Abstract
Aim
To determine whether immunotherapy with HPV6 L1 virus like particles (VLPs) without adjuvant (VLP immunotherapy) reduces recurrence of genital warts following destructive therapy.
Trial design
A randomized placebo controlled blinded study of treatment of recurrent genital warts amenable to destructive therapy, conducted independently in Australia and China.
Methods
Patients received conventional destructive therapy of all evident warts together with intramuscular administration of 1, 5 or 25 µg of VLP immunotherapy, or of placebo immunotherapy (0.9% NaCl), as immunotherapy at week 0 and week 4. Primary outcome, assessed at week 8, was recurrence of visible warts.
Results
Of 33 protocol compliant Brisbane recipients of placebo immunotherapy, 11 were disease free at two months, and a further 9 demonstrated reduction of > 50% in total wart area. Wart area reduction following destructive treatment correlated with prior duration of disease. Among 102 protocol compliant Brisbane recipients of VLP immunotherapy, disease reduction was significantly greater than among the placebo immunotherapy (50% ± s.e.m. 7%) recipients for subjects receiving 5 µg or 25 µg of VLP immunotherapy/dose (71% ± s.e.m.7%) but not for those receiving 1 µg VLP immunotherapy/dose (42% ± 7%). Of 52 protocol compliant placebo immunotherapy recipients in Wenzhou, 37 were disease free at two months, and a further 8 had > 50% disease reduction. Prior disease duration was much shorter in Wenzhou subject (8.1 ± 1.1 mo) than in Brisbane subjects (53.7 ± 5.5 mo). No significant reduction in mean wart area was observed for the 168 Wenzhou protocol compliant subjects who also received VLP immunotherapy.
Conclusions
This study confirms the findings in a previous open label trial that administration of VLP immunotherapy may assist in clearance of recurrent genital warts in patients for whom destructive therapy is unsuccessful and that unsuccessful destructive therapy is more common with increasing prior disease duration.
Keywords: :
Introduction
Genital warts are caused by infection with human papillomavirus type 6 (HPV6) or less commonly the closely related HPV 11.Citation1,Citation2 While benign, these lesions commonly persist, with less than half of immunocompetent patients resolving infection within six months.Citation3 Despite use of destructive therapies including electrocautery, cryotherapy, or application of podophyllotoxin or trichloroacetic acid, disease recurrence is common.Citation4 However, generation of specific immunity to papillomavirus proteins appears important for clearance, as immunosuppressed individuals with impaired cell mediated immunity clear genital warts more slowly, and more commonly have recurrence after treatment.Citation5
The major papillomavirus protein L1 self assembles into virus like particles, which, together with alum based adjuvants, are the basis of vaccines licensed for use for prevention of HPV infection.Citation6 L1 virus like particles, in animal models, can induce strong cell mediated immune responses including cytotoxic T-cell responses if delivered without adjuvant.Citation7,Citation8 A phase 1 open label safety trial of unadjuvanted HPV6 VLPs (VLP immunotherapy) in patients with treatment refractory genital warts had observed regression of disease that would not have been expected from previous studies of therapy in similar patients.Citation9 Therefore a randomized placebo controlled trial was undertaken to establish whether VLP immunotherapy could reduce the recurrence of genital warts following locally destructive therapy.
Results
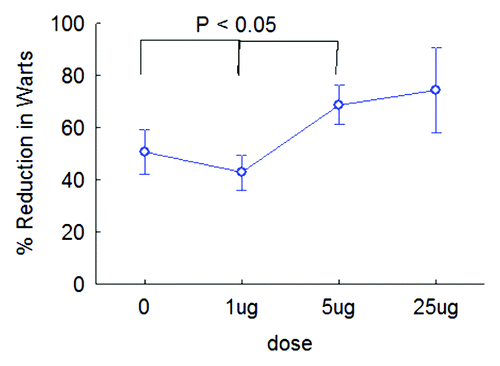
Subjects presenting with recurrent genital warts were treated with a destructive therapy according to the current standard practice at each of the trial sites (Brisbane, Australia and Wenzhou, China), and were randomized to receive one of three dose levels of VLP immunotherapy or a placebo immunotherapy, on two occasions one month apart. The primary study endpoint was recurrent disease two months after destructive treatment, and per protocol this was evaluated independently for each delivered VLP immunotherapy dose and trial site. VLP immunotherapy at the two higher doses (5 μg and 25 μg) was associated with less disease recurrence two months after treatment for patients recruited to the Australian trial site, as assessed by number of responding subjects () and by percentage reduction in disease from pre-treatment (). No significant effect of any dose of VLP immunotherapy was observed at the Chinese site ().
Table 1. Subject demographics at recruitment
Figure 2. VLP immunotherapy is effective where destructive therapy alone is insufficient to clear warts. Reduction in wart area 2 mo after therapy is shown for subjects recruited to the Australian site according to vaccine dose. Correlation of wart area reduction with dose (generalized linear modeling: f = 2.71; p < 0.05).
Known predictors of poor outcome from destructive wart treatment were examined, as they might account for the difference in outcome between different sites. Age and gender distribution was similar between Chinese and Australian sites (), and neither age (f = 0.684; p = 0.4) nor gender (f = 0.736; p = 0.4) predicted outcome after treatment. The extent of presenting disease was significantly less at the Chinese site (). However, analysis of disease recurrence following VLP or placebo immunotherapy therapy for all subjects from both sites showed no difference according to the extent of disease at recruitment (f = 0.863; p = 0.3). Disease duration prior to treatment was significantly shorter for Chinese than for Australian subjects (). Across all subjects and all treatments, outcome was not as good for subjects with long disease duration prior to study recruitment (f = 7.07; p < 0.01).
Table 2. Outcome analysis by trial site
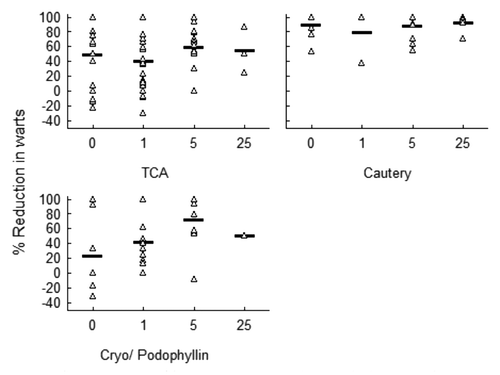
Primary destructive therapy at the Chinese site was exclusively cautery, whereas other options were also used at the Australian site (). Different treatment choices gave different probabilities of disease recurrence. Choice of treatment was associated with duration of disease, but not with initial lesion area (). Treatment outcome for Chinese subjects, all treated exclusively with cautery, and for those Australian subjects treated with cautery, was similar, despite the differences in initial disease duration and lesion size between these groups. The current study was not designed to examine the contribution of choice of destructive treatment to the effectiveness of VLP immunotherapy. However, benefit of VLP immunotherapy was most apparent in those patients receiving less effective destructive therapies (). Poorest outcome without VLP immunotherapy was seen for patients treated with cryotherapy and in this group there was a significant association between dose of VLP immunotherapy and better disease outcome (t test, independent variables; 33.4% reduction (0, 1 µg) v 69.3% reduction (5, 25 µg); t = 2.62; p < 0.02) .
Table 3. Destructive wart treatment used by site and vaccine dose
Table 4. Destructive treatment as a determinant of disease outcome (Australia)
Figure 3. VLP immunotherapy is effective where destructive therapy alone is insufficient to clear warts. Reduction in wart area 2 mo after therapy is shown for subjects recruited to the Australian site by vaccine dose according to primary treatment modality: cautery, TCA(trichloroacetic acid) or other: cryotherapy or podophyllotoxin. Treatment relationship for subjects treated with cryotherapy or podophyllotoxin: immunotherapy compared with placebo p < 0.05 (Mann-Whitney U test).
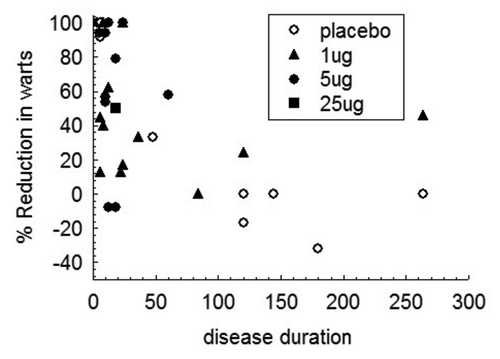
Data were then examined for evidence that non-random distribution of destructive therapy between different study patient dosage groups, as a consequence of different presenting history, might result in apparent efficacy of particular doses of VLP immunotherapy. Recipients of 5 µg or more of VLP immunotherapy and treated with other than trichloroacetic acid (TCA) or cautery, had significantly shorter duration of disease when compared with similarly treated patients receiving lower VLP immunotherapy doses (0, 1 µg 75.1 ± 18.9 mo; n = 14; 5, 25 µg 16.5 months ± 3.7 SE months n = 20; t = 2.56, p < 0.02) and shorter disease duration was associated with better outcome following VLP immunotherapy (). However, no significant difference in disease duration was seen between dose groups, for subjects treated by TCA or cautery (0, 1 µg 49.5 ± 6.8 mo; 5, 25 µg 66.8 ± 14.7 mo).
Figure 4. Response to VLP immunotherapy according to duration of disease for subjects treated by cryotherapy or podophyllotoxin. Reduction in wart area 2 mo after therapy is shown according to vaccine dose and disease duration for subjects recruited to the Australian site.
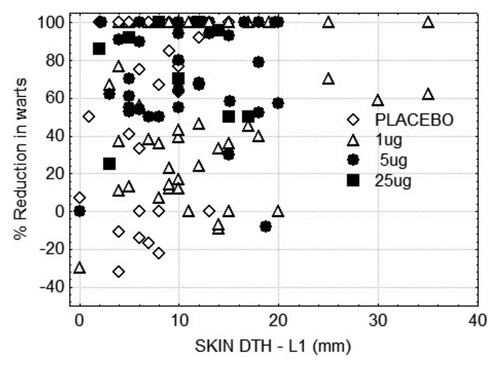
Delayed type hypersensitivity to HPV L1 protein was observed in all subjects, including placebo immunotherapy recipients (6.5 ± 0.6 mm), and significantly greater DTH was induced by each dose of VLP immunotherapy (1 μg: 12.0 ± 1.1 mm; 5 μg: 10.7 ± 0.8 mm; 25 μg: 9.6 ± 1.8 mm). There was no correlation between induced DTH and clinical outcome ().
Figure 5. Response to VLP immunotherapy according to delayed type hypersensitivity reaction to L1 protein measured after vaccination. For all Brisbane subjects, skin reactivity to HPV VLPs was measured 48 h after intradermal injection of 1 μg of HPV VLPs.
No serious adverse events at least possibly related to VLP immunotherapy were reported during the study, and no reactions to vaccine other than grade 1 local reactogenicity were recorded or self-reported by patients.
Discussion
This study demonstrates that administration of two doses of 5 µg or more of a VLP immunotherapy based on the L1 capsid protein of HPV6 was associated with a significantly reduced rate of recurrence of genital warts after conventional destructive therapy, and reduced burden of wart disease (). While VLP immunotherapy as a sole therapy was tested in our earlier study with encouraging results,Citation9 discussion with patients indicated that any immunotherapy for warts would need to be offered along with immediate treatment of the warts, and our preclinical studies had shown that immunotherapy in skin was more effective if local inflammation were present at the time of induced immunity.Citation10 Thus, a combined study of immunotherapy and conventional destructive treatment was developed.
Post hoc analysis of the results suggested that the benefit of VLP immunotherapy was not uniformly observed, but rather was restricted to patients treated with cryotherapy or podophyllotoxin, which on their own were relatively ineffective (). This association may explain the difference in observed VLP immunotherapy efficacy between the Australian and the Chinese study sites, as all Chinese subjects were treated with cautery, and had a good outcome without further intervention with VLP immunotherapy.
The nature of the cellular immune response required for clearance of genital warts in humans is unknown, though studies of clearance of cervical cancer associated persisting HPV infections suggest that helper T-cell responses to viral non-structural proteins correlate with virus clearance,Citation11 and the VLP immunotherapy intervention used in this study induced significant delayed type hypersensitivity to L1 protein(), which is a measure of induced CD4 T-cell mediated response to antigen. Natural resolution of dog and rabbit papillomavirus associated wartsCitation12 is also associated with CD4 mediated DTH responses to papillomavirus antigens. The major capsid protein, L1, is the only protein to which measurable humoral immune responses are commonly found in the course of genital wart infections in humans,Citation13 and we also observed weak DTH responses to L1 in placebo recipients suggesting that either wart disease or destructive treatment induces some cell mediated immunity, albeit insufficient for disease resolution. L1 protein is predominantly expressed in the superficial layers of the epidermis in the course of natural infection, where it would not be thought susceptible to cell mediated immune responses of the sort induced by VLP immunotherapy.Citation7 However, sufficient L1 protein for susceptibility to cell mediated immunity is presented by immature cells,Citation13,Citation14 and protein from immatureCitation14,Citation15 epithelial cells can be cross presented from skin by vascular endothelial cells to enable epithelial rejection.Citation16 As local inflammation has been shown important for local function of immune effector cells induced by vaccination,Citation17 the current study was designed to use VLPs as immunotherapy following conventional destructive therapy which was expected to induce the necessary local inflammation. Cryotherapy and podophyllotoxin, which on their own were relatively ineffective as therapy, are associated with evident local inflammation.
The current study failed to observe an association of recurrence with subject age or gender, or with the extent of disease at presentation, as has been observed in previous studies.Citation18,Citation19 Post hoc analysis of the results suggested that the benefit of VLP immunotherapy was greater in those with shorter disease duration (), in keeping with the concept that patients with prolonged refractory disease are more likely to have general or wart specific immune deficiencies that would be unlikely to respond to specific immunotherapy. Less effective destructive therapies (cryotherapy or podophyllotoxin) were improved with VLP immunotherapy in this trial. Demonstrating a therapeutic benefit for other treatments (cautery or trichloroacetic acid) may have been limited by the two month trial period. However, while a longer follow up would have been desirable, the two month follow up was a compromise between the desire to obtain a high percentage of patients reaching the defined study exit, and thus avoiding the bias associated with the tendency of patients with recurrence to return preferentially, and the desire for a clinically meaningful end point . In future studies of immunotherapy, an “off study” survey of participants at a longer time point after treatment would be warranted. In conclusion, immunotherapy warrants further trial as part of the management of recurrent genital warts being treated with destructive therapy, as a possible strategy to reduce the failure rate of primary destructive therapy.
Methods
Study design and patients
The study was a randomized placebo controlled dose ranging study of a VLP immunotherapy administered to patients undergoing destructive therapy for genital warts. Eligible subjects were those 18 y or over presenting with visible external genital wart disease of at least three months duration and treated with destructive therapy on at least one prior occasion that in the opinion of the treating physician could be removed by a single treatment course with a destructive therapy. Subjects were eligible if they were not immunosuppressed, had no other anogenital disease requiring treatment, and consented to both further destructive treatment and experimental immunotherapy. Patients were recruited and treated at two sexual health clinics in Brisbane, Australia and at two hospitals in Wenzhou, China. Demographics of recruited patients are shown in . Patients were randomly allocated to receive immunotherapy with HPV6b L1 virus like particles (VLP immunotherapy) or placebo immunotherapy (0.9% saline dispensed in a similar container) administered without adjuvant intramuscularly to the upper arm on two occasions one month apart, the first experimental treatment administered at the same visit as destructive therapy for their warts. Patients were randomized in blocks of 4 to receive VLP immunotherapy (three patients) or placebo immunotherapy (1 patient). Three dose levels of VLP immunotherapy were tested (1 μg, 5 μg and 25 μg). For each immunotherapy dose, a cohort of 40 patients of each gender was recruited at each study site (Brisbane and Wenzhou). After monitoring board review approval of safety data for each dose, recruitment at the next dose level for that gender at that site was commenced. Recruitment rates required modification of study design during the study such that once one site had recruited 40 evaluable patients of one gender for a dose level, recruitment for the next dose level was started at both sites. Sample size was designed to give an 80% probability of detecting a real 50% difference in outcome with an assumption of 50% cure through destructive therapy alone.
The study was conducted to good clinical practice (GCP) standards, with external data monitoring according to a protocol approved by the institutional ethics committees of each hospital and university at each site. The study was registered with the Australian Clinical Trials registry (registration number ACTRN 012605000674639 date submitted: 20/10/2005) prior to commencement of the study.
Randomization and masking
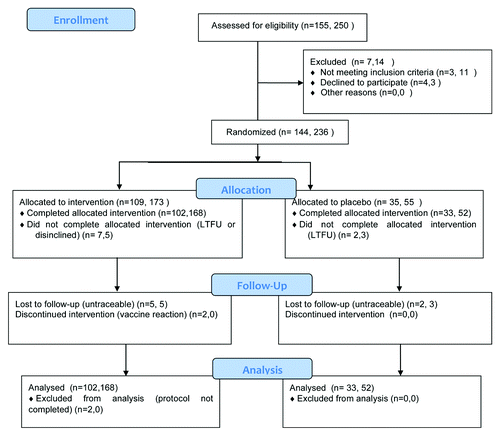
Consecutive patients attending the participating clinics for treatment of genital warts were offered participation if they fulfilled the wart related entry criteria, and were then screened for disqualifying conditions including abnormal hematology or biochemistry, a recent vaccination or evidence of immunosuppression. Immunotherapy was allocated to eligible patients sequentially by randomization number using consecutively numbered vials of stated dose, within which placebo vials were included in a predefined sequence generated by random number table, to which the data monitors, patients and investigators remained blinded until database lock. In Brisbane, of 151 patients accepting pre-screening (), 3 were not eligible (1 elevated CK, one recent influenza vaccine, 1 elevated LFTs), and 4 who met the entry criteria withdrew consent. The remaining 144 were assigned a randomization number. Nine patients failed to complete the protocol after randomization. Seven subjects failed to keep to the appointment schedule and were not included in the analysis. Two subjects declined the second vaccine after mild (grade 1–2) local reactions to the first vaccine, and were not included in the according to protocol (ATP) analysis. In Wenzhou, of 250 patients accepting pre-screening, 11 were not eligible (2 pregnancies, 2 anemia, 3 abnormal LFTs, 3 diabetes, one allergy to vaccines), and 3 declined to participate without further reason. Eight of 236 randomized patients in Wenzhou were lost to follow up after the first visit and treatment.
Procedures
The immunotherapy consisted of 1, 5 or 25 µg of HPV6b VLPs suspended in 1 ml of 0.9% NaCl without adjuvant or preservative. VLPs were produced to good laboratory practice (GLP) standards in insect cells using recombinant baculovirus, purified as previously described (Zhang et al.Citation9), subjected to 3 Grey of γ-irradiation and stored in aliquots at -80C until use. Batch release qualifications included electron microscopic appearance, immunogenicity, and sterility.
Wart destructive therapy was administered according to the current standard wart treatment at each trial site, with all visible disease treated. At the same visit the first dose of coded vaccine or placebo was administered by intramuscular injection. Four weeks later a second dose of vaccine or placebo was administered. After four more weeks disease status was assessed, and patients in Brisbane were tested for delayed type hypersensitivity to HPV6bL1 protein by intradermal injection of 10 μg of HPV 6b VLPs. Reactivity was read as the maximal diameter of induration at the site of skin test administration 48 h after injection.
Warts were assessed at each visit by magnification assisted visual inspection and recurrence assessed with reference to photographs and chart drawings
Statistical analysis
The predefined efficacy outcome measures specified in the protocol were disease status, classified as complete responder (CR) if there was no visible disease, a partial responder (PR) if total wart area was < 50% of the original pre-treatment area, and non-responder (NR) if the area was > 50% of the original area, and the percentage reduction of wart disease area from baseline. Predefined analysis for efficacy was by vaccine dose, subject gender, and treatment site (Brisbane or Wenzhou). Secondary analysis for efficacy was by subject age, by destructive treatment modality, and by wart duration and size at presentation. All analysis presented is for subjects treated according to protocol. Statistical analysis was conducted using Statistica V9 (Statsoft, Tuscon), using the ANOVA package for univariate and the General Linear Modeling package for multivariate analyses. Outcome comparisons were evaluated by Mann-Whitney U-test or t-test according to the nature of the data distribution.
Acknowledgments
This work was funded by grants from the National Health and Medical Research Council of Australia and the Wellcome Foundation to I.F. and J.L.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Contributors
From Princess Alexandra Sexual Health: Judy Nunn, Ricki Davis and Dee Archbold.
From Gold Coast Sexual Health Clinic: Fiona Clark and Denis Lester.
From Diamantina Institute: Yvonne Gautam.
From Wenzhou: Ms Huang Chao Xia, Mr Wang Rong Yue.
References
- Lacey CJ, Lowndes CM, Shah KV. Chapter 4: Burden and management of non-cancerous HPV-related conditions: HPV-6/11 disease. Vaccine 2006; 24:Suppl 3 S3 - , 35-41; http://dx.doi.org/10.1016/j.vaccine.2006.06.015; PMID: 16950016
- Garland SM, Steben M, Sings HL, James M, Lu S, Railkar R, et al. Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J Infect Dis 2009; 199:805 - 14; http://dx.doi.org/10.1086/597071; PMID: 19199546
- Insinga RP, Dasbach EJ, Elbasha EH, Liaw KL, Barr E. Incidence and duration of cervical human papillomavirus 6, 11, 16, and 18 infections in young women: an evaluation from multiple analytic perspectives. Cancer Epidemiol Biomarkers Prev 2007; 16:709 - 15; http://dx.doi.org/10.1158/1055-9965.EPI-06-0846; PMID: 17416761
- Jablonska S. Traditional therapies for the treatment of condylomata acuminata (genital warts). Australas J Dermatol 1998; 39:Suppl 1 S2 - 4; PMID: 9842092
- de la Fuente SG, Ludwig KA, Mantyh CR. Preoperative immune status determines anal condyloma recurrence after surgical excision. Dis Colon Rectum 2003; 46:367 - 73; http://dx.doi.org/10.1007/s10350-004-6557-6; PMID: 12626913
- Frazer IH. Prevention of cervical cancer through papillomavirus vaccination. Nat Rev Immunol 2004; 4:46 - 54; http://dx.doi.org/10.1038/nri1260; PMID: 14704767
- Peng SW, Frazer IH, Fernando GJ, Zhou J. Papillomavirus virus-like particles can deliver defined CTL epitopes to the MHC class I pathway. Virology 1998; 240:147 - 57; http://dx.doi.org/10.1006/viro.1997.8912; PMID: 9448699
- Kaufmann AM, Nieland JD, Jochmus I, Baur S, Friese K, Gabelsberger J, et al. Vaccination trial with HPV16 L1E7 chimeric virus-like particles in women suffering from high grade cervical intraepithelial neoplasia (CIN 2/3). Int J Cancer 2007; 121:2794 - 800; http://dx.doi.org/10.1002/ijc.23022; PMID: 17721997
- Zhang LF, Zhou J, Chen S, Cai LL, Bao QY, Zheng FY, et al. HPV6b virus like particles are potent immunogens without adjuvant in man. Vaccine 2000; 18:1051 - 8; http://dx.doi.org/10.1016/S0264-410X(99)00351-5; PMID: 10590325
- Bhat P, Mattarollo SR, Gosmann C, Frazer IH, Leggatt GR. Regulation of immune responses to HPV infection and during HPV-directed immunotherapy. Immunol Rev 2011; 239:85 - 98; http://dx.doi.org/10.1111/j.1600-065X.2010.00966.x; PMID: 21198666
- van der Burg SH, de Jong A, Welters MJ, Offringa R, Melief CJ. The status of HPV16-specific T-cell reactivity in health and disease as a guide to HPV vaccine development. Virus Res 2002; 89:275 - 84; http://dx.doi.org/10.1016/S0168-1702(02)00196-X; PMID: 12445667
- Jain S, Moore RA, Anderson DM, Gough GW, Stanley MA. Cell-mediated immune responses to COPV early proteins. Virology 2006; 356:23 - 34; http://dx.doi.org/10.1016/j.virol.2006.07.032; PMID: 16949120
- Wikström A, Eklund C, von Krogh G, Lidbrink P, Dillner J. Antibodies against human papillomavirus type 6 capsids are elevated in men with previous condylomas. APMIS 1997; 105:884 - 8; http://dx.doi.org/10.1111/j.1699-0463.1997.tb05098.x; PMID: 9393560
- Bellone S, El-Sahwi K, Cocco E, Casagrande F, Cargnelutti M, Palmieri M, et al. HPV16 VLP L1-Specific CD8+ Cytotoxic T Lymphocytes (CTL) are equally effective as E7-Specific CD8+ CTL in killing autologous HPV16 Positive Tumor Cells in Cervical Cancer Patients: Implications for L1 dendritic cell-based Therapeutic Vaccines. The Journal of Virology 2009; 38:JVI
- Passmore JA, Milner M, Denny L, Sampson C, Marais DJ, Allan B, et al. Comparison of cervical and blood T-cell responses to human papillomavirus-16 in women with human papillomavirus-associated cervical intraepithelial neoplasia. Immunology 2006; 119:507 - 14; http://dx.doi.org/10.1111/j.1365-2567.2006.02465.x; PMID: 17026720
- Valujskikh A, Lantz O, Celli S, Matzinger P, Heeger PS. Cross-primed CD8(+) T cells mediate graft rejection via a distinct effector pathway. Nat Immunol 2002; 3:844 - 51; http://dx.doi.org/10.1038/ni831; PMID: 12172545
- Zhong J, Hadis U, De Kluyver R, Leggatt GR, Fernando GJ, Frazer IH.. TLR7 stimulation augments T effector-mediated rejection of skin expressing neo-self antigen in keratinocytes. Eur. J Immunol 2008; 38:73 - 81
- Rasi A, Soltani-Arabshahi R, Khatami A. Cryotherapy for anogenital warts: factors affecting therapeutic response. Dermatol Online J 2007; 13:2; PMID: 18318999
- Czelusta AJ, Evans T, Arany I, Tyring SK. A guide to immunotherapy of genital warts: focus on interferon and imiquimod. BioDrugs 1999; 11:319 - 32; http://dx.doi.org/10.2165/00063030-199911050-00004; PMID: 18031142