Abstract
Prophylactic human papillomavirus (HPV) vaccines are now available and vaccination programs are being widely implemented, targeting adolescent girls prior to sexual debut. Since the risk of HPV exposure persists throughout a woman’s sexual life, the duration of protection provided by vaccination is critical to the overall vaccine effectiveness. We report the long-term efficacy and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine (Cervarix®) up to 8.4 y after the first vaccine dose.
In an initial placebo-controlled study performed in US, Canada and Brazil, women aged 15–25 y with normal cervical cytology, HPV-16/18 seronegative by ELISA, DNA-negative for 14 oncogenic HPV types by PCR, received either the HPV-16/18 vaccine or placebo (n = 1,113). Subjects were followed up to 6.4 y after the first dose (n = 776). We report an additional 2-y follow-up for women enrolled from the Brazilian centers from the initial study (n = 436).
During the current follow-up study (HPV-023, NCT00518336), no new infection or lesions associated with HPV-16/18 occurred in the vaccine group. Vaccine efficacy over the entire follow-up (up to 8.4 y) was 95.1% (84.6, 99.0) for incident infection, 100% (79.8, 100) for 6-mo persistent infection, 100% (56.1, 100) for 12-mo persistent infection and 100% (< 0, 100) for CIN2+ associated with HPV-16/18. All women in the vaccine group remained seropositive to both HPV-16/18, with antibody titers for total and neutralizing antibodies remaining several-folds above natural infection levels. The safety profile was clinically acceptable for both vaccine and control groups. This is, to date, the longest follow-up study for a licensed cervical cancer vaccine.
Introduction
Persistent infection with an oncogenic human papillomavirus (HPV) type is a necessary step in the pathogenesis of cervical neoplasia.Citation1 Among all 40 HPV types that are known to infect human mucosa, more than 14 types are oncogenic (HPV-16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68/73). At least 70% of cervical cancer cases are caused by oncogenic types HPV-16 and HPV-18.Citation2,Citation3,Citation4 In addition, it is now recognized that the spectrum of HPV-associated cancers extends beyond cervical cancer, with HPV being the major etiological agent in squamous cell carcinoma of the anus and a significant contributor to a major proportion of squamous cell carcinoma of the vulva, vagina, penis, mouth and oro-pharynx.Citation5 A vaccine that could provide long-term protection against infection and disease caused by oncogenic HPV types would be of great value.
At this point in time, HPV vaccination is understood to primarily provide protection against HPV infection through the generation of high levels of HPV-specific neutralizing antibodies.Citation6-Citation8 It is hypothesized that neutralizing antibodies bind to HPV’s outer shell and prevent infection of host cells.Citation9-Citation11
Prophylactic HPV vaccines are now available and vaccination programs are being widely implemented, with young adolescent girls being the primary target group for most programs. However, the risk of HPV infection persists throughout a woman’s sexual life. Therefore the duration of protection provided by HPV vaccination is critical to the overall vaccine effectiveness.
The HPV-16/18 AS04-adjuvanted vaccine (HPV-16/18 vaccine, Cervarix®*; GlaxoSmithKline (GSK) Biologicals) (hereafter referred to as the ‘HPV-16/18 vaccine’) has been designed to provide long-term protection against HPV-16 and HPV-18 infections through the generation of a sustained immune response, including high and sustained levels of neutralizing antibodies.Citation12,Citation13
Here we report an interim analysis of a long-term follow-up study with efficacy and immunogenicity data up to a maximum of 8.4 y after initial vaccination.
Results
The primary vaccination study HPV-001 (NCT00689741), enrolled a total of 1,113 women from North America (n = 607) and Brazil (n = 506).Citation14 Of the initial cohort, 776 women (including 448 women from the Brazilian centers) continued in the follow-up study HPV-007 (NCT00120848) up to 6.4 y after the first vaccination.Citation15 Women from the Brazilian centers were invited to participate in the current study HPV-023 (NCT00518336). A total of 436 women agreed to continue,Citation13 and most (n = 433; 99.3%) completed the current study to the time of the present interim analysis which represents up to 8.4 y post initial vaccination (). The retention of subjects in the Brazilian cohort was particularly good, with 448 (88.5%) in the follow-up study and 436 (85.2%) in the current study. The dropouts were mainly subjects lost to follow-up (e.g., due to address changes).
Figure 1. Study design. HPV-001: NCT00689741; HPV-007: NCT00120848; HPV-023: NCT00518336; V = HPV-16/18 vaccine (HPV-16/18 AS04-adjuvanted vaccine); ATP = According-to-protocol; M0 = Month 0 = time of randomization; M1 = Month 1; M6 = Month 6. N = Number of subjects.
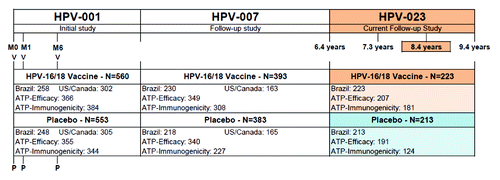
The study population of HPV-023 only included women from the Brazilian centers. However it can be considered as generally representative of the original HPV-001 study population in terms of baseline demographic characteristics. Mean age at entry to the current study was 26.5 y and the population was racially diverse.
The mean follow-up time from the start of the initial vaccination in study HPV-001 up to the current interim analysis time point was 7.9 y (2,902.6 d; standard deviation: 102.5 d), with a maximum duration of 8.4 y (3,074 d). A total of 398 women were included in the according-to-protocol (ATP) efficacy cohort and 305 in the ATP immunogenicity cohort (). Demographic characteristics of these cohorts were similar to the total vaccinated cohort (TVC) (data not shown).
Efficacy against incident and persistent infection
No cases of incident HPV-16 and/or HPV-18 (HPV-16/18) infection were accrued in the vaccine group, whereas five cases occurred in the placebo group during the two years of the current study. In the combined (pooled) analysis of the preceding studies and the current study covering a follow-up period of up to 8.4 y since first vaccination, the vaccine efficacy against incident HPV-16/18 infection remained high ().
Table 1. Vaccine efficacy against HPV-16/18 associated endpoints up to 8.4 y after first vaccination
No cases of persistent infection with HPV-16/18 were detected in either the vaccine or placebo groups during the two years of the current study (). In the combined analysis, vaccine efficacy against 6-mo and 12-mo persistent infection with HPV-16/18 remained 100% ().
Few incident infections with HPV-31 and HPV-45 were detected during the 2 y follow-up period. There was 1 new case of HPV-31 in the vaccine group (n = 195) compared with 5 new cases of HPV-31 in the placebo group (n = 168). For the same time period, there were 3 new cases of HPV-45 in the vaccine group (n = 201) compared with 5 new cases of HPV-45 in the placebo group (n = 174).
When analyzed for up to 8.4 y post initial vaccination, vaccine efficacy against HPV-31 and HPV-45 incident infection (combined analysis) with 95% confidence intervals (CI) was 54.1% (-3.9, 80.9) and 70.7% (22.9, 90.5), respectively. Considering “n” the number of subjects reporting at least one event of incident infection and “N” the number of subjects included in each group, the incidence rate per year was 0.9% for HPV-31 (n/N: 10/226 subjects) and 0.5% for HPV-45 (6/226 subjects) in the vaccine group. Similarly, the incidence rate per year was 1.9% for HPV-31 (19/201 subjects) and 1.7% for HPV-45 (18/205 subjects) in the control group.
Efficacy against cytohistological abnormalities
There was one case of low-grade squamous intraepithelial lesion or greater (≥ LSIL) associated with HPV-18 infection in the placebo group during the two years of the current study. There were no cases of any atypical squamous cells of undetermined significance or greater (≥ ASC-US) associated with HPV-16 or HPV-18 infection in the vaccine group for the same time period (). In the combined analysis, the observed vaccine efficacy against ≥ ASC-US associated with HPV-16 or HPV-18, up to 8.4 y post initial vaccination, remained high (). In the combined analysis, the vaccine efficacy against ≥ ASC-US and ≥ LSIL associated with oncogenic HPV types was 34.3% (5.3, 54.7) and 49.7% (20.8, 68.6), respectively.
No cases of histologically confirmed cervical intraepithelial neoplasia grade 1 or greater (CIN1+) or CIN grade 2 or greater (CIN2+) associated with either HPV-16 or HPV-18 occurred in study HPV-023 in either group (). In the combined analysis, the vaccine efficacy against CIN1+ and CIN2+ associated with oncogenic HPV types was 68.5% (17.2, 89.8) and 40.8% (-105.3, 84.8), respectively.
Immunogenicity
All women in the vaccine group were seropositive for anti-HPV-16 and anti-HPV-18 IgG antibodies, as measured by enzyme-linked immunosorbent assay (ELISA), at the time of the current analysis. In the placebo group, over the same time period of approximately 8 y, 17.9% and 16.1% of subjects became seropositive for antibodies against HPV-16 and HPV-18, respectively, due to natural infection.
High and sustained levels of anti-HPV-16 and anti-HPV-18 IgG antibodies were maintained in the vaccine group, having reached a plateau approximately 18 mo after the initial vaccination (). Up to 8.4 y after the initial vaccination, anti-HPV-16 and anti-HPV-18 IgG antibodies were at least 10-fold higher than antibody levels achieved following clearance of a natural infection as evaluated in a large efficacy study (HPV-008, NCT00122681) conducted in women 15–25 y of age ().Citation16
Figure 2. Immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine up to 8.4 y after first vaccination: seropositivity rates and geometric mean titers for a) anti-HPV-16 and b) anti-HPV-18 antibodies measured by ELISA (ATP cohort). Figures above the bars are the seropositivity rates for the corresponding timepoint; *Horizontal line represents the IgG antibody level in women from a phase III efficacy study (HPV-008) who had cleared a natural infection before enrolment. IgG GMTs corresponding to natural infection in study HPV-008 were 29.8 EL.U/mL (95% CI: [28.5; 31.0]) for HPV-16 and 22.6 EL.U/mL (95% CI: [21.6; 23.6]) for HPV-18; measured by ELISACitation16; Data are shown for the women enrolled in the Brazilian centers for the initial, first follow-up, and current studies; PRE: prevaccination.
![Figure 2. Immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine up to 8.4 y after first vaccination: seropositivity rates and geometric mean titers for a) anti-HPV-16 and b) anti-HPV-18 antibodies measured by ELISA (ATP cohort). Figures above the bars are the seropositivity rates for the corresponding timepoint; *Horizontal line represents the IgG antibody level in women from a phase III efficacy study (HPV-008) who had cleared a natural infection before enrolment. IgG GMTs corresponding to natural infection in study HPV-008 were 29.8 EL.U/mL (95% CI: [28.5; 31.0]) for HPV-16 and 22.6 EL.U/mL (95% CI: [21.6; 23.6]) for HPV-18; measured by ELISACitation16; Data are shown for the women enrolled in the Brazilian centers for the initial, first follow-up, and current studies; PRE: prevaccination.](/cms/asset/8981fde8-6ad7-4eed-963d-303884b5af17/khvi_a_10918865_f0002.gif)
All women in the vaccine group (included in the subset for pseudovirion-based neutralization assay (PBNA) testing) were also neutralizing antibody seropositive to both vaccine antigens at the time of the current analysis. In the placebo group, none of the subjects were seropositive for neutralizing antibodies against HPV-16 and HPV-18 in the current study.
The antibody kinetics of the neutralizing antibodies displayed a similar time course since initial vaccination to antibodies measured by ELISA for both HPV-16 and HPV-18 (). In the vaccine group, neutralizing antibody levels showed a plateau that began approximately 18 mo post initial vaccination and remained sustained for up to 8.4 y. Neutralizing antibodies for HPV-16 and HPV-18 were at least 4-fold-higher in the vaccine group compared with those measured after clearance of natural infection as obtained from study HPV-010 (NCT00423046) performed in women 18–45 y of age ().Citation17
Figure 3. Immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine up to 8.4 y after first vaccination: seropositivity rates and geometric mean titers for a) anti-HPV-16 and b) anti-HPV-18 antibodies measured by PBNA (ATP cohort). Figures above the bars are the seropositivity rates for the corresponding timepoint; *Horizontal line represents the IgG antibody level in women from a phase III efficacy study (HPV-010) who had cleared a natural infection before enrolment. IgG GMTs corresponding to natural infection in study HPV-010 were 180.1 ED50 (95% CI: [153.3; 211.4]) for HPV-16 and 137.3 ED50 (95% CI: [112.2; 168.0]) for HPV-18; measured by PBNA).Citation17 Data are shown for the women enrolled in the Brazilian centers for the initial, first follow-up, and current studies; PRE: prevaccination.
![Figure 3. Immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine up to 8.4 y after first vaccination: seropositivity rates and geometric mean titers for a) anti-HPV-16 and b) anti-HPV-18 antibodies measured by PBNA (ATP cohort). Figures above the bars are the seropositivity rates for the corresponding timepoint; *Horizontal line represents the IgG antibody level in women from a phase III efficacy study (HPV-010) who had cleared a natural infection before enrolment. IgG GMTs corresponding to natural infection in study HPV-010 were 180.1 ED50 (95% CI: [153.3; 211.4]) for HPV-16 and 137.3 ED50 (95% CI: [112.2; 168.0]) for HPV-18; measured by PBNA).Citation17 Data are shown for the women enrolled in the Brazilian centers for the initial, first follow-up, and current studies; PRE: prevaccination.](/cms/asset/3cb00712-a38c-4ff4-bbb7-666cf3807088/khvi_a_10918865_f0003.gif)
Safety
The safety analysis was performed on the TVC on data collected from the end of study HPV-007 up to Month 24 of study HPV-023 for this interim analysis.
The safety profile was clinically acceptable for both vaccine and control groups (). Ten (4.5%) women in the vaccine group and seven (3.3%) in the placebo group experienced a serious adverse event (SAE). None were considered related to the study vaccine or placebo.
Table 2. Safety of the HPV-16/18 AS04-adjuvanted vaccine during the two years of follow-up in HPV-023 (total vaccinated cohort)
The percentage of subjects reporting medically significant adverse events (AEs) (i.e., AEs prompting emergency room or physician visits that are not related to common diseases or SAEs) during the two year follow-up in this current study was 17.9% in the vaccine group (48 events) and 11.3% in the placebo group (31 events) (). No clinical pattern of medically significant AEs was observed. Each of the individual preferred terms occurred mostly once.
The percentage of women experiencing a new onset chronic disease (NOCD) up to Month 24 in study HPV-023 was 2.2% in the vaccine group (five subjects) and 0.9% in the placebo group (two subjects) (). Among these, two women experienced symptoms classified as a potential New Onset Autoimmune Disease (hypothyroidism and vitiligo). These two cases remain blinded with respect to treatment allocation as the study is still ongoing ().
A total of 72 pregnancies were reported by 68 subjects (i.e., 4 subjects have reported two pregnancy outcomes), with 53 normal infants born (26 in the vaccine group and 27 in the placebo group) during the 2-y follow-up in the current study. Ten pregnancies were ongoing at the time of this analysis. There were five spontaneous abortions (3 in the vaccine group and 2 in the placebo group) including one missed abortion. Four abnormal pregnancy outcomes (ectopic pregnancy, elective termination with no apparent congenital anomaly, molar pregnancy and still birth with no apparent congenital anomaly) were also reported. At this time of analysis, the study blind is still in place, it is not possible to state whether these occurred in women in the vaccine or placebo group.
Discussion
Despite low but continuing exposure to HPV infection in the current study, no breakthrough cases of HPV-16/18 infection or cytohistological endpoints associated with HPV-16 or HPV-18 occurred in subjects who had received the HPV-16/18 vaccine in the primary study. Five cases of incident infection with HPV-16/18 and one case of cytological abnormality (≥ LSIL) associated with HPV-18 were observed during the 2-y follow-up period, all in the placebo group.
In the combined analysis covering the whole 8.4 y follow-up, vaccine efficacy against either incident or persistent infection with HPV-16/18 was high. As persistent oncogenic HPV infection has been shown to correlate with lesions, efficacy against more severe lesions can be expected.Citation18
Corresponding vaccine efficacy against cytohistopathology associated with HPV-16 or HPV-18 was high, with no cases in the vaccine group. All cases occurred in the placebo group (7 CIN1+ cases and 3 CIN2+ cases).
It should be noted that women with an endpoint (virological, cytological or histopathological) associated with an HPV type in the preceding studies (HPV-001/007) were censored from analyses related to the same endpoint in the current study (HPV-023). As a result of the high vaccine efficacy, this occurred more often in the placebo group than in the vaccine group. Therefore, for all endpoints, after natural attrition, the number of subjects in study HPV-023 was higher in the vaccine group than in the placebo group. Due to this imbalanced number of subjects at risk (vaccine group > placebo group) and other limitations such as the limited sample size, the limited length of follow-up period at this interim analysis (2 y) and the lower prevalence of non-vaccine HPV types, the focus for the efficacy endpoints, especially those associated with all oncogenic HPV types, is on the combined analysis of HPV-001/007/023 data. The combined analyses of the preceding studies and the current study presented here were not affected by censoring, and therefore provide a global overview of the HPV-16/18 vaccine effect up to 8.4 y post initial vaccination.
Vaccine efficacy against HPV-31 and HPV-45 incident infection seemed to be sustained through to the 8.4 y time point, post initial vaccination, as calculated for the combined analysis. Again, due to the small sample size and the limited follow-up period, only a few new events (incident/persistent infections, cytological abnormalities or histopathological lesions) associated with individual oncogenic HPV types were observed at the time of this analysis. In doing so, power is limited when it comes to the calculation of efficacy estimates against both HPV-31 and HPV-45. It is therefore not possible to fully conclude on long-term protection against non-vaccine types HPV-31 and HPV-45, which are less prevalent than HPV-16 or HPV-18 in this interim analysis. The large phase III program with this vaccine is ongoing and includes evaluation of vaccine efficacy against non-vaccine types.
As discussed by De Carvalho et al., the vaccine efficacy endpoints were kept the same in study HPV-007 and the current study as in study HPV-001 to allow for both combined analyses of data from all three studies and separate analyses of data from each individual study.Citation13 Study procedures and laboratory methodology were similar in all three studies to maintain consistency.
High, sustained efficacy for up to 8.4 y against HPV-16/18 infections and cytohistological endpoints was associated with high and persistent levels of IgG and neutralizing antibodies against HPV-16 and HPV-18. All women in the vaccine group were seropositive to HPV-16 and HPV-18 as measured by both ELISA and PBNA at the time of this analysis. Furthermore, both IgG and neutralizing antibody levels were several folds higher than those following after natural infection in other studies.Citation16,Citation17
It is currently understood that neutralizing antibodies are likely to play a major role in vaccine-derived protection against HPV infection since passive immunization is protective in animal models.Citation6,Citation7,Citation19 HPV L1 VLP vaccines are highly immunogenic, with serum neutralizing antibodies persisting for at least eight years, as demonstrated in this study. Earlier studies among vaccinated individuals have shown a strong correlation between the total IgG measured by ELISA in this study and the PBNA, which measures neutralizing antibodies.Citation20
Statistical modeling predicts slow decay of vaccine induced antibodies for at least 20 y with the assumption that if long-term persistence of antibodies has a similar relevance for protection to that observed with some other vaccines, then a booster may not be needed until considerable time has elapsed post vaccination.Citation21
Although no correlate of protection has yet been defined for either HPV-16 or HPV-18, the persistence of high levels of antibodies to both vaccine antigens in this study appears to be directly related with the persistence of high levels of vaccine efficacy. The antibody kinetics summarized in this study correlate with sustained antibody production, and is likely to indicate both the generation of long-lived plasma cells and the induction of memory B-cells that replenish the plasma cell pool.Citation12 The AS04-adjuvant system in the GSK HPV-16/18 vaccine formulation is likely to be a key factor in its sustained immunogenicity.Citation22
Experience with other vaccines has suggested that the scale of the humoral response, together with memory B-cell and T-cell induction are both important for long-term protection.Citation23,Citation24
In conclusion, high vaccine efficacy against HPV-16/18 infection and cytohistological endpoints associated with HPV-16/18 was observed in a combined analysis of the preceding and current studies. No breakthrough cases of infection with HPV-16/18 and no cases of cytohistological endpoints associated with HPV-16 or HPV-18 were observed during the first two years of the current study in the vaccinated group. The HPV-16/18 vaccine provided high and sustained levels of IgG and neutralizing antibodies against HPV-16 and HPV-18 with all women being seropositive 8.4 y post initial vaccination. Furthermore, antibody titers for total IgG and neutralizing antibodies remained several-folds above natural infection levels. The HPV-16/18 vaccine continues to demonstrate a clinically acceptable safety profile. This is the longest study to date of a licensed HPV vaccine containing the two most frequently observed oncogenic HPV types, HPV-16 and HPV-18. The study will continue for a further year.
Materials and Methods
Study objectives
The primary objective was to assess long-term vaccine efficacy in the prevention of incident cervical infection with HPV-16/18 in adolescents and young women. Secondary objectives were to evaluate vaccine efficacy against incident infection with each or any oncogenic HPV type, persistent infection with HPV-16/18 or with each or any oncogenic HPV type, and to evaluate vaccine efficacy against cytological and histopathological abnormalities associated with HPV-16 or HPV-18 or with each or any oncogenic HPV type. Other objectives included the long-term vaccine immunogenicity and safety.Citation13
Study design and participants
The method of the initial study and follow-up studies has been described previously.Citation13-Citation15 In brief, women were originally enrolled into an initial double-blind, randomized, multicenter study (HPV-001).Citation14 Women were recruited from North America (US, Canada) and Brazil. From this initial study, women who received all three doses of vaccine or placebo and whose treatment allocation remained blinded were invited to take part in a follow-up study (HPV-007) whose final results were published after 6.4 y of follow-up.Citation15 Of those, women participating at Brazilian study centers were invited to continue into the current study (HPV-023). Total study duration will be approximately nine years from administration of the first vaccine/placebo dose in study HPV-001, at which time a final analysis will be performed. Here, we report data from an interim analysis, up to 8.4 y post initial vaccination ().
Healthy young women (aged 15–25 y) with normal cervical cytology at screening, who were HPV-16 and HPV-18 seronegative by ELISA and DNA-negative for 14 oncogenic HPV types (16/18/31/33/35/39/45/51/52/56/58/59/66/68) by SPF10-DEIA/LiPA25-PCR system (SPF10-LIPA25) (version 1, Laboratory Biomedical Products, Rijswijk, Netherlands), were enrolled into study HPV-001.Citation14
In study HPV-001, women were randomized 1:1 to either GSK Biologicals’ HPV-16/18 AS04-adjuvanted vaccine or placebo given at a 0, 1, 6 mo schedule. The vaccine and placebo have been described previously.Citation14 Treatment allocation has remained blinded throughout the initial, follow-up and current studies as described previously.Citation13 Similar to studies HPV-001 and HPV-007, and according to the study protocol of each study site, women in study HPV-023 continued to receive gynecological care according to Brazilian standards. The Brazilian standard of care at the time of the study involved an annual pap smear, and if any result was abnormal was followed by colposcopy and treatment.
Subjects that received the placebo in study HPV-001 and who have remained blinded throughout the studies are to be offered a crossover vaccination course (0, 1 and 6 mo) with the HPV-16/18 vaccine at the completion of the current study.
The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines.
Procedures and endpoints
Procedures and endpoints concerning virology and cytohistopathology, immunogenicity and safety are described by De Carvalho et al.Citation13
Statistics
Two interim analyses after one and two years of follow-up in the current study were specified by the study protocol and are identical in method. These statistical methods, including the adjustment of the α value, have been described previously.Citation13 The efficacy analyses presented here include data from the first two years of the current study.
The immunogenicity data are shown from the initial, follow-up and current studies for the Brazilian cohort only and are presented to illustrate the kinetics of the immune response to the vaccine antigens, as measured by both ELISA and PBNA. Safety data are presented for the first two years of the current study.Citation13
Vaccine efficacy was calculated against the following clinical endpoints associated with oncogenic HPV types: incident infection, 6-mo and 12-mo persistent infection and cytohistogical abnormalities (≥ ASC-US, ≥ LSIL, CIN1+ and CIN2+). The conditional exact method was used to estimate vaccine efficacy and exact 95% CIs around the rate ratio (ratio of the event rates in the vaccinated vs. placebo group). The calculation took into account the follow-up time of the subjects within each group. Vaccine efficacy was defined as 1 minus the rate ratio.
In addition, a descriptive combined (pooled) analysis between efficacy data from the Brazilian cohort from study HPV-001, from the Brazilian cohort of study HPV-007 and data from the current study was performed (for each virological endpoint, vaccine efficacy estimate was calculated and 95% CI provided), with a total follow-up period of up to 8.4 y after first vaccination.
Primary analyses of efficacy were performed on the ATP cohort for efficacy for virological endpoints (incident and persistent infection), and on the TVC for efficacy of cytohistological endpoints (≥ ASC-US, ≥ LSIL, CIN1+ and CIN2+). Definitions of ATP and TVC are provided as a footnote in . Primary analyses of immunogenicity were performed on the ATP cohort for immunogenicity, while the primary safety analyses were performed on the TVC. The safety results are presented in such a way it takes the blind at the subject level into account.
| Abbreviations: | ||
| AE | = | adverse event |
| ASC-US | = | atypical squamous cells of undetermined significance |
| ATP | = | according-to-protocol |
| CI | = | confidence interval |
| CIN1+ | = | cervical intraepithelial neoplasia grade 1 or greater |
| CIN2+ | = | cervical intraepithelial neoplasia grade 2 or greater |
| DNA | = | deoxyribonucleic acid |
| ELISA | = | enzyme-linked immunosorbent assay |
| HPV | = | human papillomavirus |
| HPV-001 | = | the primary vaccination study (NCT00689741) |
| HPV-007 | = | the follow-up study (NCT00120848) |
| HPV-023 | = | the current study (NCT00518336) |
| HPV-16/18 vaccine | = | GSK Biologicals’ HPV-16/18 AS04-adjuvanted vaccine, Cervarix® |
| GSK | = | GlaxoSmithKline |
| LiPA | = | line probe assay |
| LSIL | = | low-grade squamous intraepithelial lesion |
| NOCD | = | new onset chronic disease |
| PBNA | = | pseudovirion-based neutralization assay |
| PCR | = | polymerase chain reaction |
| SAE | = | serious adverse event |
| TVC | = | total vaccinated cohort |
| US | = | United States |
Acknowledgments
We would like to thank the study participants and the staff members of the study sites of this study. Laboratory work was provided by W. Quint, L.-J. Van Doorn and A. Molijn (DDL Diagnostic Laboratory, Voorburg, The Netherlands); R. Luff, M. McNeeley, E. Alt, B. Iskaros, A. Limaye, X. Jarin, C. Provenzano and B. Winkler (Quest Diagnostics, Teterboro, NJ, USA); A. Meurée, R. Crudenaire and S. Poncelet (GlaxoSmithKline Biologicals, Rixensart, Belgium). From GlaxoSmithKline Biologicals, we also thank A. Vanneuville and M. Lojo Suarez for global study management and Stéphanie Genevrois for scientific writing of the clinical study report. Finally, we would like to thank Catherine Streeton (Streeton Associates) who provided medical writing services on behalf of GlaxoSmithKline Biologicals, and Jean-Michel Heine (Keyrus Biopharma) and Denis Sohy (Business and Decision) for editorial assistance and manuscript coordination.
Disclosure of Potential Conflicts of Interest
C. Roteli-Martins, P. Naud, P. De Borba, J. Teixeira and N. De Carvalho received funding and/or equipment/administrative support through their institutions from GlaxoSmithKline Biologicals. C. Roteli-Martins, P. Naud, P. De Borba, J. Teixeira and N. De Carvalho received support for travel to meetings from GlaxoSmithKline Biologicals. C. M Roteli-Martins, P. De Borba, J. Teixeira and N. De Carvalho received fees for participation in advisory boards and/or lectures from GlaxoSmithKline Biologicals. P. Naud received consulting fee/honorarium from GlaxoSmithKline Biologicals. N. Sanchez, T. Zahaf, B. Geeraerts and D. Descamps are employees of GlaxoSmithKline Biologicals, N. Sanchez and D. Descamps hold stock options from GlaxoSmithKline Biologicals.
Note
*Cervarix® is a registered trademark of the GlaxoSmithKline group of companies.
References
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12 - 9; http://dx.doi.org/10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F; PMID: 10451482
- Muñoz N, Bosch FX, Castellsagué X, Díaz M, de Sanjose S, Hammouda D, et al. Against which human papillomavirus types shall we vaccinate and screen? The international perspective. Int J Cancer 2004; 111:278 - 85; http://dx.doi.org/10.1002/ijc.20244; PMID: 15197783
- de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, et al, Retrospective International Survey and HPV Time Trends Study Group. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 2010; 11:1048 - 56; http://dx.doi.org/10.1016/S1470-2045(10)70230-8; PMID: 20952254
- Bosch FX, Burchell AN, Schiffman M, Giuliano AR, de Sanjose S, Bruni L, et al. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine 2008; 26:Suppl 10 K1 - 16; http://dx.doi.org/10.1016/j.vaccine.2008.05.064; PMID: 18847553
- Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine 2006; 24: S3/11–S3/25; PMID: 16949997; DOI: 10.1016/j.vaccine.2006.05.111.
- Christensen ND, Reed CA, Cladel NM, Han R, Kreider JW. Immunization with viruslike particles induces long-term protection of rabbits against challenge with cottontail rabbit papillomavirus. J Virol 1996; 70:960 - 5; PMID: 8551636
- World Health Organization (WHO). Human papillomavirus and HPV vaccines: technical information for policy-makers and health professionals. 2007. Available at: http://www.who.int/reproductivehealth/publications/cancers/IVB_07.05/en/index.html. Accessed 22 September.
- Einstein MH, Schiller JT, Viscidi RP, Strickler HD, Coursaget P, Tan T, et al. Clinician’s guide to human papillomavirus immunology: knowns and unknowns. Lancet Infect Dis 2009; 9:347 - 56; http://dx.doi.org/10.1016/S1473-3099(09)70108-2; PMID: 19467474
- Stanley M, Lowy DR, Frazer I. Chapter 12: Prophylactic HPV vaccines: underlying mechanisms. Vaccine 2006; 24:Suppl 3 S3 - , 106-13; http://dx.doi.org/10.1016/j.vaccine.2006.05.110; PMID: 16949996
- Day PM, Gambhira R, Roden RB, Lowy DR, Schiller JT. Mechanisms of human papillomavirus type 16 neutralization by l2 cross-neutralizing and l1 type-specific antibodies. J Virol 2008; 82:4638 - 46; http://dx.doi.org/10.1128/JVI.00143-08; PMID: 18305047
- Stanley M. HPV - immune response to infection and vaccination. Infect Agent Cancer 2010; 5:19; http://dx.doi.org/10.1186/1750-9378-5-19; PMID: 20961432
- Schwarz TF, Leo O. Immune response to human papillomavirus after prophylactic vaccination with AS04-adjuvanted HPV-16/18 vaccine: improving upon nature. Gynecol Oncol 2008; 110:Suppl 1 S1 - 10; http://dx.doi.org/10.1016/j.ygyno.2008.05.036; PMID: 18653222
- De Carvalho N, Teixeira J, Roteli-Martins CM, Naud P, De Borba P, Zahaf T, et al. Sustained efficacy and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine up to 7.3 years in young adult women. Vaccine 2010; 28:6247 - 55; http://dx.doi.org/10.1016/j.vaccine.2010.07.007; PMID: 20643092
- Harper DM, Franco EL, Wheeler CM, Ferris DG, Jenkins D, Schuind A, et al, GlaxoSmithKline HPV Vaccine Study Group. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet 2004; 364:1757 - 65; http://dx.doi.org/10.1016/S0140-6736(04)17398-4; PMID: 15541448
- GlaxoSmithKline Vaccine HPV-007 Study Group. Sustained efficacy and immunogenicity of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6.4 years. Lancet 2009; 374:1975 - 85; http://dx.doi.org/10.1016/S0140-6736(09)61567-1; PMID: 19962185
- Paavonen J, Jenkins D, Bosch FX, Naud P, Salmerón J, Wheeler CM, et al, HPV PATRICIA study group. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet 2007; 369:2161 - 70; http://dx.doi.org/10.1016/S0140-6736(07)60946-5; PMID: 17602732
- Einstein MH, Baron M, Levin MJ, Chatterjee A, Edwards RP, Zepp F, et al, HPV-010 Study Group. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18-45 years. Hum Vaccin 2009; 5:705 - 19; http://dx.doi.org/10.4161/hv.5.10.9518; PMID: 19684472
- Paavonen J, Naud P, Salmerón J, Wheeler CM, Chow S-N, Apter D, et al, HPV PATRICIA Study Group. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 2009; 374:301 - 14; http://dx.doi.org/10.1016/S0140-6736(09)61248-4; PMID: 19586656
- Suzich JA, Ghim SJ, Palmer-Hill FJ, White WI, Tamura JK, Bell JA, et al. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc Natl Acad Sci U S A 1995; 92:11553 - 7; http://dx.doi.org/10.1073/pnas.92.25.11553; PMID: 8524802
- Dessy FJ, Giannini SL, Bougelet CA, Kemp TJ, David M-P, Poncelet SM, et al. Correlation between direct ELISA, single epitope-based inhibition ELISA and pseudovirion-based neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the AS04-adjuvanted HPV-16/18 cervical cancer vaccine. Hum Vaccin 2008; 4:425 - 34; http://dx.doi.org/10.4161/hv.4.6.6912; PMID: 18948732
- David MP, Van Herck K, Hardt K, Tibaldi F, Dubin G, Descamps D, et al. Long-term persistence of anti-HPV-16 and -18 antibodies induced by vaccination with the AS04-adjuvanted cervical cancer vaccine: modeling of sustained antibody responses. Gynecol Oncol 2009; 115:Suppl S1 - 6; http://dx.doi.org/10.1016/j.ygyno.2009.01.011; PMID: 19217149
- Garcon N, Leo O. Innate immunity and vaccine adjuvants: from concept to the development of a unique adjuvant system AS04 used for the formulation of a human papillomavirus (HPV) vaccine. Current Cancer Therapy Reviews 2010; 6:126 - 37; http://dx.doi.org/10.2174/157339410791202574
- Banatvala J, Van Damme P, Oehen S. Lifelong protection against hepatitis B: the role of vaccine immunogenicity in immune memory. Vaccine 2000; 19:877 - 85; http://dx.doi.org/10.1016/S0264-410X(00)00224-3; PMID: 11115711
- Stanley M. HPV vaccines: are they the answer?. Br Med Bull 2008; 88:59 - 74; http://dx.doi.org/10.1093/bmb/ldn037; PMID: 18940889