drifted A/H1N1 or A/H3N2 influenza strains, explaining in this way the persistence of these memory T cells at sizeable frequency over time.Citation40,Citation41 The lower threshold for activation of memory T cells can explain the finding that the adjuvant had only a limited impact on already primed CD4+ T cells, like the ones reactive to seasonal antigens in adults. In this study, it is likely that the main effect of MF59® was exerted on the generation of an immunogenic environment in the draining lymph nodes required for an optimal B-cell recruitment and activation leading to the enhanced antibody response consistently observed in the subjects immunized with MF59-adjuvanted vaccines.Citation38
The overall safety and tolerability profiles were as expected in this population and in line with previous studies using MF59-adjuvanted vaccines.Citation25 Systemic tolerability was similar across groups, whereas mild to moderate solicited local reactions were more common with vaccines formulated with higher doses of MF59. Although high antigen dose vaccination was expected to result in increased incidence of solicited reactions,Citation15 results of this study found no significant increase in either local or systemic reactions with doubling of H3N2 antigen content. It is unknown whether this finding would be confirmed with even higher antigen doses, or indeed if doses of the other antigens were increased. The majority of solicited reactions were mild to moderate, with few severe reactions (0–2%). There was no dose-related pattern for spontaneous AEs and only two SAEs were reported and were not related to the study vaccine. No premature study withdrawals due to AEs occurred in any vaccination group. All together, the safety results confirm the good safety and tolerability profile of Fluad and MF59®.Citation25
In conclusion, this study shows, that, despite a detectable increase in the frequency of mild to moderate local reactions, the standard formulation of Fluad had the best benefit-risk profile in older adults, with better and more rapid immunogenicity and similar overall safety and tolerability, compared with TIV formulations with lower MF59® content and/or higher H3N2 antigen doses.
Materials and Methods
This multicenter, randomized, observer-blind study was conducted at one site in Poland (Krakow), one site in Belgium (Gent) and four sites in Germany (Zentrallabor, Würzburg, Potsdam, Hamburg), between October 2008 and February 2009. The immunogenicity objectives of this study were as follows: (1) To assess whether any of the vaccine formulations with lower MF59® content and/or higher H3N2 content are capable of eliciting noninferior or superior immunogenicity to the licensed ATIV in the elderly (Fluad); (2) To evaluate the impact of H3N2 antigen level on H3N2-specific antibody responses and on H1N1 and B strain antibody titers; (3) To assess the dose-response relationship of MF59®; (4) To assess immunogenicity of the study vaccine groups against the European Committee for Medicinal Products for Human Use (CHMP) criteria; (5) To assess the impact of different doses of MF59 and/or H3N2 dose on CD4+ T-lymphocyte responses to vaccine antigens (in a subset of participants).
One further objective was the assessment of the immunogenicity and safety of intradermal vaccine formulations administered to two separate study groups which will be reported in a separate paper. Safety and tolerability objectives were to evaluate solicited local and systemic reactions and spontaneously reported adverse events (AEs) and serious AEs (SAEs) in response to the study vaccine formulations.
Subjects
A total of 450 healthy volunteers aged ≥ 65 y were enrolled in the study, of which 357 were randomized to the eight intramuscular (IM) vaccination groups (range, 43–47 per group) covered in this paper. The main exclusion criteria were immunization with any influenza vaccine within 6 mo before study enrollment, immunization with any experimental influenza vaccine containing adjuvant within 2 y before study enrollment, a body mass index ≥ 35 kg/m2, hypersensitivity to vaccine components, laboratory-confirmed influenza disease within 12 mo before study enrollment, receipt of any other vaccine or investigational agent within 30 d before study enrollment, infection requiring systemic antibiotic or antiviral therapy within 14 d before study enrollment, serious disease, fever (≥38°C) within 7 d before study enrollment, or an impaired or altered immune system.
Treatments and procedures
The eight study groups (A to H) as listed in included combinations of standard (15 µg) and high (30 µg) H3N2 antigen dose (H3N2st and H3N2hi, respectively) and 0%, 25%, 50%, or 100% of the MF59® dose (MF590, MF5925 MF5950 and MF59100, respectively) that is approved in Europe for elderly adults. Subsets of 18 subjects per group (first 180 enrolled subjects from one study center) were included in the cell-mediated immunity (CMI) analyses. The vaccine formulation received by subjects in reference group G (MF59100 H3N2st) was identical to Fluad. The vaccine formulation received by subjects in reference group A (MF590 H3N2st), was identical to the licensed seasonal TIV, Agrippal® (Novartis Vaccines and Diagnostics). All participants received a single 0.5-mL IM vaccine dose on Day 1. All vaccines were administered in the deltoid muscle, preferably in the nondominant arm. Immunogenicity assessments were performed on Day 1 (prevaccination), Day 8 (one week postvaccination) and Day 22 (three weeks postvaccination). Blood samples of 15 mL (for subset participants included in CMI analyses, 70 mL per sample) were obtained by venipuncture at each time point. Serum was separated from clotted blood by centrifugation and stored at −18°C or below until shipped to the Novartis Vaccines Clinical Serology Laboratory in Marburg, Germany, for immunogenicity analysis. Peripheral blood mononuclear cells were isolated from heparinized blood samples by Ficoll gradient centrifugation and were frozen and stored in liquid nitrogen until used for CMI analysesCitation39 at the Centre for Vaccinology at Ghent University Hospital Ghent, Belgium.
Table 5. Vaccine formulations, study groups and number of subjects per group.
Safety assessments were performed on Day 1 (prevaccination baseline to 30 min after vaccination) and Days 8 and 22. Solicited local and systemic adverse reactions were to be recorded on a diary card for seven consecutive days following vaccination. All spontaneous reports of AEs and concomitant medications were to be collected throughout the entire study period and reported at the subsequent study visit. Any SAE was to be reported immediately to the study sponsor.
Vaccines
Vaccine formulations for H3N2st (groups A, C, E, and G) contained 15 µg of haemagglutinin from each of the influenza strains A/Brisbane/59/2007 (H1N1), A/Uruguay/716/2007 (H3N2) and B/Florida/4/2006, as recommended for the 2008–09 influenza season in the Northern Hemisphere. The H3N2hi groups (groups B, D, F and H) contained an additional 15 µg of A/Brisbane/10/2007 H3N2 haemagglutinin. A standard dose of MF59®, as used in one 0.5-mL dose of the commercially available seasonal influenza vaccine Fluad, contains 9.75 mg squalene. Formulations containing lower doses of MF59® were achieved by bedside dilution of ATIV with non-adjuvanted TIV. This was achieved by manual serial dilution of adjuvanted TIV with non-adjuvanted TIV: 50% MF59® dose formulations were achieved by 1:1 dilution of full dose MF59® TIV with non-adjuvanted TIV and 25% MF59® dose formulations were achieved by taking a 50% MF59 TIV formulation and mixing this 1:1 with non-adjuvanted TIV. Final volume for administration of all IM formulations was 0.5 mL.
Immunogenicity assessment
Antibody titers in serum samples collected at baseline and at Days 8 and 22 after vaccination were measured by hemagglutination inhibition (HI), according to standard methods.Citation42 HI antibody responses on Days 1, 8 and 22 were expressed as geometric mean titers (GMTs) and geometric mean ratios (GMRs) of the postvaccination to prevaccination titer (Day 8/Day 1 titer and Day 22/Day 1 titer); seroprotection rates, defined as the percentage of subjects with HI titers ≥40; and seroconversion rates, defined as percentage of subjects per group achieving at least a 4-fold increase in HI titer from a seropositive prevaccination titer (≥10) or a rise from <10 to ≥40 in those who were originally seronegative. HI assays were performed using the homologous vaccine antigen strains A/Brisbane/59/2007 (H1N1), A/Uruguay /716/2007 (H3N2) and B/Florida/4/2006. Frequency and functional profile of CD4+ T cells specific for vaccine antigens were assessed by polychromatic flow cytometry as previously described.Citation39 Production of any nonoverlapping combination of interleukin-2 (IL-2), interferon-γ (IFNγ) and tumor necrosis factor-α (TNFα) was analyzed after pulsing vaccinees’ peripheral blood mononuclear cells in vitro with vaccine subunit antigens from either A/Brisbane/59/07 (H1N1) or A/Brisbane/10/07 (H3N2), or with a library of peptides (18-mers overlapping by ten) spanning the whole H3 protein from A/Brisbane/10/2007 (H3N2).Citation39
Safety and tolerability assessment
Subjects were provided with diary cards and asked to record the occurrence and severity of a predefined set of solicited adverse local or systemic reactions for 7 d and any other symptoms or illnesses (“unsolicited reactions”) for 21 d following vaccine administration. Solicited local reactions included pain at the site of injection, erythema, induration, swelling and ecchymosis. Pain at the injection site was also assessed by a 100-mm visual analog scale, ranging from zero (best imaginable health state) to 100 (worst imaginable health state). Pain scores included the averaged mean score over seven consecutive days and the maximum score. Solicited systemic reactions were headache, chills, fatigue, arthralgia, malaise, myalgia, nausea, sweating, vomiting, diarrhea, fever (≥38°C), use of analgesic or antipyretic medication and events causing the subject to remain at home. Local and systemic reactions were graded for severity according to standardized scales, following the Center for Biologics Evaluation and Research guidance.Citation43 Accordingly, the severity of adverse reactions was categorized as none, mild (grade 1), moderate (grade 2), severe (grade 3), or potentially life threatening (grade 4). Unsolicited reactions (adverse events) were evaluated by the investigator as “mild,” “moderate,” or “severe” in severity and were further categorized as serious or not serious in accordance with ICH Guidance E2A standard definitions.
Statistical analysis
Statistical evaluation was performed using SAS® version 9.1 software (SAS Institute).
Sample size
Assuming log-normal distributed antibody titers, a common SD of 0.7 of log10 titers and a two-sided type I error of 5%, there was 80% power to demonstrate significance with a sample size of 42 per group if the difference between groups was at least factor 3.0. Taking advantage of group pooling, a sample size of 88 subjects per group led to a minimum relevant difference of factor 2.0.
Populations
Immunogenicity analyses were run on the per-protocol set, which included all enrolled subjects who received the vaccine correctly, provided evaluable serum samples at relevant time points and had no major protocol deviations. Safety was analyzed for all subjects exposed to study vaccines.
Immunogenicity
Log10-transformed antibody titers at Day 22 were used in all analyses as a dependent variable, and strain-specific prevaccination titers served as independent covariate in all analysis of covariance (ANCOVA) models. Contingent on the specific study objective, further qualitative factors were included in the ANCOVA models. GMTs, GMRs and their two-sided 95% CIs, as well as P values for testing noninferiority and superiority hypotheses were derived from the models. Noninferiority and superiority margins of 0.67 and 1.00, respectively, were prespecified and significance was declared if the one-sided P value was <0.025. A two-sided P value <0.05 was required for the analysis of differences. Vaccine groups were combined and pooled by MF59® dose [group AB (MF590), group CD (MF5925), group EF (MF5950) and group GH (MF59100)] or H3N2 antigen dose [group ACEG (H3N2st) and group BDFH (H3N2hi)].
Objective 1
To identify whether combinations with lower MF59 content and/or higher H3N2 content were capable of eliciting noninferior/superior immunogenicity to MF59100 vaccination groups, strain-specific antibodies to A/H1N1 and B strains (vaccination groups AB, CD and EF), H3N2st strain (groups A, C and E) and H3N2hi strain (groups B, D, F and H) were tested for noninferiority/superiority to reference vaccine groups with MF59100 adjuvant (i.e., groups GH [H1N1 and B comparisons] or G [H3N2 comparisons]) using ANCOVA with vaccine group as qualitative factor. Noninferiority analyses were predefined, but superiority analyses were conducted post hoc.
Objective 2
To assess the impact of H3N2 antigen content on H3N2, H1N1, and B antibody response, ANCOVA models were applied with MF59 adjuvant dose (MF5925, MF5950 and MF59100) and H3N2 antigen dose (H3N2st and H3N2hi) as qualitative factors. Additional analyses (F-test) were performed after the removal of nonsignificant effects.
Objective 3
The dose-response relationship of MF59® adjuvant was evaluated using linear regression analyses with MF59® adjuvant dose as independent quantitative factor and ANCOVA models to assess the differences between adjuvant doses.
Objective 4
Immunogenicity data were also analyzed based on HI licensure criteria according to the European Medicines Agency recommendations (CPMP/BWP/214/96).Citation44 For subjects aged >60 y, the following criteria apply (1) percentage of subjects with seroconversion or at least 4-fold increase in HI antibody is >30%; (2) percentage of subjects achieving an HI titer ≥ 1:40 is >60%; and (3) the GMR is >2. At least one of three criteria must be fulfilled for each strain.
Objective 5
For the CMI analyses, all available data were included. Frequencies of vaccine-specific CD4+ T cells were compared between non-adjuvanted TIV and ATIV dose-escalating arms. For each antigen and response variable, separated by vaccine group and visit, means, differences and corresponding CIs were calculated using analysis of variance with vaccine group as qualitative factor adjusted for baseline.
Safety and tolerability
Data were summarized by vaccine group. To determine the impact of MF59® dose on the safety and tolerability profile, percentages of subjects with total local and systemic adverse reactions were compared applying Poisson regression analyses.
| Abbreviations: | ||
| AE | = | adverse event |
| ANCOVA | = | analysis of covariance |
| ATIV | = | adjuvanted trivalent influenza vaccine |
| CHMP | = | European Committee for Medicinal Products for Human Use |
| CMI | = | cell-mediated immunity |
| GMR | = | geometric mean ratio |
| GMT | = | geometric mean titer |
| HI | = | haemagglutinin inhibition |
| ID | = | intradermal |
| IFN | = | interferon |
| IL-2 | = | interleukin-2 |
| IM | = | intramuscular |
| SAE | = | serious adverse event |
| SC | = | seroconversion |
| SI | = | significant increase SP, seroprotection |
| TIV | = | trivalent influenza vaccine |
Acknowledgments
The authors wish to thank Dr. Patricia de Groot, CHC Europe, for her assistance in preparing the manuscript on behalf of Novartis, Inc. and Dr. Nicolaos Gaitatzis, NVD Marburg, for the serological analysis of the samples. ClinicalTrials.gov Identifier: NCT00848848
Disclosure of Potential Conflicts of Interest
Della Cioppa, Nicolay, Lindert, Castellino, Galli, Groth and Del Giudice are Novartis employees.
Funding Statement
This study was supported by funds provided by Novartis Vaccines and Development.
Author Contributions
Della Cioppa, Nicolay, Lindert, Castellino, Galli, Groth and Del Giudice designed the experiments and interpreted results. Nicolay analyzed the data. Leroux-Roels and Clement conducted the study, the CMI analyses and interpreted data. All authors contributed to the manuscript.
References
- Fleming DM, Elliot AJ. The impact of influenza on the health and health care utilisation of elderly people. Vaccine 2005; 23:Suppl 1 S1 - 9; http://dx.doi.org/10.1016/j.vaccine.2005.04.018; PMID: 15908058
- McElhaney JE. The unmet need in the elderly: designing new influenza vaccines for older adults. Vaccine 2005; 23:Suppl 1 S10 - 25; http://dx.doi.org/10.1016/j.vaccine.2005.04.019; PMID: 15908062
- Pitman RJ, Melegaro A, Gelb D, Siddiqui MR, Gay NJ, Edmunds WJ. Assessing the burden of influenza and other respiratory infections in England and Wales. J Infect 2007; 54:530 - 8; http://dx.doi.org/10.1016/j.jinf.2006.09.017; PMID: 17097147
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289:179 - 86; http://dx.doi.org/10.1001/jama.289.2.179; PMID: 12517228
- Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine 2006; 24:1159 - 69; http://dx.doi.org/10.1016/j.vaccine.2005.08.105; PMID: 16213065
- Hobson D, Curry RL, Beare AS, Ward-Gardner A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond) 1972; 70:767 - 77; http://dx.doi.org/10.1017/S0022172400022610; PMID: 4509641
- McElhaney JE, Ewen C, Zhou X, Kane KP, Xie D, Hager WD, et al. Granzyme B: Correlates with protection and enhanced CTL response to influenza vaccination in older adults. Vaccine 2009; 27:2418 - 25; http://dx.doi.org/10.1016/j.vaccine.2009.01.136; PMID: 19368783
- Murasko DM, Bernstein ED, Gardner EM, Gross P, Munk G, Dran S, et al. Role of humoral and cell-mediated immunity in protection from influenza disease after immunization of healthy elderly. Exp Gerontol 2002; 37:427 - 39; http://dx.doi.org/10.1016/S0531-5565(01)00210-8; PMID: 11772530
- Potter CW, Oxford JS. Determinants of immunity to influenza infection in man. Br Med Bull 1979; 35:69 - 75; PMID: 367490
- Remarque EJ. Influenza vaccination in elderly people. Exp Gerontol 1999; 34:445 - 52; http://dx.doi.org/10.1016/S0531-5565(99)00007-8; PMID: 10433399
- Ginaldi L, De Martinis M, D'Ostilio A, Marini L, Loreto MF, Corsi MP, et al. The immune system in the elderly: I. Specific humoral immunity. Immunol Res 1999; 20:101 - 8; http://dx.doi.org/10.1007/BF02786466; PMID: 10580635
- Targonski PV, Jacobson RM, Poland GA. Immunosenescence: role and measurement in influenza vaccine response among the elderly. Vaccine 2007; 25:3066 - 9; http://dx.doi.org/10.1016/j.vaccine.2007.01.025; PMID: 17275144
- Couch RB, Winokur P, Brady R, Belshe R, Chen WH, Cate TR, et al. Safety and immunogenicity of a high dosage trivalent influenza vaccine among elderly subjects. Vaccine 2007; 25:7656 - 63; http://dx.doi.org/10.1016/j.vaccine.2007.08.042; PMID: 17913310
- Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis 2009; 200:172 - 80; http://dx.doi.org/10.1086/599790; PMID: 19508159
- Keitel WA, Atmar RL, Cate TR, Petersen NJ, Greenberg SB, Ruben F, et al. Safety of high doses of influenza vaccine and effect on antibody responses in elderly persons. Arch Intern Med 2006; 166:1121 - 7; http://dx.doi.org/10.1001/archinte.166.10.1121; PMID: 16717175
- Banzhoff A, Nacci P, Podda A. A new MF59-adjuvanted influenza vaccine enhances the immune response in the elderly with chronic diseases: results from an immunogenicity meta-analysis. Gerontology 2003; 49:177 - 84; http://dx.doi.org/10.1159/000069172; PMID: 12679609
- Sindoni D, La Fauci V, Squeri R, Cannavo G, Bacilieri S, Panatto D, et al. Comparison between a conventional subunit vaccine and the MF59-adjuvanted subunit influenza vaccine in the elderly: an evaluation of the safety, tolerability and immunogenicity. J Prev Med Hyg 2009; 50:121 - 6; PMID: 20099444
- de Bruijn I, Meyer I, Gerez L, Nauta J, Giezeman K, Palache B. Antibody induction by virosomal, MF59-adjuvanted, or conventional influenza vaccines in the elderly. Vaccine 2007; 26:119 - 27; http://dx.doi.org/10.1016/j.vaccine.2007.10.051; PMID: 18063446
- Tosh PK, Poland GA. Emerging vaccines for influenza. Expert Opin Emerg Drugs 2008; 13:21 - 40; http://dx.doi.org/10.1517/14728214.13.1.21; PMID: 18321146
- Holland D, Booy R, De Looze F, Eizenberg P, McDonald J, Karrasch J, et al. Intradermal influenza vaccine administered using a new microinjection system produces superior immunogenicity in elderly adults: a randomized controlled trial. J Infect Dis 2008; 198:650 - 8; http://dx.doi.org/10.1086/590434; PMID: 18652550
- Belshe RB. The need for quadrivalent vaccine against seasonal influenza. Vaccine 2010; 28:Suppl 4 D45 - 53; http://dx.doi.org/10.1016/j.vaccine.2010.08.028; PMID: 20713260
- Rudenko LG, Arden NH, Grigorieva E, Naychin A, Rekstin A, Klimov AI, et al. Immunogenicity and efficacy of Russian live attenuated and US inactivated influenza vaccines used alone and in combination in nursing home residents. Vaccine 2000; 19:308 - 18; http://dx.doi.org/10.1016/S0264-410X(00)00153-5; PMID: 10930686
- Treanor JJ, Mattison HR, Dumyati G, Yinnon A, Erb S, O'Brien D, et al. Protective efficacy of combined live intranasal and inactivated influenza A virus vaccines in the elderly. Ann Intern Med 1992; 117:625 - 33; PMID: 1530193
- O'Hagan DT, Rappuoli R, De Gregorio E, Tsai T, Del Giudice G. MF59 adjuvant: the best insurance against influenza strain diversity. Expert Rev Vaccines 2011; 10:447 - 62; http://dx.doi.org/10.1586/erv.11.23; PMID: 21506643
- Pellegrini M, Nicolay U, Lindert K, Groth N, Della Cioppa G. MF59-adjuvanted versus non-adjuvanted influenza vaccines: integrated analysis from a large safety database. Vaccine 2009; 27:6959 - 65; http://dx.doi.org/10.1016/j.vaccine.2009.08.101; PMID: 19751689
- U.S. Food and Drug Administration. Press Release (2009). FDA Approves A High Dose Seasonal Influenza Vaccine Specifically Intended for People Ages 65 and Older: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm195483.htm.
- Banzhoff A, Gasparini R, Laghi-Pasini F, Staniscia T, Durando P, Montomoli E, et al. MF59-adjuvanted H5N1 vaccine induces immunologic memory and heterotypic antibody responses in non-elderly and elderly adults. PLoS ONE 2009; 4:e4384; http://dx.doi.org/10.1371/journal.pone.0004384; PMID: 19197383
- Keitel W, Groth N, Lattanzi M, Praus M, Hilbert AK, Borkowski A, et al. Dose ranging of adjuvant and antigen in a cell culture H5N1 influenza vaccine: safety and immunogenicity of a phase 1/2 clinical trial. Vaccine 2010; 28:840 - 8; http://dx.doi.org/10.1016/j.vaccine.2009.10.019; PMID: 19835829
- Arguedas A, Soley C, Abdelnour A, Sales V, Lindert K, Cioppa GD, et al. Assessment of the safety, tolerability and kinetics of the immune response to A/H1N1v vaccine formulations with and without adjuvant in healthy pediatric subjects from 3 through 17 years of age. Hum Vaccin 2011; 7; http://dx.doi.org/10.4161/hv.7.1.13411; PMID: 21285531
- Vesikari T, Pellegrini M, Karvonen A, Groth N, Borkowski A, O'Hagan DT, et al. Enhanced immunogenicity of seasonal influenza vaccines in young children using MF59 adjuvant. Pediatr Infect Dis J 2009; 28:563 - 71; http://dx.doi.org/10.1097/INF.0b013e31819d6394; PMID: 19561422
- Cate TR, Rayford Y, Nino D, Winokur P, Brady R, Belshe R, et al. A high dosage influenza vaccine induced significantly more neuraminidase antibody than standard vaccine among elderly subjects. Vaccine. Feb 25;28(9):2076-9.
- Kilbourne E. Inacitvated Influenza Vaccines. In: Plotkin A MEe, editor. Vaccines. Philadelphia: Saunders; 1994. 565–81.
- Hehme N, Engelmann H, Kunzel W, Neumeier E, Sanger R. Pandemic preparedness: lessons learnt from H2N2 and H9N2 candidate vaccines. Med Microbiol Immunol (Berl) 2002; 191:203 - 8; http://dx.doi.org/10.1007/s00430-002-0147-9; PMID: 12458361
- Li R, Fang H, Li Y, Liu Y, Pellegrini M, Podda A. Safety and immunogenicity of an MF59-adjuvanted subunit influenza vaccine in elderly Chinese subjects. Immun Ageing 2008; 5:2; http://dx.doi.org/10.1186/1742-4933-5-2; PMID: 18289372
- Tsai T, Brestrich G, Nacci L, Knuf M, Wutzler P, Karvonen A, et al. Hemaglutinin inhibition and microneutralization antibodies in infants and children receiving seasonal trivalent influenza vaccine (TIV) or MF59-adjuvanted TIV (ATIV). Poster presented at the 29th annual European Society for Pediatric Infectious Disease (ESPID) meeting, 7-11 June 2011, The Hague, the Netherlands.
- Bocharov G, Quiel J, Luzyanina T, Alon H, Chiglintsev E, Chereshnev V, et al. Feedback regulation of proliferation vs. differentiation rates explains the dependence of CD4 T-cell expansion on precursor number. Proc Natl Acad Sci USA 2011; 108:3318 - 23; http://dx.doi.org/10.1073/pnas.1019706108; PMID: 21292990
- Quiel J, Caucheteux S, Laurence A, Singh NJ, Bocharov G, Ben-Sasson SZ, et al. Antigen-stimulated CD4 T-cell expansion is inversely and log-linearly related to precursor number. Proc Natl Acad Sci USA 2011; 108:3312 - 7; http://dx.doi.org/10.1073/pnas.1018525108; PMID: 21292989
- Dormitzer PR, Galli G, Castellino F, Golding H, Khurana S, Del Giudice G, et al. Influenza vaccine immunology. Immunol Rev 2011; 239:167 - 77; http://dx.doi.org/10.1111/j.1600-065X.2010.00974.x; PMID: 21198671
- Galli G, Medini D, Borgogni E, Zedda L, Bardelli M, Malzone C, et al. Adjuvanted H5N1 vaccine induces early CD4+ T cell response that predicts long-term persistence of protective antibody levels. Proc Natl Acad Sci USA 2009; 106:3877 - 82; http://dx.doi.org/10.1073/pnas.0813390106; PMID: 19237568
- Cusick MF, Wang S, Eckels DD. In vitro responses to avian influenza H5 by human CD4 T cells. J Immunol 2009; 183:6432 - 41; http://dx.doi.org/10.4049/jimmunol.0901617; PMID: 19841175
- Roti M, Yang J, Berger D, Huston L, James EA, Kwok WW. Healthy human subjects have CD4+ T cells directed against H5N1 influenza virus. J Immunol 2008; 180:1758 - 68; PMID: 18209073
- Rowe T, Abernathy RA, Hu-Primmer J, Thompson WW, Lu X, Lim W, et al. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J Clin Microbiol 1999; 37:937 - 43; PMID: 10074505
- Center for Biological Evaluation and Research. Guidance for industry: clinical data needed to support the licensure of pandemic influenza vaccines. Silver Spring, MD: Food and Drug Administration, May 2007.
- Committee for proprietary medicinal products. Note for guidance on harmonisation of requirements for influenza vaccines. 12 March 1997, CPMP/BWP/214/96. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003945.pdf.