Abstract
TUTI-16 is a synthetic universal HIV-1 Tat epitope vaccine, designed to induce anti-Tat antibodies that block the function of circulating Tat, an HIV encoded protein secreted by HIV-1 infected cells. Circulating Tat activates CD4 T cells, permitting HIV replication and sustained viremia. Safety, immunogenicity and antiretroviral potential of TUTI-16 were explored in a randomized double-blind dose-escalating study in asymptomatic treatment-naïve HIV-1 infected subjects. TUTI-16 was safe, with mild local and systemic injection-related adverse reactions, but the antibody response was barely detectable. Surprisingly, a highly statistically significant reduction of HIV-1 viral load was found in the lowest 30 μg vaccine dose group (p < 0.01) but not at the higher doses. We posited that an anti-Tat antibody response below the limit of detection inhibited HIV viral load at this dose, an effect nullified at higher vaccine doses by activating cytokines induced by adjuvant components in TUTI-16. To clarify this immunogenicity/activation conundrum open label immunogenicity studies were performed in healthy HIV uninfected and aviremic ART-controlled HIV-infected subjects. These established that (1) healthy HIV negative subjects had robust antibody responses, maximal with 1 mg TUTI-16, (2) ART-controlled aviremic HIV infected subjects had similarly robust antibody responses, and (3) adjuvant-induced increases of HIV viral load did not occur in the presence of ART. These studies provided us a basis for the design of a protocol to explore the therapeutic potential of TUTI-16 vaccination to provide drug free control of HIV-1 viremia.
Introduction
Current antiretroviral therapy is effective in preventing progression to AIDS and deathCitation1 but HIV persistence dictates the necessity for lifelong use of ART. Early ART initiation is also effective in preventing transmission of HIVCitation2 but again, the burden of lifetime use of ART remains. The goal of long-term drug-free remission remains elusive,Citation3 as does the goal of a prophylactic HIV vaccine.Citation4,Citation5 A major problem for HIV vaccine development is the high variability of HIV antigenicity at first encounter, compounded by emergence of resistant antigenic variants driven by the selective pressure of the initial host immune response.Citation6 Following initial partial control of viral load by the host immune response an HIV set point is established and this level is a strong correlate for HIV-1 transmissionCitation7,Citation8 and progression to AIDS.Citation9
In 1996 we reviewed the evidence that extracellular HIV Tat protein was essential to maintain HIV set point viremia and proposed the notion of an anti-Tat AIDS vaccine.Citation10 There is strong evidence that Tat, secreted from HIV infected cells,Citation11 circulates in the blood,Citation12 is taken up by cellsCitation13 and is a powerful activator of CD4+ T cells,Citation14,Citation15 rendering them permissive for productive HIV replication, an activity blocked by anti-Tat antibodies.Citation14,Citation15 Thus Tat vaccination could potentially provide an anti-toxin approach to controlling HIV set point viremia.
We embarked on a strategy to develop an immunogenic and universally reactive HIV Tat B cell epitope vaccine to block circulating Tat protein. Tat protein displays the extensive sequence variation characteristic of most HIV proteins so we sought, and found, an immunodominant B cell epitope, Tat 4–12 (VDPRLEPWK), that was relatively conserved, with only 8-fold antigenic variation within Tat sequences from all HIV-1 clades,Citation16,Citation17 a manageable variation for vaccine development. Furthermore, the development of resistance to such an epitope vaccine would be improbable because no selection mechanism exists to direct Tat sequence evolution within an infected individual; Tat is not present on the HIV virion and a B cell epitope would not be present on the surface of HIV infected cells and would not induce a T cell response. Positive selection of Tat for function must occur at the evolutionary bottleneck of HIV transmission,Citation6 since effective transmission requires a fully functional Tat sequence.
To validate our hypothesis we conducted a placebo-controlled study in rhesus macaques. Tat epitope-immunized monkeys challenged with a non-pathogenic simian/human chimeric virus (SHIV33) developed acute viral counts that were indistinguishable from control-immunized monkeys whereas the chronic viral counts showed a statistically significant reduction in viral load in vaccinated monkeys (p = 0.008).Citation18 We postulated that Tat had no demonstrable role in the viremia of acute HIV-1 infection, with activation of HIV replication being provided at this stage by a reported systemic cytokine storm initiated at first contact by the innate immune system.Citation19 However, Tat appeared critical to the subsequent development and maintenance of HIV set point viremia following subsistence of activating cytokines. In contrast, recombinant Tat immunization controlled SHIV89.6P infection in cynomologous monkeys,Citation20 a disparity explained by SHIV89.6P being an acutely pathogenic virus with a disease course dissimilar to HIV. Viral control in this study was associated with T cell immune responses and not with anti-Tat antibody levels. The viral control seen with recombinant Tat in non-human studies was not reproduced in human studies wherein recombinant Tat was safe and modestly immunogenic but did not lower HIV viral loads.Citation21,Citation22
To circumvent the problem of extreme antigenic variation in HIV proteins, including Tat, we created a universally reactive synthetic HIV-1 Tat epitope vaccine, termed TUTI-16, by synthesizing a “wobble” peptide epitope sequence that induced antibodies reactive with all eight variant Tat epitope sequences known to occur in Tat, and by incorporating strong adjuvant components suitable for use in humans.Citation23
For the present first-in-humans study of this candidate prophylactic vaccine we first studied its use in untreated HIV-infected subjects, with established HIV set point viral load, to enable an assessment of whether Tat epitope immunization would lower the viral load and, if so, the antibody level required. Accordingly, the study addressed (1) the safety of TUTI-16, (2) anti-Tat antibody responses to immunization, and (3) the effect of immunization on HIV viral counts and CD4+ T cell levels. The unexpected findings of severely suppressed antibody responses to Tat and putative TLR2 adjuvant activation of HIV viremia led us to explore, in an open-label secondary study, TUTI-16 immunogenicity in (1) healthy HIV uninfected subjects, to clarify whether suppression of antibody response to TUTI-16 in treatment naïve HIV-1 infected subjects was specific to the untreated HIV state and (2) in aviremic ART-controlled HIV infected subjects to determine if ART control of HIV improved antibody responses and prevented the adjuvant induction of HIV replicationCitation24,Citation25 that we postulated in treatment naïve HIV-1 infected subjects at the higher vaccine doses.
Results
Study population and safety evaluations
Randomized placebo-controlled double blind study in treatment-naïve HIV infected subjects
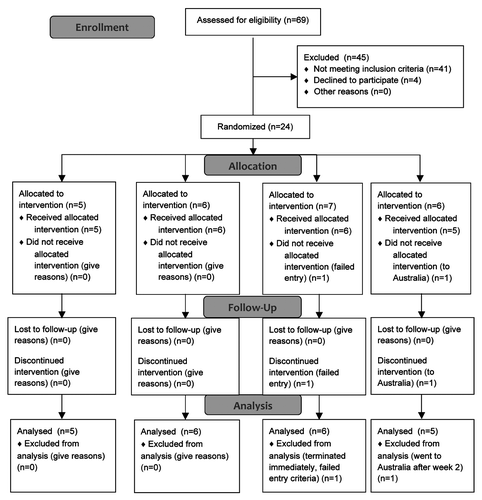
Twenty-two subjects completed the study through 20 weeks (). They comprised 5 receiving placebo; 6 receiving 30 μg TUTI-16; 6 receiving 100 μg TUTI-16; and 5 receiving 600 μg TUTI-16. No severe or serious adverse reactions occurred and the injection related adverse reactions were all transient and mild with the exception of one subject who had moderate fatigue and weakness (). ART was initiated in 2/5 placebo treated subjects after 12 weeks, while still in the blinded phase, at the behest of their personal physicians. None of the 17 TUTI-16 recipients were placed on ART during the study (p = 0.043, Fisher’s exact T test, two-tailed) ().
Table 1. Baseline demographics and injection related adverse reactions
Open label studies in healthy uninfected subjects and ART controlled HIV infected subjects
All open label subjects were male. The five HIV subjects on ART receiving 200 μg TUTI-16 were aged 45 ± 6 y (mean ± SD); all were positive for HIV-1/2 antibodies and all had HIV RNA counts < 20 copies/mL. The duration of antiretroviral treatment was 13 ± 7 y (mean ± SD). One subject had mild redness at the injection site and 2 subjects had transient mild elevations of AST and ALT liver enzymes 3 weeks after the second immunization. The 10 healthy HIV seronegative subjects were aged 35 ± 6 y (mean ± SD); One subject receiving 200 μg TUTI-16 had mild redness and tenderness at the injection site lasting several days and 3 subjects receiving 1.0 mg had flu-like symptoms for several days.
Anti-Tat antibodies
Randomized Study
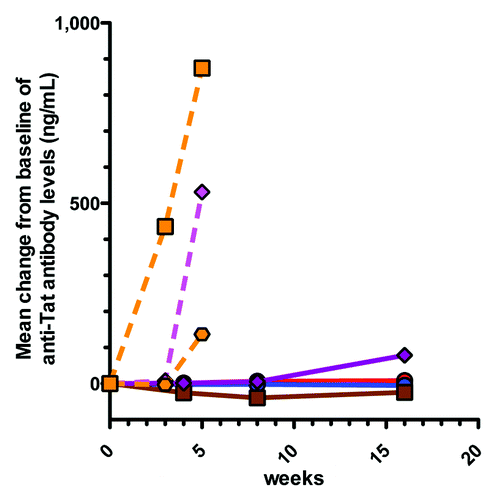
Ten of 22 (45%) entry serums in the double blind study had antibody levels ranging from 43–485 ng/mL and these were unevenly distributed between the groups with 8/10 being within the 100 μg and 600 μg TUTI-16 group. The open groups also had 1/5 subjects each with antibody detected at entry. We do not know the significance of these background antibodies that occurred in both HIV and uninfected subjects and may be related to cross-reactive autoantibodies.Citation26 With this background in mind we analyzed change from baseline to determine if immunization-induced increases in antibody had occurred. For these calculations serums below 40 ng/mL were assigned a baseline value of 20 ng/mL (midpoint of 0–40 ng/mL range). There were no significant antibody responses in the placebo, 30 μg and 100 μg groups of the randomized study and the 600 μg group alone showed a mean increase from baseline, 78 ng/mL at 16 weeks (). The low numbers of serums with measurable antibody responses precluded statistical analysis.
Figure 2. Mean changes from baseline of anti-Tat antibody concentrations following TUTI-16 immunization. Treatment naïve HIV subjects (solid lines): placebo red circle, 30 μg TUTI-16 blue circle, 100 μg TUTI-16 brown square, 600 μg TUTI-16 plum diamond. Healthy HIV seronegative subjects (dashed lines): 200 μg TUTI-16 orange hexagonal, 1.0 mg TUTI-16 orange square; ART controlled HIV subjects: 200 μg TUTI-16 magenta diamond. Treatment naïve HIV randomized study immunizations at 0, 4 and 12 weeks; open label immunizations at 0 and 3 weeks.
Open Label Study
In contrast, the ART controlled HIV-1 group (200 μg TUTI-16) had an increase of 531 ± 465 (mean ± SEM) ng/mL (2/5 responders), the healthy uninfected group (200 μg TUTI-16) had an increase of 137 ± 36 (mean ± SEM) ng/mL (4/5 responders) and the healthy uninfected group (1.0 mg TUTI-16) had an increase of 859 ± 401 (mean ± SEM) ng/mL (5/5 responders) (). All the HIV seronegative subjects remained HIV seronegative after TUTI-16 immunization.
Changes in HIV-1 viral load
Randomized study
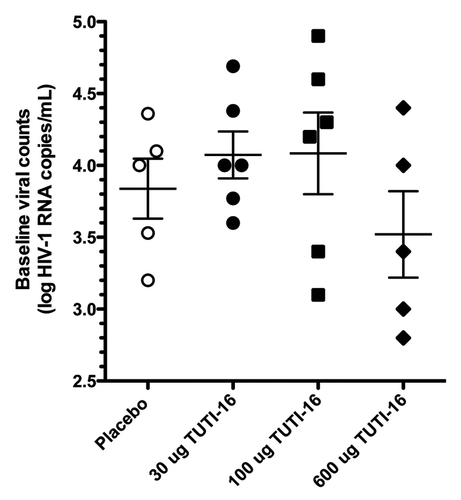
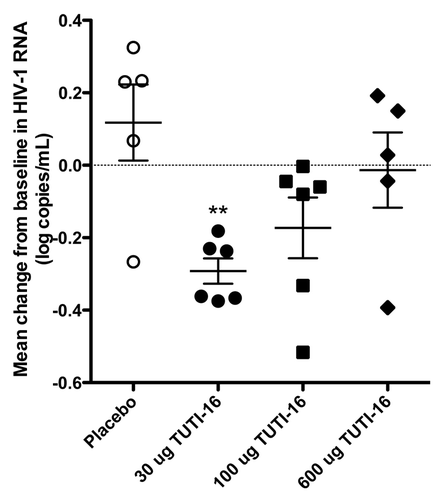
Baseline plasma HIV count (log HIV RNA copies /mL) was 3.8 ± 0.21, 4.1 ± 0.16, 4.1 ± 0.28 and 3.5 ± 0.30 (mean ± SEM) for the placebo, 30 μg, 100 μg and 600 μg TUTI-16 groups, respectively (), differences that were not statistically significant (p = 0.36, one-way ANOVA), establishing successful randomization. The change from baseline (mean ± SEM) was 0.12 ± 0.10 (n = 5), -0.29 ± 0.03 (n = 6), -0.17 ± 0.08 (n = 6) and -0.01 ± 0.10 (n = 5) HIV RNA copies/mL for placebo, 30 μg, 100 μg, and 600 μg TUTI-16 groups, respectively (). The one-way ANOVA statistic for decline in HIV viral load was statistically significant (p = 0.014) with the 30 μg TUTI-16 group alone being significantly lower than placebo (p < 0.01, post testing with Bonferroni’s multiple comparison test). With successful randomization at entry and blinded placebo comparison, the P value of < 0.01 was striking although confirmation with larger subject numbers would be desirable. Viral load data in two placebo subjects who initiated antiretroviral treatment after 12 weeks was censored for weeks 16 and 20.
Open label study
The five open label HIV-1 subjects on ART had < 20 HIV RNA copies/mL before, during and after immunization.
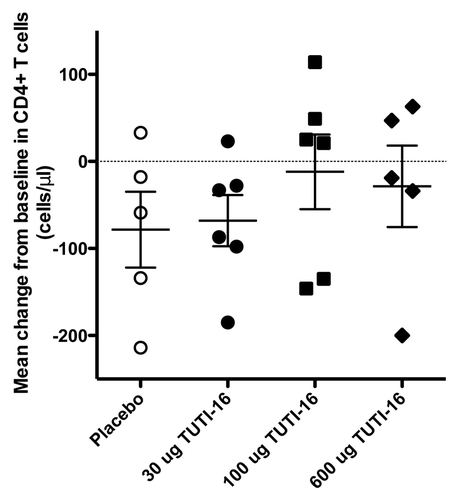
Changes in CD4+ T cell counts
Randomized Study
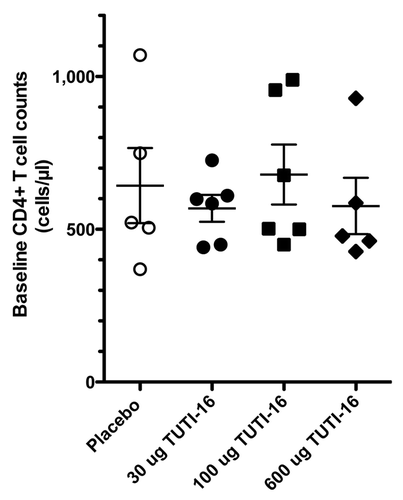
Entry levels of CD4+ T cells (mean ± SEM cells/μl) were 643 ± 123, 568 ± 44, 679 ± 98 and 576 ± 92 for the placebo, 30 μg, 100 μg and 600 μg TUTI-16 groups, respectively (), not statistically significant (p = 0.35, one way ANOVA), establishing successful randomization. The changes from baseline (mean ± SEM) were -78 ± 44 (n = 5), -68 ± 29 (n = 6), -12 ± 43(n = 6) and -29 ± 47 (n = 5) CD4+ T cell/μl for placebo, 30 μg, 100 μg and 600 μg TUTI-16 groups, respectively (). There were no statistically significant between group differences (p = 0.62, one-way ANOVA).
Tepiumab universal anti-Tat epitope monoclonal antibody standard Tepiumab affinities on recombinant Tat variants
The KD results on recombinant Tat variants with epitope sequence variations were: VDPRLEP, 3.60E-12; VDPNLEP, 2.70E-12; VDPKLEP 1.00E-11; VDPSLEP 1.50E-10; VDPNLDP, 4.00E-13 (variable amino acids in bold type). These data established that tepiumab was of high affinity and a valid Tat ELISA standard for all the phenotypic variants of this Tat epitope.
Discussion
The present phase 1/2a exploratory study in asymptomatic ART naïve HIV subjects was initiated to determine if TUTI-16 immunization would lower the HIV viral load in infected subjects and, with a future prophylactic study in mind, the target antibody level needed to control HIV viral load. These objectives were achieved with the findings of (1) reduction in HIV viral load compared with placebo at 30 μg TUTI-16 but diminishing with increasing TUTI-16 doses and (2) the occurrence of this antiretroviral activity at putative antibody increases below the 40 ng/mL LOD of the assay. Anti-Tat antibodies induced by the vaccine, but below the LOD of the assay, provide a plausible explanation for the reduction of HIV viral load and efficacy of an anti-Tat antibody response; notably, antibody levels below 40 ng/mL would still be likely to be stoichiometrically effective in blocking Tat since the median level of circulating Tat in HIV is < 0.1 ng/mL.Citation28
The diminished antiretroviral effect of TUTI-16 immunization in the higher dose groups requires explanation since higher vaccine doses would be expected to be associated with increased antibody levels and correspondingly lower HIV viral loads. TUTI-16 contains a Toll like receptor 2 (TLR2) agonist designed to enhance the antibody response.Citation23-Citation25,Citation29,Citation30 However this action would also induce cells of the innate immune system to secrete cytokines that activate CD4+ T cells and enhance HIV VL.Citation23,Citation29,Citation30 Activating cytokines activate CD4 T cells, making them permissive for productive HIV infection in a Tat independent manner, both in vitroCitation31,Citation32 and in vivo.Citation33,Citation34 We posit that with increasing TUTI-16 doses the dose dependent increases of cytokines activate CD4+ T cells to increase the HIV viral load and obscure the underlying suppression of Tat-dependent HIV replication by anti-Tat antibodies.
Antibody responses in treatment naïve HIV infected subjects were highly suppressed compared with our previous findings in rodentsCitation23 and our present demonstration of robust TUTI-16 immunogenicity in healthy uninfected HIV subjects suggest untreated HIV infection and possibly circulating TatCitation35 as the cause of this immunosuppression. The robust antibody response and acceptable injection related adverse reaction profile found in the open label healthy uninfected group at 1 mg dosing suggest that this would be an optimal dose for exploring the potential of TUTI-16 as a prophylactic vaccine. On the other hand, to develop a practical regimen for treating HIV infected subjects, the present findings suggest that control of HIV viral load using ART should precede immunization, to overcome the dual problems of poor immunogenicity and adjuvant induction of Tat-independent increases of HIV viral load in viremic HIV infected subjects. The finding that ART controlled aviremic HIV infected subjects had robust antibody responses and, importantly, remained aviremic during and after TUTI-16 immunization, suggests that ART control of HIV viral load prior to and during a prime/boost immunization regimen may obviate the problems of poor immunogenicity and adjuvant activation of Tat-independent increase in HIV viral load. This regimen could provide a feasible approach to exploring the use of TUTI-16 as a therapeutic HIV-1 vaccine, especially if initiated early at first diagnosis.
In summary, this study established that immunization with TUTI-16 was safe over the 30 μg to 1 mg dose range tested but resulted in a barely detectable antibody response in ART naïve HIV subjects. Nevertheless, statistically significant reduction of HIV viral load, albeit with a low number of test subjects, established proof of concept that anti-Tat antibodies lower HIV viral load, with the antibody threshold level needed being below 40 ng/mL. Antibody responses to a 1 mg TUTI-1 prime and 3 week boost regime in healthy HIV seronegative subjects were robust, with a mean antibody level at least 20-fold greater than the threshold for lowering HIV viral load, providing encouragement to explore the potential of TUTI-16 as a prophylactic vaccine. TUTI-16 immunization of ART naïve HIV infected subjects is contraindicated but a stratagem of ART control of HIV-1 viral load prior to and during immunization may offer a workable approach to explore therapeutic use of TUTI-16 to establish long-term drug-free control of HIV viral load in infected subjects.
Methods
Participants, blinding and protocol design – Randomized study
Asymptomatic ART naïve HIV infected (> 6 mo) male or female subjects 18–50 y of age, with plasma viral loads of ≥ 3,000 ≤ 100,000 HIV RNA copies/mL and CD4+ T cell counts ≥ 500/mm3 were selected from February to October 2009. Subjects were sequentially accrued into three cohorts of 8, each cohort consisting of 6 active and 2 placebo subjects assigned in a double blind fashion according to a code developed and maintained by Damiano Consulting Associates, Inc. The assignments remained unknown to the sponsor, patients and all personnel at the clinic site. Active subjects in each cohort received 30 μg, 100 μg and 600 μg TUTI-16, respectively, for cohorts 1, 2 and 3. Dosing levels were based on rodent immunogenicity studiesCitation23 and pre-clinical safety studies (data not shown). Clinical and laboratory safety evaluations 2 weeks after each cohort completed the first injections were assessed before dose escalation to the next cohort was instituted. Immunizations were at day 0, week 4 and week 12, with clinical and laboratory monitoring through week 20.
Open study
Three 5-subject open label groups were added subsequently to assess immunogenicity in HIV-1 subjects on ART with undetectable HIV plasma viral counts, 200 μg dose, 5 subjects, and healthy HIV uninfected subjects at two doses, 200 μg, 5 subjects, and 1.0 mg, 5 subjects. Immunizations were at day 0 and week three with antibody assessments at day 0, week 3 and week 5, a protocol used previously in rodent studies.Citation23
Vaccine preparation, formulation and injection
TUTI-16 was synthesized under cGMP by Bachem AB and provided as a lyophilized powder. The final formulation was in 4.5 mg/mL mannitol in water for injection and the blinded vials were prepared by Frontage Laboratories by weighing out, under aseptic conditions, 4.5 mg mannitol in each vial, with addition of TUTI-16 to provide 30, 100 or 600 μg doses in the active vials or no further additions for placebo. The vials were stored at -20°C. They were reconstituted with water for injection and injected subcutaneously in the deltoid region through a medical Millex-GV filter, 0.2 μm, 33 mm to achieve terminal sterilization. Vaccine stability was validated for the lyophilized and solution stages and for passage through the filter.
Laboratory parameters
Hunter Laboratories, CA (randomized study) and Spectra Laboratories, NJ (open label groups) performed the biochemistry and hematology safety panels and urinalysis and provided plasma HIV-1 RNA levels and flow cytometric analyses of CD4+ T cells. Anti-Tat antibody levels were measured at Molecular Diagnostic Services, Inc.
Sandwich ELISA for anti-Tat antibodies
A sandwich ELISA used recombinant Tat (B consensus sequence) Genscript, Piscataway, NJ) for capture, a high affinity human IgG1 λ anti-Tat monoclonal antibody (HIV tepiumab, formerly E1–4-E9)Citation27 for the standard curve, and a horseradish peroxidase- conjugated goat anti-human IgG antibody (KPL, Gathersburg, MD) for the detector. The assay had an LOD of 0.4 ng/mL in assay buffers, but serums needed 1/100 dilution to reduce background found in HIV seronegative control serums,Citation26,Citation27 yielding a final LOD of 40 ng/mL serum.
Affinity determinations of tepiumab
Tepiumab is a human IgG1 λ monoclonal antibody. The KD (M) of tepiumab was determined by surface plasmon resonance (Biacore 3,000) (Precision Antibody, Columbia MD) on five recombinant Tat proteins containing the naturally occurring variations in the Tat epitope 4–10 sequence.
Statistical analysis
GraphPad Prism 5 for Mac OS X (Version 5.0d) was used for statistical computations. Two-tailed Fisher’s exact test was used for contingency comparisons. One way analysis of variance (ANOVA) was used for comparisons of entry levels of log HIV RNA copies/mL and CD4+ T cell counts. Mean changes from baseline for log HIV viral counts (copies of HIV RNA/mL) and CD4+ T cell counts (cells/μl) over the 20 week study period were calculated by establishing a mean change from baseline for each subject during the 20 week study period and assessing between treatment group differences by one way ANOVA and post testing with Bonferroni’s multiple comparison test between placebo and each TUTI-16 dose group if the overall statistic was p < 0.05. p < 0.05 was considered statistically significant. All log notations are log10. Statistical comparisons of differences in antibody responses were not performed because distributions were not normal and the n values of serums containing measurable antibodies were too low for non-parametric statistics.
| Abbreviations: | ||
| ANOVA | = | analysis of variance |
| ART | = | antiretroviral therapy |
| HIV | = | HIV-1 |
| LOD | = | limit of detection |
| SD | = | standard deviation |
| SEM | = | standard error of mean |
| TUTI-16 | = | Thymon Universal Tat Immunogen-16 |
Acknowledgments
We thank the participants for their participation in this study, Eve Damiano for her invaluable regulatory guidance, the staff of the Conant Medical Group, that conducted the double blind study, Marcus Conant and Chris Eden; the staff at Clinilabs, Inc., that performed the open label studies, Malika Pasha, Mardik Donikyan, Emily Muller, Brian Pondracz, Ray Ramondi; and Michael Willet and Melissa Carbon of Ready Clinical, for their meticulous care and expertise. Harvey Motulsky of GraphPad Sofware Inc. and Alan Fisher provided valued statistical support. We also thank Katherine Berkousen, Brenda Baldwin and the staff at FDA/CBER/OVRR for their valued guidance throughout this development.
GG developed the concepts and the protocol, with assistance from Eve Damiano, supervised the trial, performed the statistics, with support from Alan Fisher, and wrote the paper. JJC developed and performed the sandwich ELISA for measuring antibodies to Tat and wrote the assay section in Methods.
This study was performed under IND 1374 from CBER, USFDA, and ethical review from the Western Institutional Review Board, Olympia, WA (randomized study) and the New England Institutional Review Board, Newton, MA (open label study). All subjects signed approved informed consent forms before admission into the study. The randomized study was conducted at the Conant Medical Group, San Francisco, CA and the open label study at Clinilabs, Inc., New York, NY. Clinicaltrials.gov identifiers # NCT00848211, # NCT01144026.
Disclosure of Potential Conflicts of Interest
An IRS Qualified Therapeutic Discovery Grant was the only external funding provided for this study. GG is the founder of Thymon LLC, a virtual company formed to develop an HIV vaccine, and the inventor on 8 issued US patents related to HIV-1 Tat that are assigned to Thymon LLC.
References
- Ray M, Logan R, Sterne JA, Hernández-Díaz S, Robins JM, Sabin C, et al, HIV-CAUSAL Collaboration. The effect of combined antiretroviral therapy on the overall mortality of HIV-infected individuals. AIDS 2010; 24:123 - 37; http://dx.doi.org/10.1097/QAD.0b013e3283324283; PMID: 19770621
- Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al, HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493 - 505; http://dx.doi.org/10.1056/NEJMoa1105243; PMID: 21767103
- Trono D, Van Lint C, Rouzioux C, Verdin E, Barré-Sinoussi F, Chun TW, et al. HIV persistence and the prospect of long-term drug-free remissions for HIV-infected individuals. Science 2010; 329:174 - 80; http://dx.doi.org/10.1126/science.1191047; PMID: 20616270
- Girard MP, Osmanov S, Assossou OM, Kieny MP. Human immunodeficiency virus (HIV) immunopathogenesis and vaccine development: a review. Vaccine 2011; 29:6191 - 218; http://dx.doi.org/10.1016/j.vaccine.2011.06.085; PMID: 21718747
- McMichael AJ, Borrow P, Tomaras GD, Goonetilleke N, Haynes BF. The immune response during acute HIV-1 infection: clues for vaccine development. Nat Rev Immunol 2010; 10:11 - 23; http://dx.doi.org/10.1038/nri2674; PMID: 20010788
- Brumme ZL, Walker BD. Tracking the culprit: HIV-1 evolution and immune selection revealed by single-genome amplification. J Exp Med 2009; 206:1215 - 8; http://dx.doi.org/10.1084/jem.20091094; PMID: 19487418
- Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, et al, Rakai Project Study Group. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N Engl J Med 2000; 342:921 - 9; http://dx.doi.org/10.1056/NEJM200003303421303; PMID: 10738050
- Royce RA, Seña A, Cates W Jr., Cohen MS. Sexual transmission of HIV. N Engl J Med 1997; 336:1072 - 8; http://dx.doi.org/10.1056/NEJM199704103361507; PMID: 9091805
- Mellors JW, Rinaldo CRJ Jr., Gupta P, White RM, Todd JA, Kingsley LA. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 1996; 272:1167 - 70; http://dx.doi.org/10.1126/science.272.5265.1167; PMID: 8638160
- Goldstein G. HIV-1 Tat protein as a potential AIDS vaccine. Nat Med 1996; 2:960 - 4; http://dx.doi.org/10.1038/nm0996-960; PMID: 8782444
- Chang HC, Samaniego F, Nair BC, Buonaguro L, Ensoli B. HIV-1 Tat protein exits from cells via a leaderless secretory pathway and binds to extracellular matrix-associated heparan sulfate proteoglycans through its basic region. AIDS 1997; 11:1421 - 31; http://dx.doi.org/10.1097/00002030-199712000-00006; PMID: 9342064
- Westendorp MO, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, et al. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature 1995; 375:497 - 500; http://dx.doi.org/10.1038/375497a0; PMID: 7539892
- Vendeville A, Rayne F, Bonhoure A, Bettache N, Montcourrier P, Beaumelle B. HIV-1 Tat enters T cells using coated pits before translocating from acidified endosomes and eliciting biological responses. Mol Biol Cell 2004; 15:2347 - 60; http://dx.doi.org/10.1091/mbc.E03-12-0921; PMID: 15020715
- Li CJ, Ueda Y, Shi B, Borodyansky L, Huang L, Li YZ, et al. Tat protein induces self-perpetuating permissivity for productive HIV-1 infection. Proc Natl Acad Sci U S A 1997; 94:8116 - 20; http://dx.doi.org/10.1073/pnas.94.15.8116; PMID: 9223324
- Sahaf B, Atkuri K, Heydari K, Malipatlolla M, Rappaport J, Regulier E, et al. Culturing of human peripheral blood cells reveals unsuspected lymphocyte responses relevant to HIV disease. Proc Natl Acad Sci U S A 2008; 105:5111 - 6; http://dx.doi.org/10.1073/pnas.0712363105; PMID: 18364393
- Goldstein G, Tribbick G, Manson K. Two B cell epitopes of HIV-1 Tat protein have limited antigenic polymorphism in geographically diverse HIV-1 strains. Vaccine 2001; 19:1738 - 46; http://dx.doi.org/10.1016/S0264-410X(00)00393-5; PMID: 11166899
- Ruckwardt TJ, Tikhonov I, Berg S, Hatfield GS, Chandra A, Chandra P, et al. Sequence variation within the dominant amino terminus epitope affects antibody binding and neutralization of human immunodeficiency virus type 1 Tat protein. J Virol 2004; 78:13190 - 6; http://dx.doi.org/10.1128/JVI.78.23.13190-13196.2004; PMID: 15542671
- Goldstein G, Manson K, Tribbick G, Smith R. Minimization of chronic plasma viremia in rhesus macaques immunized with synthetic HIV-1 Tat peptides and infected with a chimeric simian/human immunodeficiency virus (SHIV33). Vaccine 2000; 18:2789 - 95; http://dx.doi.org/10.1016/S0264-410X(00)00085-2; PMID: 10812220
- Stacey AR, Norris PJ, Qin L, Haygreen EA, Taylor E, Heitman J, et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol 2009; 83:3719 - 33; http://dx.doi.org/10.1128/JVI.01844-08; PMID: 19176632
- Maggiorella MT, Baroncelli S, Michelini Z, Fanales-Belasio E, Moretti S, Sernicola L, et al. Long-term protection against SHIV89.6P replication in HIV-1 Tat vaccinated cynomolgus monkeys. Vaccine 2004; 22:3258 - 69; http://dx.doi.org/10.1016/j.vaccine.2004.03.009; PMID: 15308348
- Ensoli B, Fiorelli V, Ensoli F, Lazzarin A, Visintini R, Narciso P, et al. The preventive phase I trial with the HIV-1 Tat-based vaccine. Vaccine 2009; 28:371 - 8; http://dx.doi.org/10.1016/j.vaccine.2009.10.038; PMID: 19879233
- Longo O, Tripiciano A, Fiorelli V, Bellino S, Scoglio A, Collacchi B, et al. Phase I therapeutic trial of the HIV-1 Tat protein and long term follow-up. Vaccine 2009; 27:3306 - 12; http://dx.doi.org/10.1016/j.vaccine.2009.01.090; PMID: 19208456
- Goldstein G, Chicca JJ 2nd. A universal anti-HIV-1 Tat epitope vaccine that is fully synthetic and self-adjuvanting. Vaccine 2010; 28:1008 - 14; http://dx.doi.org/10.1016/j.vaccine.2009.10.129; PMID: 19931501
- Hübschle T, Mütze J, Mühlradt PF, Korte S, Gerstberger R, Roth J. Pyrexia, anorexia, adipsia, and depressed motor activity in rats during systemic inflammation induced by the Toll-like receptors-2 and -6 agonists MALP-2 and FSL-1. Am J Physiol Regul Integr Comp Physiol 2006; 290:R180 - 7; http://dx.doi.org/10.1152/ajpregu.00579.2005; PMID: 16154916
- Okusawa T, Fujita M, Nakamura J, Into T, Yasuda M, Yoshimura A, et al. Relationship between structures and biological activities of mycoplasmal diacylated lipopeptides and their recognition by toll-like receptors 2 and 6. Infect Immun 2004; 72:1657 - 65; http://dx.doi.org/10.1128/IAI.72.3.1657-1665.2004; PMID: 14977973
- Rodman TC, Pruslin FH, To SE, Winston R. Human immunodeficiency virus (HIV) Tat-reactive antibodies present in normal HIV-negative sera and depleted in HIV-positive sera. Identification of the epitope. J Exp Med 1992; 175:1247 - 53; http://dx.doi.org/10.1084/jem.175.5.1247; PMID: 1373758
- Hinkula JSY, Devito C, Sonesson E, Goldstein G, Carlsson R. Human recombinant IgG1 anti-Tat antibodies inhibit HIV-1 viral replication in vitro. In: 12th Conference on retroviruses and opportunistic infections; paper #549; 2005; 2005.
- Westendorp MO, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, et al. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature 1995; 375:497 - 500; http://dx.doi.org/10.1038/375497a0; PMID: 7539892
- Zeng W, Ghosh S, Lau YF, Brown LE, Jackson DC. Highly immunogenic and totally synthetic lipopeptides as self-adjuvanting immunocontraceptive vaccines. J Immunol 2002; 169:4905 - 12; PMID: 12391202
- Alphs HH, Gambhira R, Karanam B, Roberts JN, Jagu S, Schiller JT, et al. Protection against heterologous human papillomavirus challenge by a synthetic lipopeptide vaccine containing a broadly cross-neutralizing epitope of L2. Proc Natl Acad Sci U S A 2008; 105:5850 - 5; http://dx.doi.org/10.1073/pnas.0800868105; PMID: 18413606
- Li CJ, Ueda Y, Shi B, Borodyansky L, Huang L, Li YZ, et al. Tat protein induces self-perpetuating permissivity for productive HIV-1 infection. Proc Natl Acad Sci U S A 1997; 94:8116 - 20; http://dx.doi.org/10.1073/pnas.94.15.8116; PMID: 9223324
- Sahaf B, Atkuri K, Heydari K, Malipatlolla M, Rappaport J, Regulier E, et al. Culturing of human peripheral blood cells reveals unsuspected lymphocyte responses relevant to HIV disease. Proc Natl Acad Sci U S A 2008; 105:5111 - 6; http://dx.doi.org/10.1073/pnas.0712363105; PMID: 18364393
- Stanley SK, Ostrowski MA, Justement JS, Gantt K, Hedayati S, Mannix M, et al. Effect of immunization with a common recall antigen on viral expression in patients infected with human immunodeficiency virus type 1. N Engl J Med 1996; 334:1222 - 30; http://dx.doi.org/10.1056/NEJM199605093341903; PMID: 8606717
- Staprans SI, Hamilton BL, Follansbee SE, Elbeik T, Barbosa P, Grant RM, et al. Activation of virus replication after vaccination of HIV-1-infected individuals. J Exp Med 1995; 182:1727 - 37; http://dx.doi.org/10.1084/jem.182.6.1727; PMID: 7500017
- Cohen SS, Li C, Ding L, Cao Y, Pardee AB, Shevach EM, et al. Pronounced acute immunosuppression in vivo mediated by HIV Tat challenge. Proc Natl Acad Sci U S A 1999; 96:10842 - 7; http://dx.doi.org/10.1073/pnas.96.19.10842; PMID: 10485913