Abstract
Background and objectives
Antibody persistence and immune memory against hepatitis A (HAV) in adults in a low endemicity country, 15 y after immunisation with two doses of HAV vaccine has been demonstrated. This communication provides additional information on antibody persistence up to Year 17 from two of the longest follow-up studies [NCT00289757/NCT00291876].
Methods
In two double-blind primary studies, healthy adults aged 17−40 y and 21−40 y, respectively received two doses of the HAV vaccine following a 0,6 mo or 0,12 mo schedule. Anti-HAV antibody concentrations were measured using an enzyme-linked immunoassay (cut-off: 15mIU/ml) at Year 16 and Year 17. Subjects who became seronegative (anti-HAV < 15mIU/ml) since previous reporting were offered a challenge dose, with anti-HAV antibody concentration measurements at Day 14 and Day 30 thereafter.
Results
At Year 17, 100% and 96.7% of subjects remained seropositive for anti-HAV antibodies following the 0, 6 mo and 0, 12 mo regimens, respectively (GMCs: 278mIU/ml and 369mIU/ml). One subject who became seronegative at Year 16 received a HAV challenge dose within the next 12 mo and mounted an anamnestic response. The challenge dose was well-tolerated.
Conclusions
Both HAV immunisation regimens (0,6 mo and 0,12 mo) induced persistence of vaccine-induced antibodies against HAV for at least 17 y after primary vaccination.
Introduction
Hepatitis A (HAV) disease burden continues to be a cause of concern for public health authorities worldwide with approximately 1.5 million clinically-confirmed cases occurring annually.Citation1 The adult population is increasingly at risk of contracting HAV infection as they remain susceptible for a longer duration and are increasingly exposed to the virus by traveling to HAV endemic regions.Citation2,Citation3 As a consequence, HAV, so far thought to be a benign, mostly self-limiting and rarely fatal disease,Citation1 will become more symptomatic as pathology worsens with adult age.
Active immunization is a well-established, safe and effective method of conferring protection against HAV infectionsCitation1,Citation4-Citation6 with previous studies evaluating two or three dose regimens reporting long-term immunity ranging up to at least 15 y,Citation6-Citation11 and mathematical modeling studies predicting persistence of detectable levels of anti-HAV antibodies up to at least 20−25 y.Citation12-Citation14
Two studies (A and B) aimed to assess the immunogenicity against HAV in adults with a two-dose primary vaccination (0, 6 mo or 0, 12 mo schedule) using a monovalent, inactivated HAV vaccine. After 15 y of follow-up (Study A: n = 62; Study B: n = 128), > 97% of subjects were seropositive for anti-HAV antibodies, while six subjects who became seronegative (anti-HAV antibody concentration < 15mIU/ml) received a HAV challenge dose and mounted anamnestic responses.Citation10 The manuscript presents additional data from two of the longest follow-up studies assessing persistent immunity against HAV up to Year 17, also documenting the anamnestic response to a HAV challenge dose in subjects who became seronegative after Year 15.
Results
At Year 17, 63 and 124 subjects from Study A and Study B, respectively returned for follow-up. Of these, 45 and 91 subjects were included in the Year 17 long-term according-to-protocol (LT-ATP) immunogenicity cohort. The reasons for eliminations are presented in .
Table 1. Number of subjects included in the LT-ATP cohort at Year 17 with reasons for elimination
At Year 17, mean ages of subjects in the LT-ATP cohort of the studies A and B were respectively: 44.2 y (range: 34 to 56 y) and 47.4 y (range: 39 to 57 y); 75.6% and 74.7% of subjects, respectively were female and all subjects were of Caucasian heritage. The demographic characteristics in the long-term total cohort were comparable to those in the LT-ATP cohort at Year 17. In addition, the LT-ATP cohorts and the long-term total cohorts at Year 17 were considered to be comparable to the original cohorts of the respective primary studies in line with the post-hoc analyses performed at the Year 15 time point which confirmed the comparability of the Year 15 cohorts to the respective primary study cohorts in terms of demographic characteristics (age and gender) and post-Dose 2 anti-HAV antibody concentrations.Citation10
Seventeen years after primary vaccination, in the LT-ATP cohort, 100% (95% CI: 92.1−100%) of subjects in Study A and 96.7% (90.7−99.3%) of subjects in Study B remained seropositive; the corresponding anti-HAV antibody Geometric Mean Concentrations (GMCs) were 278 (207−374 mIU/ml) and 369 (294−464 mIU/ml), respectively. In the long-term total cohort at Year 17, all subjects in both studies (Study A: 100% [95% CI: 94.3−100%]; Study B: 100% [95% CI: 97.1−100%]) were seropositive for anti-HAV antibodies (this cohort included one subject who became seronegative at Year 16, subsequently received a HAV challenge dose within the next 12 mo). The corresponding anti-HAV antibody GMCs in the two studies (Study A and Study B) at the Year 17 time point were 331mIU/ml (95% CI: 250−439 mIU/ml) and 406 mIU/ml (95% CI: 333−494 mIU/ml), respectively. The anti-HAV seropostivity rates in the long-term total cohort and the LT-ATP cohort at all time points from primary vaccination up to Year 17 are presented in . The anti-HAV antibody evolution across all long-term follow-up time points is presented in .
Table 2. Percentage of subjects seropositive for anti-HAV antibodies at each yearly follow-up time point up to 17 y (Long-term Total cohort and LT ATP cohort)
Figure 1. Evolution of anti-HAV antibody geometric mean concentration across the yearly follow-up time points. *Serum samples up to Year 11 were tested with ELISA kits, while serum samples from Years 12 to 17 were tested with EIA kits. Blood samples at Year 11 were re-tested with the new assay kit and results were compared with the previous assay kit. Anti-HAV seropositivity cut-offs: Up to Year 11: ≥ 20 mIU/ml; Year 12 to Year 17: ≥ 15 mIU/ml
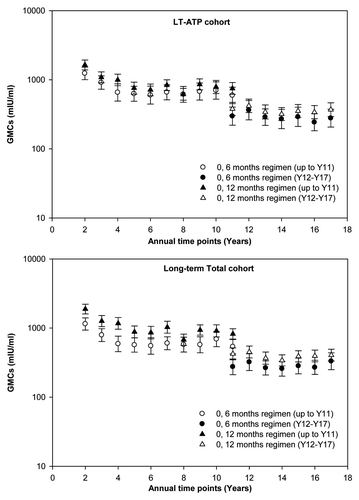
All subjects except one (belonging to Study B; 0, 12 mo regimen) had anti-HAV antibody concentration > 15 mIU/ml at Year 16. This subject became seronegative at Year 16 and was subsequently offered a challenge dose within the next 12 mo. One month after receiving the challenge dose, this subject mounted an anamnestic response to the challenge dose (pre-challenge antibody concentration: 16 mIU/ml; Day 14 post-challenge dose: 3222 mIU/ml; Day 30 post-challenge dose: 4894 mIU/ml). The challenge dose was well-tolerated; the subject did not report any adverse event other than minor injection site pain that did not interfere with day-to-day activities.
Discussion
Data from two of the longest follow-up studies in adults with a two-dose regimen of GSK Biologicals’ monovalent HAV vaccine (either a 0, 6 mo or 0, 12 mo regimen) have reported persistence of anti-HAV antibodies up to at least 15 y after primary vaccination.Citation10 This communication aimed to extend the follow-up period to the next two annual time points, in order to share up-to-date data on the longevity of anti-HAV immune response in adults following two different dosing regimens of this study vaccine.
At Year 17, all subjects except one remained seropositive for anti-HAV antibodies indicating that anti-HAV antibodies and immune response to two priming doses of HAV vaccine persisted for at least 17 y. This subject who became seronegative at Year 16 (Study B: 0, 12 mo regimen) received a HAV challenge dose and mounted an anamnestic response one month later. The challenge dose was well-tolerated.
In conclusion, both regimens of HAV vaccine—standard 0, 6 mo regimen and extended 0, 12 mo regimen, conferred long-term immunity and induced persistent vaccine-induced anti-HAV antibodies for up to at least 17 y after primary vaccination. This confirmed the flexibility in the priming immunization regimen for HAV vaccines. Presence of immune memory could be shown in one subject, 17 y after primary immunization. This data could have plausible implications on public health policy decisions with respect to selection of convenient dosing schedules to increase compliance and completion rates as well as in evaluation of the requirement for a booster dose in adults.
Materials and Methods
Study design, subjects and study vaccine
In the two double-blind, randomized primary studies, healthy adults aged 17−40 y and 21−40 y received two doses of Havrix™ [GSK Biologicals, Belgium; 1 ml dose contained at least 1440El.U of HAV (strain HM175)] following a standard 0, 6 mo or an extended 0, 12 mo schedule.Citation15 Subjects who completed their primary vaccination regimen and expressed willingness to participate in the follow-up phase were eligible to participate in these long-term follow-up studies (NCT00289757/NCT00291876). Subjects who became seronegative (anti-HAV antibody concentration < 15 mIU/ml) after the Year 15 time point, were eligible to receive a challenge dose of the same vaccine in the deltoid of the non-dominant arm.
Written informed consent was obtained from subjects prior to blood sampling at each yearly visit. The study was conducted as per Good Clinical Practice, the Declaration of Helsinki and applicable local regulations; all study-related documents were approved by the Ethics Review Committee of the Antwerp University Hospital.
Immunogenicity assessments
Anti-HAV antibody concentrations in serum samples were collected at Year 16 and Year 17 time points. Seronegative subjects who received a HAV challenge dose also provided serum samples prior to, at Day 14 and Day 30 after the challenge dose. Measurements relied on a commercially available enzyme-linked immunoassay (Enzygnost®, DADE Behring; cut-off: 15mIU/ml). Anamnestic response for anti-HAV antibodies was defined as post-challenge dose anti-HAV antibody concentration ≥ 15 mIU/ml in subjects who were seronegative before the challenge dose.
Assessment of safety
For the challenge dose, solicited local and general adverse events and unsolicited adverse events were recorded during the 4-d and 30-d post-vaccination follow-up periods. Serious adverse events (SAEs) considered by the investigator to be causally-related to vaccination were recorded throughout the study period.
Statistical analyses
Descriptive immunogenicity analyses were performed on the LT-ATP cohort for immunogenicity and long-term total cohort at yearly time points and the safety analyses were performed on the long-term total cohort.
The long-term total cohort included all subjects who belonged to the total cohort in the primary study and returned at the annual time points at Years 16 and 17. The LT-ATP immunogenicity cohort included all subjects from the ATP cohort in the primary study who had not received any additional HAV vaccine or abnormal increase in anti-HAV antibody concentrations since the previous time point. The anti-HAV seropositivity rate and corresponding GMCs were calculated with 95%. The GMCs were calculated by taking the anti-log of the mean of log-transformed concentrations (calculated for seropositive subjects).
These statistical analyses were performed using Statistical Analysis Software versions 9.1 and 9.2 for Years 16 and 17, respectively.
| Abbreviations: | ||
| CI | = | Confidence Interval |
| GMC | = | Geometric Mean Concentration |
| HAV | = | Hepatitis A |
| (LT)-ATP | = | Long-term According-To-Protocol |
| SAE | = | Serious Adverse Event |
Acknowledgments
The authors would like to thank all subjects for their valued participation in this study and the study physician Dr. Froukje Kafeja; the study nurse Serge Broodhaers; and Bert Peeters and Jef Vanpellicom who assisted in tracing of subjects throughout the follow-up period (Vaccine and Infectious Disease Institute, Centre for the Evaluation of Vaccination, Antwerp, Belgium). The authors would also like to acknowledge Jyoti Kumari for performing statistical analyses, Avishek Pal for medical writing assistance and Manjula K for publication coordination (all employed by GSK Biologicals).
Trademark statement
Havrix is a trademark of the GlaxoSmithKline group of companies. Enzygnost is a registered trade mark of DADE Behring.
Role of funding source
GlaxoSmithKline Biologicals was the funding source and was involved along with the investigators in all stages of the study conduct and analyses. GlaxoSmithKline Biologicals took in charge all costs associated with the development and the publishing of the present manuscript. All authors had full access to the data and had final responsibility to submit for publication. They received no remuneration for this work.
Disclosure of Potential Conflicts of Interest
The authors disclose the following conflict of interest: KVH declares that his institution (University of Antwerp) has obtained grants to conduct the study and he was supported to travel to meetings in order to present the study results; outside the scope of this study as well, his institution has received research grants, fees for consultancy and educational presentations on vaccines provided by KVH from different vaccine manufacturers. PC is employed at GSK Biologicals; MM is employed by GSK Biologicals as a consultant (from the CRO CHILTERN); KH is employed at GSK Biologicals and also has stock ownership at GSK Biologicals. PVD acts as chief and principal investigator for clinical trials conducted on behalf of the University of Antwerp, for which the University obtains research grants from vaccine manufacturers; speaker’s fees for presentations on vaccines are paid directly to an educational fund held by the University of Antwerp. PVD receives no personal remuneration for this work.
References
- World Health Organization (WHO). 2000. Hepatitis A position paper. Wkly Epidemiol Rec 75:38–42. World Health Organisation, Dept. of Communicable diseases surveillance and Response. WHO/CDC/CSR/LYO/2002.2: Hepatitis B. Available: http://www.who.int/csr/disease/hepatitis/HepatitisB_whocdscsrlyo2002_2.pdf. [Accessed on August 05, 2011].
- Andersson KL, Friedman LS, Hepatitis A. A travelling target. Arch Intern Med 2010; 20:1818 - 9; http://dx.doi.org/10.1001/archinternmed.2010.412
- Askling HH, Rombo L, Andersson Y, Martin S, Ekdahl K. Hepatitis A risk in travelers. J Travel Med 2009; 16:233 - 8; http://dx.doi.org/10.1111/j.1708-8305.2009.00307.x; PMID: 19674261
- André F, Van Damme P, Safary A, Banatvala J. Inactivated hepatitis A vaccine: immunogenicity, efficacy, safety and review of official recommendations for use. Expert Rev Vaccines 2002; 1:9 - 23; http://dx.doi.org/10.1586/14760584.1.1.9; PMID: 12908508
- Nothdurft HD. Hepatitis A vaccines. Expert Rev Vaccines 2008; 7:535 - 45; http://dx.doi.org/10.1586/14760584.7.5.535; PMID: 18564009
- Van Herck K, Van Damme P. Prevention of hepatitis A by Havrix: a review. Expert Rev Vaccines 2005; 4:459 - 71; http://dx.doi.org/10.1586/14760584.4.4.459; PMID: 16117704
- Hammitt LL, Bulkow L, Hennessy TW, Zanis C, Snowball M, Williams JL, et al. Persistence of antibody to hepatitis A virus 10 years after vaccination among children and adults. J Infect Dis 2008; 198:1776 - 82; http://dx.doi.org/10.1086/593335; PMID: 18976095
- López EL, Contrini MM, Mistchenko A, Debbag R. Long-term immunity after two doses of inactivated hepatitis A vaccine, in Argentinean children. Pediatr Infect Dis J 2010; 29:568 - 70; PMID: 20195189
- Van Herck K, Van Damme P, Lievens M, Stoffel M. Hepatitis A vaccine: indirect evidence of immune memory 12 years after the primary course. J Med Virol 2004; 72:194 - 6; http://dx.doi.org/10.1002/jmv.10574; PMID: 14695659
- Van Herck K, Jacquet JM, Van Damme P. Antibody persistence and immune memory in healthy adults following vaccination with a two-dose inactivated hepatitis A vaccine: long-term follow-up at 15 years. J Med Virol 2011; 83:1885 - 91; http://dx.doi.org/10.1002/jmv.22200; PMID: 21915861
- Van Herck K, Van Damme P. Inactivated hepatitis A vaccine-induced antibodies: follow-up and estimates of long-term persistence. J Med Virol 2001; 63:1 - 7; http://dx.doi.org/10.1002/1096-9071(200101)63:1<1::AID-JMV1000>3.0.CO;2-U; PMID: 11130881
- Rendi-Wagner P, Korinek M, Winkler B, Kundi M, Kollaritsch H, Wiedermann U. Persistence of seroprotection 10 years after primary hepatitis A vaccination in an unselected study population. Vaccine 2007; 25:927 - 31; http://dx.doi.org/10.1016/j.vaccine.2006.08.044; PMID: 17005304
- Van Herck K, Beutels P, Van Damme P, Beutels M, Van den Dries J, Briantais P, et al. Mathematical models for assessment of long-term persistence of antibodies after vaccination with two inactivated hepatitis A vaccines. J Med Virol 2000; 60:1 - 7; http://dx.doi.org/10.1002/(SICI)1096-9071(200001)60:1<1::AID-JMV1>3.0.CO;2-H; PMID: 10568755
- Van Herck K, Van Damme P. Inactivated hepatitis A vaccine-induced antibodies: follow-up and estimates of long-term persistence. J Med Virol 2001; 63:1 - 7; http://dx.doi.org/10.1002/1096-9071(200101)63:1<1::AID-JMV1000>3.0.CO;2-U; PMID: 11130881
- Van Damme P, Matheï C, Thoelen S, Meheus A, Safary A, André FE. Single dose inactivated hepatitis A vaccine: rationale and clinical assessment of the safety and immunogenicity. J Med Virol 1994; 44:435 - 41; http://dx.doi.org/10.1002/jmv.1890440422; PMID: 7897376