Abstract
As progress toward global poliovirus eradication continues, more and more countries are moving away from use of oral poliovirus vaccines (OPV) to inactivated poliovirus vaccines (IPV) in national vaccination schedules. Reduction of antigen dose in IPV could increase manufacturing capacity and facilitate the change from OPV to IPV. Combination vaccines reduce the number of injections required to complete vaccination, thus playing an important role in maintaining high vaccine coverage with good public acceptability. Three formulations of a combined, candidate hexavalent diphtheria-tetanus-whole cell pertussis-hepatitis B-inactivated poliovirus-Hemophilus influenzae type b conjugate vaccine (DTPw-HBV-IPV/Hib, GlaxoSmithKline Biologicals) differing only in IPV antigen content (full-dose, half-dose and one-third dose as compared with available stand-alone IPV vaccines), were evaluated when administered to healthy toddlers. Controls received separately administered licensed DTPw-HBV/Hib and IPV vaccines. Immunogenicity was assessed before and one month after vaccination. Safety and reactogenicity data were assessed for 30 d after vaccination. A total of 312 Filipino children were vaccinated in their second year of life. Each DTPw-HBV-IPV/Hib formulation was non-inferior to control in terms of pre-defined criteria for IPV immunogenicity. Post-vaccination GMTs against each poliovirus type were increased between 4.2- and 37.9-fold over pre-vaccination titers. Non-inferiority to other vaccine antigens was also demonstrated. The safety profile of the 3 DTPw-HBV-IPV/Hib formulations resembled licensed DTPw-HBV/Hib Kft and IPV in terms of the frequency and intensity of adverse reactions after vaccination. Further investigation of DTPw-HBV-IPV/Hib containing reduced quantity of IPV antigen for primary vaccination in infants is warranted.
This study is registered at www.clinicaltrials.gov NCT number: NCT01106092
Introduction
The World Health Organization (WHO) recommends that all infants be routinely vaccinated against diphtheria, tetanus, pertussis, hepatitis B, poliomyelitis, Hemophilus influenzae type B (Hib) and measles.Citation1,Citation2 Despite continued gains in global vaccine coverage, WHO estimates that in 2009, 23.2 million children did not receive three doses of diphtheria-tetanus-pertussis (DTP) vaccine, and that 1.7 million children less than 5 y of age died from vaccine-preventable diseases.Citation3
With the global coverage of DTP3 (three doses of DTP in infancy) now over 80%,Citation3 incorporating additional antigens to DTP vaccines is an effective approach to integrating new vaccines into existing schedules. Combination vaccines reduce the number of injections required to complete vaccination, minimize clinic visits and reduce costs associated with vaccine transport and storage. Importantly, use of combination vaccines improves vaccination coverage rates and the timeliness of vaccination.Citation4,Citation5 DTP has been combined with hepatitis B surface antigen as DTPw-HBV, which may also be mixed with Hib conjugate vaccines (DTPw-HBV/Hib). Combinations of DTPw with Hib and inactivated poliovirus vaccine (IPV) are also available (DTPw-IPV/Hib). No hexavalent vaccine that incorporates all of these antigens (DTPw-HBV-IPV/Hib) is currently available.
Poliomyelitis is a highly infectious disease caused by any one of three poliovirus serotypes. Since the introduction of the Global Polio Eradication Initiative in 1988, the number of poliomyelitis cases has decreased from 350,000 per year in 1988, to 1,783 cases in 2009.Citation3 Much of this gain is attributable to the widespread use of oral live-attenuated poliovirus vaccines (OPV), which have been the cornerstone of polio prevention in most countries since the 1960s. However, in countries where polio has already been eradicated, and in the years following global polio eradication, the risk of vaccine-associated paralytic polio and of polio outbreaks due to circulating vaccine-derived polioviruses means that continued use of OPV is untenable.Citation6 In these settings, population immunity against poliomyelitis will be maintained through IPV.Citation6 Since the production capacity of IPV for worldwide distribution is limited, reduction of the amount of IPV antigen in each vaccine dose would increase capacity and facilitate the shift from OPV to IPV.
A candidate hexavalent DTPw-HBV-IPV/Hib vaccine (GlaxoSmithKline Biologicals [GSK]) combines the antigens of two vaccines licensed by GSK: IPV (Poliorix™) and DTPw-HBV/Hib (Zilbrix™/Hib). The development of the hexavalent vaccine included the feasibility of reducing the quantity of IPV antigens in the DTPw-HBV-IPV/Hib vaccine.
The objectives of this phase II, randomized, partially double-blind dose-range study were to evaluate the immunogenicity, reactogenicity and safety of a booster dose of three DTPw-HBV-IPV/Hib formulations which differed in IPV antigen content (full dose, half-dose and one-third dose, ) compared with licensed DTPw-HBV/Hib and IPV administered separately. This was the first administration of the novel DTPw-HBV-IPV/Hib formulation in humans. The study was conducted in healthy toddlers between 12 and 24 mo of age.
Table 1. Composition of study vaccines
Results
Subjects and demography
A total of 312 subjects were enrolled and vaccinated (78 in each group). One subject withdrew consent and failed to complete the study. All other subjects completed the study and all (n = 311) were included in the ATP immunogenicity cohort. The mean age of all subjects was 17.7 mo and 54.2% were males. The study groups were comparable in terms of demographic characteristics at enrolment ().
Table 2. Demographic characteristics of the total vaccinated cohort
Immunogenicity
Response to poliovirus vaccine
The primary objectives of the study were to demonstrate non-inferiority in terms of immunogenicity, of each DTPw-HBV-IPV/Hib formulation compared with the Control group in terms of anti-polio seroprotection rates and in terms of anti-polio geometric mean antibody titer (GMT) increases (at least a 2-fold increase). Both primary objectives were met for each DTPw-HBV-IPV/Hib group, for each of the three poliovirus types ().
Figure 1. Comparison between groups in the immune response to poliovirus types 1, 2 and 3 (ATP immunogenicity cohort): (A) difference in seroprotection rates between the control group and each DTPa-HBV-IPV/Hib formulation (Formulations 1–3); (B) GMT ratios (post divided by pre-vaccination titers) for anti-poliovirus types 1, 2 and 3. (A) The upper limits of the standardized asymptotic 95% CI on the group difference in the percentage of subjects with anti-poliovirus types 1, 2 and 3 titers ≥ 8 are ≤ 10 (predefined criteria for non-inferiority indicated by bold horizontal line); (B) The lower limits of the two-sided 95% CI on the geometric mean of the individual ratios (post- over pre-vaccination titers) for anti-poliovirus types 1, 2 and 3 antibodies are ≥ 2 (predefined criteria for immunogenicity indicated by bold horizontal line). Form 1 received DTPw-HBV-IPV(full dose)/Hib; Form 1/2 received DTPw-HBV-IPV(1/2 dose)/Hib; Form 1/3 received DTPw-HBV-IPV(1/3 dose)/Hib; Controls received DTPw-HBV/Hib Kft + IPV.
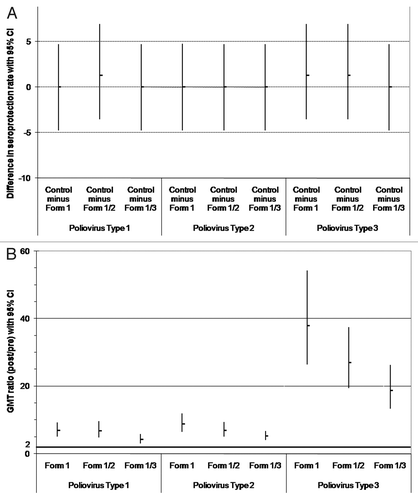
Before the booster dose the majority of subjects in each group (between 89.7% and 100%) had seroprotective antibody concentrations against each of the three poliovirus types. One month after the booster dose, between 98.7% and 100% of subjects had seroprotective antibodies against each poliovirus type (). In all groups, post-vaccination GMTs against each poliovirus type increased between 4.2- and 37.9-fold over pre-vaccination levels ().
Table 3. Seroprotection/seropositivity rates and antibody GMCs before and one month post-booster (ATP cohort for immunogenicity)
Anti-poliovirus types 1, 2 and 3 seroconversion rates (seroconversion was defined as post-booster antibody titer ≥ 1:8 in initially seronegative subjects, at least a 4-fold increase in post-booster titer in initially seropositive subjects, or a titer greater than the highest dilution tested [1:8,192] in subjects with pre-booster antibody titers < 8,192) were at least 76.9% in all 4 groups and reached a maximum of 97.4% (). All but two initially seronegative subjects seroconverted after the booster dose (one remained seronegative for poliovirus types 1 and 3, and the other remained seronegative for poliovirus type 3).
Figure 2. Seroconversion rates for poliovirus types 1, 2 and 3 one month post-booster (ATP immunogenicity cohort). *Seroconversion was defined as post-booster antibody titer ≥ 1:8 in initially seronegative subjects, at least a 4-fold increase in post-booster titer in initially seropositive subjects, or a titer greater than the highest dilution tested [1:8192] in subjects with pre-booster antibody titers < 8192). Form 1 received DTPw-HBV-IPV(full dose)/Hib; Form 1/2 received DTPw-HBV-IPV(1/2 dose)/Hib; Form 1/3 received DTPw-HBV-IPV(1/3 dose)/Hib; Controls received DTPw-HBV/Hib Kft + IPV.
![Figure 2. Seroconversion rates for poliovirus types 1, 2 and 3 one month post-booster (ATP immunogenicity cohort). *Seroconversion was defined as post-booster antibody titer ≥ 1:8 in initially seronegative subjects, at least a 4-fold increase in post-booster titer in initially seropositive subjects, or a titer greater than the highest dilution tested [1:8192] in subjects with pre-booster antibody titers < 8192). Form 1 received DTPw-HBV-IPV(full dose)/Hib; Form 1/2 received DTPw-HBV-IPV(1/2 dose)/Hib; Form 1/3 received DTPw-HBV-IPV(1/3 dose)/Hib; Controls received DTPw-HBV/Hib Kft + IPV.](/cms/asset/f3542aa2-7ca4-41ce-8825-4aac9d4071a7/khvi_a_10918630_f0002.gif)
Exploratory analyses indicated that the post-booster GMTs against each poliovirus type were higher in the control group than in each of the DTPw-HBV-IPV/Hib groups, with the exception of poliovirus type 3 in Form 1 group (). Exploratory analyses indicated no differences between groups in poliovirus seroconversion rates with the exception of the seroconversion rate to poliovirus type 1, which was lower in the Form 1/3 group compared with the control group (data not shown). Note that due to the multiplicity of endpoints, statistically significant findings must be interpreted with caution.
Responses to other vaccine antigens
Before the booster dose the majority of subjects in each group had seroprotective antibody concentrations against diphtheria (range 84.4 to 88.5% ≥ 0.1 IU/ml), tetanus (range 98.7 to 100% ≥ 0.1 IU/ml) and hepatitis B (range 79.5 to 90.9% ≥ 10 mIU/ml; ). Pre-booster, between 34.6 and 45.5% were seroprotected against Hib (≥ 0.15 μg/ml) and between 63.6 and 65.8% were seropositive for anti-BPT antibodies (≥ 15 EL.U/ml).
One month after the booster dose all subjects in the 4 study groups had antibody levels consistent with seroprotection against diphtheria and tetanus, and all but two subjects were seroprotected against hepatitis B. The percentage of subjects with seroprotective antibodies against Hib was between 91.0% and 98.7%, and all subjects except one were seropositive for antibodies against BPT.
The secondary objective of the study was to show non-inferiority between each DTPw-HBV-IPV/Hib group and the control group in terms of seroprotection rates against diphtheria, tetanus and hepatitis B, and in terms of antibody GMCs for anti-BPT. All secondary objectives were met (data not shown). Additional exploratory analyses showed no statistically significant differences between groups in terms of seroprotection or seropositivity rates for these antigens.
Post-vaccination anti-PRP GMCs were statistically significantly lower in the Form 1/3 group than the control group, whereas statistically higher GMCs were observed for some DTPw-HBV-IPV/Hib groups for post-vaccination anti-diphtheria, anti-tetanus and anti-HBs antibody GMCs ().
Reactogenicity and safety
All subjects returned diary cards. The majority of subjects (up to 98.7%) reported at least one symptom or adverse event of any type, and between 33.3% and 41.0% of subjects reported an event of grade 3 intensity within the 8-d post-vaccination follow-up period.
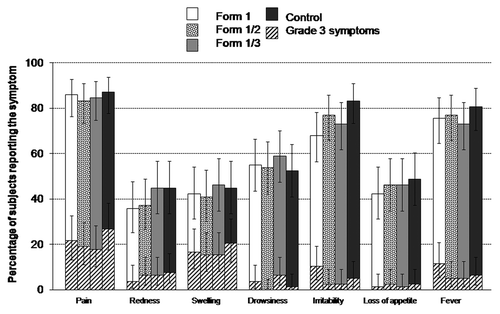
Pain, irritability and fever were the most commonly reported solicited symptoms, whereas pain and swelling were the most commonly reported events of grade 3 intensity (). The incidence of solicited local and general symptoms was similar across all 4 groups, as suggested by overlapping 95% CIs in all cases.
Figure 3. Percentage of subjects reporting solicited local and general symptoms during the 8-d post-vaccination follow-up period (total vaccinated cohort). Grade 3 was defined as: Cried when limb is moved/spontaneously painful (pain); injection site diameter > 20 mm (redness/swelling); axillary temperature > 39.0°C (fever); prevented normal daily activities (irritability/fussiness, drowsiness); not eating at all (loss of appetite). Form 1 received DTPw-HBV-IPV(full dose)/Hib; Form 1/2 received DTPw-HBV-IPV(1/2 dose)/Hib; Form 1/3 received DTPw-HBV-IPV(1/3 dose)/Hib; Controls received DTPw-HBV/Hib + IPV.
Four large injection site swellings, defined as injection site swelling of diameter > 50 mm, noticeable diffuse swelling or increased limb circumference, were reported: one subject in each the Form 1 and Form 1/3 groups and two subjects in the Control group.
The percentage of subjects reporting at least one unsolicited symptom during the 31-d follow-up period was, at most, 56.4% across the study groups. Only one grade 3 unsolicited symptom was reported by a subject in the Control group, which was considered by the investigator to be unrelated to vaccination.
Seven subjects reported serious adverse events (SAEs): one in the Form 1 group, three in the Form 1/2 group, two in the Form 1/3 group and one in the Control group. Five SAEs were reports of febrile convulsions, of which two were reported as complex febrile convulsions. All five episodes had an onset between 6 d and 28 d after vaccination. None of the SAEs were considered by the investigator to be causally related to vaccination and all the subjects recovered without sequelae.
Discussion
The present study evaluated a candidate combined hexavalent DTPw-HBV-IPV/Hib vaccine using different IPV antigen dosages for the first time in humans.
All three DTPw-HBV-IPV/Hib formulations met pre-defined criteria in terms of the response to the poliovirus antigens: seroprotection rates were non-inferior to those of the control group, and anti-poliovirus GMTs increased by more than 2-fold from pre to post vaccination. The post-vaccination anti-poliovirus GMTs in the DTPw-HBV-IPV/Hib groups correlated with the IPV antigen content, and was almost always lower than in the Control group. All GMTs were more than 100-fold above the seroprotection cut-off. All anti-poliovirus GMTs were higher than those of a booster dose of OPV administered at the same age and in the same country in a previous study.Citation7 The results suggest that reduction in the IPV antigen content in DTPw-HBV-IPV/Hib is feasible, and deserves further evaluation in other populations, including infants.
Pre-defined non-inferiority criteria in terms of responses to diphtheria, tetanus, hepatitis B and pertussis were also met for each DTPw-HBV-IPV/Hib group compared with the Control group. The results suggest no clinically meaningful impact of the presence of the IPV antigens in the candidate vaccine on the immunogenicity of the DTPw-HBV antigens.
Children enrolled in this study had not received primary vaccination against Hib. Post-vaccination, 91.0 to 98.7% of subjects had seroprotective antibodies against Hib. These seroprotection rates are in the expected range when a Hib vaccine is administered as a single dose in unprimed toddlers. There appeared to be a dose-related effect of IPV on the magnitude of the antibody responses to Hib, with the lowest seroprotection rate and anti-PRP GMC observed following vaccination with DTPw-HBV-IPV/Hib containing the lowest IPV dosage. IPV appears to enhance Hib responses when combined with acellular pertussis vaccines (DTPa).Citation8 The data from the present study suggest a possible dose-related interaction between IPV and Hib immunogenicity.
The safety profile of the 3 DTPw-HBV-IPV/Hib formulations resembled licensed DTPw-HBV/Hib and IPV in terms of the frequency and intensity of adverse reactions after vaccination. The rate of local and general adverse events in this study is consistent with published data following DTPw booster vaccination.Citation9,Citation10 Five subjects experienced febrile convulsion after vaccination. One subject had concurrent pneumonia. Febrile convulsions after DTPw vaccination typically occur within the first 24 h after vaccination.Citation11,Citation12 All cases in the present study began 6 d or later after vaccination, suggesting that they were due to causes other than vaccination.
Potential limitations of the study include the open design with respect to groups that received DTPw-HBV-IPV/Hib and separately administered DTPw-HBV/Hib and IPV which may have introduced bias in the reporting of safety endpoints. Bias in terms of laboratory outcomes is unlikely to have occurred because laboratory staff were blinded to vaccine group during the analysis.
Combination vaccines have an increasingly important role to play in maintaining public acceptance of vaccination, by minimizing the number of injections necessary for full vaccination. Combination vaccines facilitate high levels of vaccination coverage and timely vaccination by reducing the risk of vaccination deferral. Adding new antigens onto existing vaccines with high coverage levels is an efficient way of introducing new antigens into vaccination schedules. Uptake and coverage of the new antigen rapidly increases to the level of the existing vaccine, with good acceptability by parents and vaccine providers. Three formulations of the candidate combined DTPw-HBV-IPV/Hib vaccine were immunogenic when administered as a booster dose to healthy toddlers, with safety and reactogenicity profiles that resembled licensed DTPw-HBV/Hib co-administered with IPV. Further investigation of DTPw-HBV-IPV/Hib for primary vaccination in infants is warranted, including evaluation of formulations containing reduced IPV antigen content.
Methods
Study design and study subjects
The study was conducted at a single center, the Research Institute for Tropical Medicine, Manila, in the Philippines between May 2010 and September 2010 (113264, www.clinicaltrials.gov NCT01106092). Eligible toddlers were between 12 and 24 mo of age, in good health and who had received three doses of poliovirus vaccine as well as other recommended vaccines, via routine primary vaccination procedures during the first year of life.
Subjects were excluded from participation if they had received any investigational drug/vaccine within one month before the booster vaccination; if they had received any non-study vaccine within 30 d prior to booster vaccination, or planned administration during the active study period; if they were immunosuppressed from any cause; if they had a history of infection against any pathogen against which the study vaccines were targeted or had received previous booster vaccination against diphtheria, tetanus, pertussis, poliovirus, hepatitis B and/or Hib.
The study was conducted according to Good Clinical Practice and in accordance with the Declaration of Helsinki. The protocol and associated documents were reviewed and approved by the ethics committees of the participating study center. Written informed consent was obtained from the parents/guardians of all subjects before study entry.
Subjects were randomized (1:1:1:1) to one of four study groups: three groups received DTPw-HBV-IPV/Hib vaccines that differed only in the quantity of poliovirus antigen in each dose (). The study was double-blind with respect to which the DTPw-HBV-IPV/Hib formulation was administered. The control group received commercially available DTPw-HBV/Hib (Zilbrix™/Hib) and IPV (Poliorix™) vaccines administered as separate injections. Due to the different number of injections given to the control group, subjects knew if they received DTPw-HBV-IPV/Hib or DTPw-HBV/Hib + IPV.
Enrolment was undertaken in a phased manner to evaluate the safety of the new vaccine formulations. During the first phase, a maximum of two subjects were enrolled per day over one month, up to a total of 32 subjects. Randomization was then paused, and safety data were analyzed by an internal Safety Review Committee. No safety concern was raised and hence recruitment was reopened until the target sample size was attained.
Assessment of immunogenicity
Blood samples were collected from all subjects before and one month after the booster dose. Antibodies against diphtheria, tetanus, BPT, hepatitis B surface antigen (anti-HBs) and PRP were measured using standard enzyme-linked immunosorbent assays (ELISA). A microneutralization assay was used to assess antibody titers to the three poliovirus types.Citation13
Testing for anti-HBs was performed at the Center for Vaccinology Ghent University, Belgium. The poliovirus neutralization assay was undertaken at Biomnis, France. All other assays were performed at GSK Biologicals’ laboratory in Belgium using in-house or commercial assays. The serological assays used standardized, validated procedures with adequate controls. Laboratory staff were blinded to vaccine group during the analysis.
Assessment of reactogenicity and safety
Local and general symptoms were solicited using diary cards, for 8 d (Day 0–7). Parents/guardians of subjects were advised to contact the investigators in case of any large injection site reaction. Other (unsolicited) symptoms were recorded for 31 d after vaccination and SAEs were recorded for the entire study duration. Completed diary cards were collected at the time of the follow-up blood sample one month after vaccination. Any unreturned diary cards were sought from the subjects’ parents/legal guardians via the telephone or any other convenient procedure.
Statistical Analyses
Randomization was performed using an SAS program at GSK Biologicals, Belgium. Block randomization (1:1:1:1 ratio) ensured balance between the treatment arms. Treatment allocation was done using a centralized internet randomization system.
The statistician was blind to treatment group during the analysis, although due to the different number of injections, it was possible to ascertain whether subjects received a study vaccine or control. Nevertheless, the statistician was blind to all laboratory results during the analysis.
The primary (non-inferiority) immunogenicity endpoint was performed on the according-to-protocol (ATP) cohort comprising subjects who complied with protocol procedures, had received a booster dose of the study vaccine and had pre- and post-booster blood sampling data available.
A sample size of 78 subjects per group would provide 88% power (Bonferroni adjustment for β) to the overall study, assuming that 10% of subjects dropped out.
The 95% CI for seroprotection/seropositivity status, antibody GMC/GMTs to all antigens, seroconversion status in terms of poliovirus antigens and booster response to the pertussis antigens, one month after booster administration was calculated.
Each DTPw-HBV-IPV/Hib formulation was non-inferior to the control vaccines if the upper limit of standardized asymptotic 95% CI on group difference (control group minus each of the DTPw-HBV-IPV/Hib groups) was ≤ 10% in terms of seroprotection rates to anti-poliovirus 1, 2 and 3, and if the lower limit of the asymptotic 95% CI on geometric mean of individual ratios (post-over pre-booster titers) for antibodies for all three poliovirus types was ≥ 2.
Non-inferiority between groups (secondary objective) would be established if the upper limit of the standardized asymptotic 95% CI on group difference (control group minus each of the DTPw-HBV-IPV/Hib groups) was ≤ 10% in terms of seroprotection rates against diphtheria, tetanus and hepatitis B antigens and upper limit of the 95% CI of the anti-BPT GMC ratio (control group divided by each DTPw-HBV-IPV/Hib group) was ≤ 1.5.
Additional exploratory comparisons between groups were made. Vaccine groups were considered significantly different if the 95% CI for the GMC/GMT ratio (ANCOVA model including the group as fixed effect and the log-transformed pre-booster concentration as covariable) between groups did not contain the value 1, or, if the asymptotic standardized 95% CI for the difference in seroprotection/seropositivity rates between groups did not contain the value 0. Due to the multiplicity of endpoints, statistically significant findings must be interpreted with caution.
Safety and reactogenicity analysis was performed on the total vaccinated cohort comprising all vaccinated subjects. The percentage of subjects reporting symptoms was tabulated with exact 95% CI.
ZILBRIX and POLIORIX are trademarks of GlaxoSmithKline group of companies.
| Abbreviations: | ||
| μg/ml | = | micrograms per milliliter |
| ATP | = | according to protocol |
| BPT | = | Bordetella pertussis |
| CI | = | confidence interval |
| DTPw-HBV-IPV/Hib | = | diphtheria-tetanus-whole cell pertussis-hepatitis B-inactivated poliovirus and Haemophilus influenzae type b vaccine |
| DTPw-HBV/Hib | = | diphtheria-tetanus-whole cell pertussis-hepatitis B- and Haemophilus influenzae type b vaccine |
| EL.U/ml | = | ELISA units per milliliter |
| GMC | = | geometric mean antibody concentration |
| GMT | = | geometric mean antibody titer |
| IPV | = | inactivated poliovirus vaccine |
| IU/ml | = | international units per milliliter |
| mIU/ml | = | milli-international units per milliliter |
| OPV | = | live attenuated oral poliovirus vaccine |
| PRP | = | polyribosylribitol phosphate |
| SAE | = | serious adverse event |
| WHO | = | World Health Organization |
Acknowledgments
The authors would like to thank Petra Vandenberk for the study coordination, Vrushali Sanghrajka (GlaxoSmithKline Biologicals) for performing the statistical analysis, Geetha Subramanyam for early work on the manuscript, Dr Joanne Wolter (independent medical writer) for preparing the first draft of the manuscript, and Dr Julia Donnelly for editorial assistance.
Sources of Support
GlaxoSmithKline Biologicals (GSK) was the funding source and was involved in all stages of the study conduct and analysis. GSK also funded all costs associated with the development and the publishing of the present manuscript. The corresponding author had full access to the data and was responsible for submission of the publication.
Disclosure of Potential Conflicts of Interest
B.Q. received an honorarium from GSK Biologicals to conduct this study and has received research grants and travel grants from Sanofi, Novartis and GSK Biologicals. O.V.M., D.K. and S.G. are employees of GlaxoSmithKline Biologicals.
References
- World Health Organization. Immunization policy. Global programme for vaccines and immunization. Expanded Programme on Immunization. Available from: http://whqlibdoc.who.int/hq/1995/WHO_EPI_GEN_95.03_Rev.1.pdf. Accessed 22 May 2011.
- World Health Organization. WHO position paper on Haemophilus influenzae type b conjugate vaccines. (Replaces WHO position paper on Hib vaccines previously published in the Weekly Epidemiological Record). Wkly Epidemiol Rec 2006; 81:445 - 52; PMID: 17124755
- World Health Organization. Global Immunization Data. 2010 Dec; Available from: http://www.who.int/immunization_monitoring/Global_Immunization_Data.pdf Accessed 22 May 2011.
- Marshall GS, Happe LE, Lunacsek OE, Szymanski MD, Woods CR, Zahn M, et al. Use of combination vaccines is associated with improved coverage rates. Pediatr Infect Dis J 2007; 26:496 - 500; http://dx.doi.org/10.1097/INF.0b013e31805d7f17; PMID: 17529866
- Kalies H, Grote V, Verstraeten T, Hessel L, Schmitt H-J, von Kries R. The use of combination vaccines has improved timeliness of vaccination in children. Pediatr Infect Dis J 2006; 25:507 - 12; http://dx.doi.org/10.1097/01.inf.0000222413.47344.23; PMID: 16732148
- World Health Organization. Inactivated poliovirus vaccine following oral poliovirus vaccine cessation. Wkly Epidemiol Rec 2006; 81:137 - 44; PMID: 16673508
- Knuf M, Szenborn L, Moro M, Petit C, Bermal N, Bernard L, et al. Immunogenicity of routinely used childhood vaccines when coadministered with the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV). Pediatr Infect Dis J 2009; 28:Suppl S97 - 108; http://dx.doi.org/10.1097/INF.0b013e318199f61b; PMID: 19325452
- Dagan R, Poolman JT, Zepp F. Combination vaccines containing DTPa-Hib: impact of IPV and coadministration of CRM197 conjugates. Expert Rev Vaccines 2008; 7:97 - 115; http://dx.doi.org/10.1586/14760584.7.1.97; PMID: 18251697
- Gatchalian S, Reyes M, Bermal N, Chandrasekaran V, Han HH, Bock HL, et al. A new DTPw-HBV/Hib vaccine: immune memory after primary vaccination and booster dosing in the second year of life. Hum Vaccin 2008; 4:60 - 6; http://dx.doi.org/10.4161/hv.4.1.5069; PMID: 18376148
- Gatchalian SR, Ramakrishnan G, Bock HL, Lefevre I, Jacquet JM. Immunogenicity, reactogenicity and safety of three-dose primary and booster vaccination with combined diphtheria-tetanus-whole-cell pertussis-hepatitis B-reduced antigen content Haemophilus influenzae type b vaccine in Filipino children. Hum Vaccin 2010; 6:664 - 72; http://dx.doi.org/10.4161/hv.6.8.12155; PMID: 20657177
- Barlow WE, Davis RL, Glasser JW, Rhodes PH, Thompson RS, Mullooly JP, et al, Centers for Disease Control and Prevention Vaccine Safety Datalink Working Group. The risk of seizures after receipt of whole-cell pertussis or measles, mumps, and rubella vaccine. N Engl J Med 2001; 345:656 - 61; http://dx.doi.org/10.1056/NEJMoa003077; PMID: 11547719
- Cody CL, Baraff LJ, Cherry JD, Marcy SM, Manclark CR. Nature and rates of adverse reactions associated with DTP and DT immunizations in infants and children. Pediatrics 1981; 68:650 - 60; PMID: 7031583
- World Health Organization. Standard Procedure for Determining Immunity to Poliovirus using the Microneutralisation Test. 1993 WHO/EPI/GEN 93 9.