Abstract
Group A rotaviruses (RVA) are a major cause of acute gastroenteritis in children ≤ 5 y worldwide which could be prevented with two recently introduced vaccines – monovalent Rotarix (live-attenuated G1P[8] strain) and pentavalent RotaTeq (human-bovine reassortant containing serotypes G1, G2, G3, G4 and P[8]). Prior to implementation of vaccines into national immunization program we aimed to describe RVA genotype distribution in hospitalized children aged < 5 y in Estonia during 2007–2008. A total of 671 children with confirmed RVA gastroenteritis from three major pediatric hospitals were prospectively enrolled. G- and P-genotypes were detected from 124 stool samples by semi-nested reverse transcription-PCR. Severity of disease was assessed using Clark scoring system. The majority of cases (65%) occurred in infants aged 7 to 24 mo and were of moderate severity (mean Clark score 12.1 (SD 3.2)). The prevailing strain was G2P[4] (34.7%), causing significantly more cases than G4P[8] (12.9%), G1P[8] or G9P[8] (both 4.0%), G3P[8] (1.6%). Yearly differences in genotype distribution occurred, as G2P[4] (52.8%) dominated in 2007, but G4P[8] (26.9%) in 2008. One third of strains remained non-typeable. The distribution of RVA genotypes in Estonia differs from that seen in other Central and Eastern European countries, although one should bear in mind the large proportion of P-untypeable strains and natural fluctuations of dominating RVA genotypes. Nevertheless, considering the high genotype-independent efficacy of the vaccines, introduction of national immunization should be considered.
Introduction
Group A rotaviruses (RVA) are one of the leading causes of acute gastroenteritis in children ≤ 5 y around the world – more than 100 million infections, 2 million hospitalizationsCitation1 and 453,000 deaths occurred every year before the introduction of vaccines.Citation2 In European Union, 2.8 million RVA gastroenteritis (RVGE) episodes requiring only home care, 87,000 hospitalizations and 231 deaths occurred annually in children < 5 y in the prevaccine era.Citation3 Two vaccines against RVA have been licensed and introduced into immunization programs in a number of, but not in all countries due to the negative cost-benefit ratioCitation4-Citation6 – a monovalent live attenuated G1P[8] vaccine (Rotarix®, GlaxoSmithKline Biologicals) and a pentavalent human-bovine (WC3) reassortant vaccine (Rotateq®, Sanofi Pasteur MSD) containing serotypes G1, G2, G3, G4 and P[8].
There is a significant seasonal and geographical variation in the distribution of circulating RVA.Citation7 However, which role, if any, in this process plays the selective pressure of newly introduced RVA vaccines is less clear. G1P[8] still remains the most dominant, accounting for 61.6% of strains in the Australian states utilizing RotaTeq.Citation8 However, G2 genotypes have emerged as the prevailing ones in countries where vaccination with Rotarix has been performed, e.g., Belgium, Brazil and also some states in Australia.Citation8-Citation10 Therefore establishment of current situation, representing the pre-vaccination era, is necessary in Estonia to be able to spot probable vaccine-mediated changes afterwards and take actions as soon as possible.
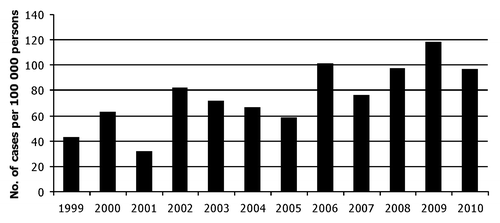
In Estonia, RVGE is a growing problem (). During recent years the incidence of reported RVGE cases has been 75–118 per 100,000 persons with hospitalization rate of 75–91% (www.terviseamet.ee/en.html). However, as for other Eastern European countries,Citation11 data on the burden of RVGE in terms of mortality, morbidity and genotypic distribution are limited. A previous survey on electropherotypes and serotypes of RVA in Estonia was conducted almost 20 y ago and showed predominance of serotype G1 (71%) followed by G4, G2 and G3 (12%, 10% and 8%, respectively).Citation12
Figure 1. Number of registered group A rotaviral gastroenteritis cases per 100 000 persons per year from 1999 to 2010 according to Estonian Health Board (www.terviseamet.ee/en.html). Note that 75–91% of cases have been hospitalized.
We aimed to describe the clinical characteristics of hospitalized RVGE and genotype distribution in Estonia during two consecutive RVA seasons (2007–2008) in order to define circulating strains prior to introduction of the vaccines into the national immunization program.
Results
Study population
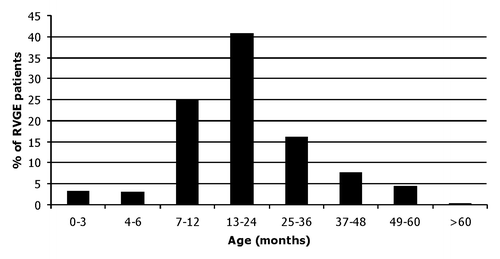
A total of 671 (347 males) patients < 5 y hospitalized with RVGE were evenly distributed between the three participating hospitals and accounted for 5.5% of all admissions below 15 y. Of all cases 14.5% were nosocomial leading to the rate of 42 per 100,000 hospitalizations. The majority of cases (65%) occurred in subjects between 7 to 24 mo () with a median age of 17 mo (IQR 11;27).
Figure 2. Age distribution of the hospitalised group A rotaviral gastroenteritis patients in Estonia in 2007 to 2008. Note that maximum value of Y axis is 45%.
In most hospitalized patients (76.9%) the severity of disease was moderate (); the mean Clark score was 12.1 (SD 3.2). Mean duration of hospitalization was 3.2 d (SD 1.9) for community-acquired RVGE. The nosocomial RVGE prolonged duration of hospitalization by 4.2 (SD 2.2) days. No admissions to intensive care unit, deaths or severe disabilities were recorded. In general, the demographic and clinical characteristics did not differ between 2007 and 2008.
Table 1. Clinical characteristics of children with group A rotaviral gastroenteritis
The monthly distribution of RVGE revealed year-round circulation of RVA strains with the peaks occurring in March (2007) and April (2008).
Group A rotavirus genotypes
The randomly selected 124 stored stool samples represent the whole population in terms of age, gender, geographical distribution, severity of disease, degree of dehydration, treatment and the percent of nosocomial infections. Of these 124 strains only 66.1% could be characterized with both genotypes; the remaining were P-, G- or fully untypeable ().
Table 2. G- and P-genotype combinations by year
“Common” RVA strains (G1P[8], G2P[4], G3P[8], G4P[8], and G9P[8]) accounted for 57.3% of cases (). The most dominant strains were G2P[4] (34.7%) and G4P[8] (12.9%). Yearly difference in prevailing genotype occurred – G2P[4] (52.8%) dominated in 2007, but G4P[8] (26.9%) in 2008.
“Uncommon” human strains, arising from the reassortment of genetic material of “common” human strains, occurred in 3.2% of samples. Other combinations, which likely result from reassortment between animal and human strains, accounted for 1.6%. Mixed infections with multiple RVA strains occurred in 4% of cases.
Similarly to the whole study, the most prevailing strain in the nosocomial infections was G2P[4] (50%), only one mixed infection occurred and 38.8% of isolated RVA were partially untypeable. In contrast to the whole population, the prevalence of G4P[8] among hospital-acquired infections was 5.6%.
Discussion
This study revealed that RVGE is a common vaccine preventable disease requiring hospitalization in Estonia, but severe disease with pronounced dehydration is rare. In the prevaccine era we demonstrated a significant variability in the distribution of G- and P- genotypes over two consecutive seasons. The most prevailing genotype combinations were G2P[4] in 2007 and G4P[8] in 2008. Our results differ from those observed in 2007/08 in other Central or Eastern European countries, where G1P[8] dominated and the level of partially or fully untypeable strains remained low (< 14%).Citation11
The disagreement between the prevalence of “common” strains in western countries (more than 90% of all RVA)Citation7 and in Estonia (57.3%) could be primarily explained by the fairly large proportion of P-untypeable strains (29%), which in fact varies from study to study. For example, while in a study in five European countries all samples were P-genotyped,Citation13 as many as 38.2% remained P-untypeable in an Italian study.Citation14 Failure to type has been attributed to natural variation due to genetic drift leading to the accumulation of point mutations. Consequent mismatches between a specific primer and its complementary region of the cDNA destabilize the primer-template duplex. As a result, primer elongation is prevented or the efficiency of the PCR is significantly reduced.Citation15 Differences in laboratory practices are probably another aspect contributing to failure to type.Citation11,Citation16 Other countries in Central and Eastern Europe have reported the rate of fully or partially non-typeable strains 0.6–13.7%.Citation11 Of these countries, three quarters have urbanization rates lower than Estonia (www.who.int/countries/en), likely resulting in more frequent human contact with animals and thus more likely presence of unusual human-animal reassortants.Citation7 However, uncommon genotypes occurred in these Central and Eastern European countries at rates < 14%.Citation11 Thus, in Estonia, the high prevalence of P-untypeable strains seems to be attributable to mismatches between primers and VP4-genes of RVA and/or the methodological aspects of the study rather than unusual P-genotypes possibly originating from animals.
An important issue that needs to be addressed before deciding which vaccine to select is the effectiveness of Rotarix against serotypically unrelated G2P[4], the dominant strain in Estonia. Usually the high prevalence of G2P[4] strains has been observed after implementation of RVA vaccines into national immunization programs, e.g., in Australia, Belgium and Brazil.Citation8,Citation10,Citation17 Subsequently, selective pressure driven by vaccines has been hypothesized, although several aspects contradict the idea. First, there is no convincing evidence that monovalent RVA vaccine does not induce immunity against fully heterotypic G2P[4].Citation18,Citation19 In addition, protection provided by Rotarix against strains without shared G- and P- genotypes was confirmed in the integrated analysis.Citation20 Second, the high rate of G2P[4] strains has been reported in countries without reimbursed vaccination – e.g., Italy, Greece, Basque Country in Spain, Bulgaria.Citation14,Citation21-Citation23 Third, in Brazil the prevalence of G2P[4] started to finally decrease in 2009, when the national vaccination coverage reached very high levels – 80–90%.Citation9 Fourth, G2P[4] strain shifts have occurred in the setting of tremendous decline in RVGE cases,Citation24-Citation26 therefore not precluding study results from biases. Fifth, if vaccines are believed to exert selective pressure toward prevalence of heterotypic strains, then how to explain the dominance of fully homotypic G1P[8] in the states of Australia utilizing RotaTeq.Citation8 Thus, despite the preponderance of G2P[4] strains in Estonia in 2007–2008 no impediment to implementation of Rotarix is justified. In addition, Rotarix has been found to be more cost-effective than RotaTeq.Citation5 Yet, as it has been stated by several authors, only long-term surveillancesCitation7,Citation8,Citation10,Citation27 and phylogenetic analysis of G2P[4] samples from vaccinated and unvaccinated childrenCitation28 can determine the true effect of vaccination on G2P[4] strain.
When the results of our study and that obtained by others are taken into consideration, question arises whether the introduction of vaccination would be cost-effective in Estonia. In the light of the natural fluctuations of RVA genotypes (also shown by us) and the heterotypic immunity vaccines induce (although it is not conclusively clear yet),Citation19,Citation29,Citation30 predicting the effectiveness of vaccination on the ground of strain distribution in the prevaccine era seems not to be legitimate. The sentiment is illustrated by the fact that many cost-effectiveness analyses conducted in western countries prior to the decision do not take into account the molecular epidemiology of RVGE.Citation4-Citation6 A cost-effectiveness study is currently being performed by the Department of Public Health of the University of Tartu to answer the question about the introduction of the vaccines into the national immunization program of Estonia. As up to now only some European countries (Finland, Greece) have found the vaccination to be economically beneficialCitation5,Citation31 and others (France, Italy, the Netherlands) have stated that the prices of the vaccines should be reduced in order to implement them,Citation4-Citation6 we should put no high expectations on the net results of cost-effectiveness analysis. Nevertheless, emphasizing the tremendous incidence of RVA-associated diarrhea among young childrenCitation1 and the recommendation of vaccination against RVA in European guidelines,Citation32 the implementation of RVA vaccines should still be considered to benefit children’s quality of life.
Some limitations of the study should be noted. The short duration of the study does not allow drawing conclusions about genotype fluctuation of RVA over several seasons. Second, the high proportion of P-untypeable strains may introduce a bias when interpreting genotype distribution. However, distribution of RVA VP4-genotypes typed with newly designed primers has been shown to correlate well with the incidences of previously successfully typed strains,Citation16 suggesting this should not be a major concern. RVA strains untypeable by PCR could be typed by sequencing the first round product,Citation16,Citation17 yet the drawbacks of sequencing technique are the cost and availability. Third, we did not genotype all samples but only a representative collection of them and finally we are aware that some although very few children may have had RVA vaccine from private market. Also, the large proportion of P-untypeable strains could be contributed to degradation of RVA RNA when stored at -20°C for months. All these may have introduced a bias, although highly unlikely a significant one, into the study. In the end, only RVA strains isolated from hospitalized children, reflecting more severe disease, were genotyped, yet vaccines have been shown to be particularly effective against severe forms of RVGE.
In conclusion, the distribution of RVA genotypes in Estonia does not resemble that seen in the other countries in Central and Eastern Europe. However, when interpreting the results one should keep in mind the large proportion of strains that could not be P-genotyped and also the natural fluctuations of dominating RVA strains described in many countries. Nevertheless, considering studies already conducted in other countries, we could expect efficacy, although not cost-effectiveness of national immunization in Estonia to be high.
Materials and methods
Study population and sampling
Between January 1, 2007 to December 31, 2008 all cases of RVGE (defined as ≥ 3 episodes of watery diarrhea during 24 h with or without vomiting with overall duration of ≤ 7 d and detection of the RVA in feces by latex-agglutination (Orion Diagnostica, catalog number 67491) or chromatographic immunoassay (Certest Biotec, catalog number X740001LF)) in children aged less than 5 y and hospitalized into three major pediatric hospitals in Estonia (Children’s Clinic of Tartu University Hospital, Merimetsa Infection Centre, and Tallinn Children’s Hospital) were registered. These hospitals cover about 80% of pediatric hospitalizations in Estonia. RVGE occurring for at least 48h after hospitalization or less than 72h after hospital discharge was considered nosocomial whereas all others as community acquired. Prior to discharge the severity of disease was assessed using the Clark scoring system.Citation33 Mild gastroenteritis was defined as a score of < 9 points, moderate as 9–16 points and severe as > 16 points.Citation34 For genotyping, approximately 5 ml of fresh stool was collected into a sterile container and stored at -80°C (not longer than 4 y) until further analyses.
About 20% of samples were selected for genotypic analysis. There was no formal randomization scheme but in the selection process an equal representation of both years and all months, both towns as well as nosocomial and community-acquired infections were considered.
Extraction of viral particles and RNA
Approximately 1 ml of 10% fecal suspension in 1x phosphate-buffered saline was prepared and centrifuged at 7600 rpm for 30 min. Supernatant was collected and stored at -20°C until viral RNA extraction by QIAamp Viral RNA Mini Kit (QIAGEN, catalog number 52906) according to the manufacturer's instructions. The extracted RNA was frozen at -20°C until genotyping, but not longer than for 6 mo.
VP4 and VP7 genotyping
RVA G- and P-genotypes were detected by semi-nested reverse transcription PCR as described previously.Citation35,Citation36 Briefly, the purified viral RNA was reverse transcribed into cDNA (cDNA) by using consensus primers specific for RVA VP7 or VP4 gene.Citation35,Citation36 Using the same primer pairs, VP7 and VP4 genes were amplified in the first round of semi-nested PCR. The second round was performed as a multiplex-PCR, where in addition to consensus primer, genotype-specific primers were used, detecting genotypes G1, G2, G3, G4, G8, G9 and P[4], P[6], P[8], P[9], P[10], P[11].Citation35-Citation37 Genotypes of individual RVA strains were identified according to the length of second round PCR product visualized by agarose gel electrophoresis.Citation37
The study protocol was approved by the Ethics Committee of the University of Tartu and informed consent was signed by parents or guardian.
| Abbreviations: | ||
| cDNA | = | complementary DNA |
| IQR | = | interquartile range |
| ND | = | not determined |
| RVA | = | group A rotaviruses |
| RVGE | = | group A rotaviral gastroenteritis |
| SD | = | standard deviation |
Acknowledgments
The study was supported by the Target Financing of Estonian Ministry of Education and Research (SF0182726s06), European Union through the European Regional Development Fund and the Archimedes Foundation, an unrestricted grant from MSD, European Social Fund’s Doctoral Studies and Internationalisation Programme DoRa. We thank Doctor Tuuli Metsvaht for her critical review of the manuscript.
Disclosure of Potential Conflicts of Interest
The authors declare no conflict of interest related to this study.
References
- Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis 2003; 9:565 - 72; http://dx.doi.org/10.3201/eid0905.020562; PMID: 12737740
- Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD, the WHO-coordinated Global Rotavirus Surveillance Network. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. [Epub ahead of print] Lancet Infect Dis 2011; 24; PMID: 22030330
- Soriano-Gabarró M, Mrukowicz J, Vesikari T, Verstraeten T. Burden of rotavirus disease in European Union countries. Pediatr Infect Dis J 2006; 25:Suppl S7 - 11; http://dx.doi.org/10.1097/01.inf.0000197622.98559.01; PMID: 16397431
- Giammanco MD, Coniglio MA, Pignato S, Giammanco G. An economic analysis of rotavirus vaccination in Italy. Vaccine 2009; 27:3904 - 11; http://dx.doi.org/10.1016/j.vaccine.2009.04.002; PMID: 19446934
- Jit M, Bilcke J, Mangen MJ, Salo H, Melliez H, Edmunds WJ, et al. The cost-effectiveness of rotavirus vaccination: Comparative analyses for five European countries and transferability in Europe. Vaccine 2009; 27:6121 - 8; http://dx.doi.org/10.1016/j.vaccine.2009.08.030; PMID: 19715781
- Mangen MJ, van Duynhoven YT, Vennema H, van Pelt W, Havelaar AH, de Melker HE. Is it cost-effective to introduce rotavirus vaccination in the Dutch national immunization program?. Vaccine 2010; 28:2624 - 35; http://dx.doi.org/10.1016/j.vaccine.2010.01.014; PMID: 20109593
- Santos N, Hoshino Y. Global distribution of rotavirus serotypes/genotypes and its implication for the development and implementation of an effective rotavirus vaccine. Rev Med Virol 2005; 15:29 - 56; http://dx.doi.org/10.1002/rmv.448; PMID: 15484186
- Kirkwood CD, Boniface K, Barnes GL, Bishop RF. Distribution of rotavirus genotypes after introduction of rotavirus vaccines, Rotarix® and RotaTeq®, into the National Immunization Program of Australia. Pediatr Infect Dis J 2011; 30:Suppl S48 - 53; http://dx.doi.org/10.1097/INF.0b013e3181fefd90; PMID: 21183840
- Carvalho-Costa FA, Volotão EdeM, de Assis RM, Fialho AM, de Andrade JdaS, Rocha LN, et al. Laboratory-based rotavirus surveillance during the introduction of a vaccination program, Brazil, 2005-2009. Pediatr Infect Dis J 2011; 30:Suppl S35 - 41; http://dx.doi.org/10.1097/INF.0b013e3181fefd5f; PMID: 21048523
- Zeller M, Rahman M, Heylen E, De Coster S, De Vos S, Arijs I, et al. Rotavirus incidence and genotype distribution before and after national rotavirus vaccine introduction in Belgium. Vaccine 2010; 28:7507 - 13; http://dx.doi.org/10.1016/j.vaccine.2010.09.004; PMID: 20851085
- Ogilvie I, Khoury H, El Khoury AC, Goetghebeur MM. Burden of rotavirus gastroenteritis in the pediatric population in Central and Eastern Europe: serotype distribution and burden of illness. Hum Vaccin 2011; 7:523 - 33; http://dx.doi.org/10.4161/hv.7.5.14819; PMID: 21422818
- Ginevskaya VA, Amitina NN, Eremeeva TP, Shirman GA, Priimägi LS, Drozdov SG. Electropherotypes and serotypes of human rotavirus in Estonia in 1989-1992. Arch Virol 1994; 137:199 - 207; http://dx.doi.org/10.1007/BF01311188; PMID: 7979994
- Forster J, Guarino A, Parez N, Moraga F, Román E, Mory O, et al, and the Rotavirus Study Group. Hospital-based surveillance to estimate the burden of rotavirus gastroenteritis among European children younger than 5 years of age. Pediatrics 2009; 123:e393 - 400; http://dx.doi.org/10.1542/peds.2008-2088; PMID: 19254975
- Grassi T, Bagordo F, Cavallaro A, Guido M, Malaventura C, Gabutti G, et al. Sequence analysis of human rotavirus strains: comparison of clinical isolates from Northern and Southern Italy. [Epub ahead of print] Eur J Clin Microbiol Infect Dis 2011; http://dx.doi.org/10.1007/s10096-011-1350-7; PMID: 21796344
- Iturriza-Gómara M, Green J, Brown DW, Desselberger U, Gray JJ. Diversity within the VP4 gene of rotavirus P[8] strains: implications for reverse transcription-PCR genotyping. J Clin Microbiol 2000; 38:898 - 901; PMID: 10655412
- Simmonds MK, Armah G, Asmah R, Banerjee I, Damanka S, Esona M, et al. New oligonucleotide primers for P-typing of rotavirus strains: Strategies for typing previously untypeable strains. J Clin Virol 2008; 42:368 - 73; http://dx.doi.org/10.1016/j.jcv.2008.02.011; PMID: 18378188
- Carvalho-Costa FA, Araújo IT, Santos de Assis RM, Fialho AM, de Assis Martins CM, Bóia MN, et al. Rotavirus genotype distribution after vaccine introduction, Rio de Janeiro, Brazil. Emerg Infect Dis 2009; 15:95 - 7; http://dx.doi.org/10.3201/eid1501.071136; PMID: 19116062
- Correia JB, Patel MM, Nakagomi O, Montenegro FM, Germano EM, Correia NB, et al. Effectiveness of monovalent rotavirus vaccine (Rotarix) against severe diarrhea caused by serotypically unrelated G2P[4] strains in Brazil. J Infect Dis 2010; 201:363 - 9; http://dx.doi.org/10.1086/649843; PMID: 20047501
- Vesikari T, Karvonen A, Prymula R, Schuster V, Tejedor JC, Cohen R, et al. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet 2007; 370:1757 - 63; http://dx.doi.org/10.1016/S0140-6736(07)61744-9; PMID: 18037080
- De Vos B, Han HH, Bouckenooghe A, Debrus S, Gillard P, Ward R, et al. Live attenuated human rotavirus vaccine, RIX4414, provides clinical protection in infants against rotavirus strains with and without shared G and P genotypes: integrated analysis of randomized controlled trials. Pediatr Infect Dis J 2009; 28:261 - 6; http://dx.doi.org/10.1097/INF.0b013e3181907177; PMID: 19289978
- Cilla G, Montes M, Gomariz M, Piñeiro L, Pérez-Trallero E. Rotavirus genotypes in children in the Basque Country (northern Spain) over a 13-year period (July 1996-June 2009). Eur J Clin Microbiol Infect Dis 2010; 29:955 - 60; http://dx.doi.org/10.1007/s10096-010-0951-x; PMID: 20490883
- Koukou D, Grivea I, Roma E, Tsioni H, Trimis G, Galanakis E, et al, Greek Rotascore Extension Study Group. Frequency, clinical characteristics, and genotype distribution of rotavirus gastroenteritis in Greece (2007-2008). J Med Virol 2011; 83:165 - 9; http://dx.doi.org/10.1002/jmv.21945; PMID: 21108355
- Mladenova Z, Korsun N, Geonova T, Iturriza-Gómara M, Group RS, Rotavirus Study Group. Molecular epidemiology of rotaviruses in Bulgaria: annual shift of the predominant genotype. Eur J Clin Microbiol Infect Dis 2010; 29:555 - 62; http://dx.doi.org/10.1007/s10096-010-0895-1; PMID: 20217165
- Buttery JP, Lambert SB, Grimwood K, Nissen MD, Field EJ, Macartney KK, et al. Reduction in rotavirus-associated acute gastroenteritis following introduction of rotavirus vaccine into Australia’s National Childhood vaccine schedule. Pediatr Infect Dis J 2011; 30:Suppl S25 - 9; http://dx.doi.org/10.1097/INF.0b013e3181fefdee; PMID: 21183837
- do Carmo GM, Yen C, Cortes J, Siqueira AA, de Oliveira WK, Cortez-Escalante JJ, et al. Decline in diarrhea mortality and admissions after routine childhood rotavirus immunization in Brazil: a time-series analysis. PLoS Med 2011; 8:e1001024; http://dx.doi.org/10.1371/journal.pmed.1001024; PMID: 21526228
- Raes M, Strens D, Vergison A, Verghote M, Standaert B. Reduction in pediatric rotavirus-related hospitalizations after universal rotavirus vaccination in Belgium. Pediatr Infect Dis J 2011; 30:e120 - 5; http://dx.doi.org/10.1097/INF.0b013e318214b811; PMID: 21436757
- Hull JJ, Teel EN, Kerin TK, Freeman MM, Esona MD, Gentsch JR, et al, National Rotavirus Strain Surveillance System. United States rotavirus strain surveillance from 2005 to 2008: genotype prevalence before and after vaccine introduction. Pediatr Infect Dis J 2011; 30:Suppl S42 - 7; http://dx.doi.org/10.1097/INF.0b013e3181fefd78; PMID: 21183839
- Gómez MM, de Mendonça MC, Volotão EdeM, Tort LF, da Silva MF, Cristina J, et al. Rotavirus A genotype P[4]G2: genetic diversity and reassortment events among strains circulating in Brazil between 2005 and 2009. J Med Virol 2011; 83:1093 - 106; http://dx.doi.org/10.1002/jmv.22071; PMID: 21503926
- Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, Abate H, Breuer T, Clemens SC, et al, Human Rotavirus Vaccine Study Group. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 2006; 354:11 - 22; http://dx.doi.org/10.1056/NEJMoa052434; PMID: 16394298
- Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, et al, Rotavirus Efficacy and Safety Trial (REST) Study Team. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med 2006; 354:23 - 33; http://dx.doi.org/10.1056/NEJMoa052664; PMID: 16394299
- Syriopoulou V, Kafetzis D, Theodoridou M, Syrogiannopoulos GA, Mantagos S, Trimis G, et al. Evaluation of potential medical and economic benefits of universal rotavirus vaccination in Greece. Acta Paediatr 2011; 100:732 - 9; http://dx.doi.org/10.1111/j.1651-2227.2010.02127.x; PMID: 21223372
- Vesikari T, Van Damme P, Giaquinto C, Gray J, Mrukowicz J, Dagan R, et al, Expert Working Group, European Society for Paediatric Infectious Diseases, European Society for Paediatric Gastroenterology, Hepatology, and Nutrition. European Society for Paediatric Infectious Diseases/European Society for Paediatric Gastroenterology, Hepatology, and Nutrition evidence-based recommendations for rotavirus vaccination in Europe: executive summary. J Pediatr Gastroenterol Nutr 2008; 46:615 - 8; http://dx.doi.org/10.1097/MPG.0b013e31816e213a; PMID: 18493224
- Clark HF, Borian FE, Bell LM, Modesto K, Gouvea V, Plotkin SA. Protective effect of WC3 vaccine against rotavirus diarrhea in infants during a predominantly serotype 1 rotavirus season. J Infect Dis 1988; 158:570 - 87; http://dx.doi.org/10.1093/infdis/158.3.570; PMID: 2842405
- Givon-Lavi N, Greenberg D, Dagan R. Comparison between two severity scoring scales commonly used in the evaluation of rotavirus gastroenteritis in children. Vaccine 2008; 26:5798 - 801; http://dx.doi.org/10.1016/j.vaccine.2008.08.030; PMID: 18786584
- Gentsch JR, Glass RI, Woods P, Gouvea V, Gorziglia M, Flores J, et al. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J Clin Microbiol 1992; 30:1365 - 73; PMID: 1320625
- Gouvea V, Glass RI, Woods P, Taniguchi K, Clark HF, Forrester B, et al. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J Clin Microbiol 1990; 28:276 - 82; PMID: 2155916
- Chouikha A, Fodha I, Ben Hadj Fredj M, Ardhaoui M, Teleb N, Brini I, et al. Relationship between electropherotypes and VP7/VP4 genotypes of group A rotaviruses detected between 2000 and 2007 in Tunisian children. Pathol Biol (Paris) 2011; 59:e43 - 8; http://dx.doi.org/10.1016/j.patbio.2009.04.008; PMID: 19481882