Abstract
Background
Recently, more clinical trials are being conducted in Africa and Asia, therefore, background morbidity in the respective populations is of interest. Between 2000 and 2007, the International AIDS Vaccine Initiative sponsored 19 Phase 1 or 2A preventive HIV vaccine trials in the US, Europe, Sub-Saharan Africa and India, enrolling 900 healthy HIV-1 uninfected volunteers.
Objective
To assess background morbidity as reflected by unsolicited adverse events (AEs), unrelated to study vaccine, reported in clinical trials from four continents.
Methods
All but three clinical trials were double-blind, randomized and placebo-controlled. Study procedures and data collection methods were standardized. The frequency and severity of AEs reported during the first year of the trials were analyzed. To avoid confounding by vaccine-related events, solicited reactogenicity and other AEs occurring within 28 d after any vaccination were excluded.
Results
In total, 2134 AEs were reported by 76% of all participants; 73% of all events were mild. The rate of AEs did not differ between placebo and vaccine recipients. Overall, the percentage of participants with any AE was higher in Africa (83%) compared with Europe (71%), US (74%) and India (65%), while the percentage of participants with AEs of moderate or greater severity was similar in all regions except India. In all regions, the most frequently reported AEs were infectious diseases, followed by gastrointestinal disorders.
Conclusions
Despite some regional differences, in these healthy participants selected for low risk of HIV infection, background morbidity posed no obstacle to clinical trial conduct and interpretation. Data from controlled clinical trials of preventive interventions can offer valuable insights into the health of the eligible population.
Introduction
In recent years, there has been increasing interest in conducting clinical research in Africa and Asia, partly because of increased investment in treatment and prevention of diseases of poverty, such as AIDS, malaria, tuberculosis and other neglected diseases.Citation1 Concerns have been raised about conducting human trials in less developed and developing countries, because of a perception that persons in these countries have higher background morbidity and/or a compromised health status. If this perception were true, it could lead to increased frequency of adverse events (AEs) unrelated to the investigational product, and difficulties in assessing the product’s safety. Nevertheless, countries and regions with high disease burden need to participate in research and development for new vaccines and drugs to assure that data are applicable to the respective populations.
Between 2000 and 2007, the International AIDS Vaccine Initiative (IAVI) sponsored 19 Phase 1 or 2A clinical trials testing different HIV-1 candidate vaccines and enrolling a total of 900 HIV-1 uninfected volunteers at low risk for HIV acquisition in the US, Europe, Eastern and Southern Africa and India. The purpose of this manuscript is to describe frequency and severity of background morbidity, as shown in unsolicited adverse events [AE, as defined per International Conference on Harmonisation—Good Clinical Practice (ICH-GCP)] collected during the first 12 mo after the initial injection of study vaccine or placebo. To avoid potential confounding effects of vaccine-related events, solicited reactogenicity and other AEs occurring within 28 d after any administration were excluded. We postulate that AEs occurring distant from vaccination are likely indicative of background morbidity. The findings support the validity of conducting early phase clinical trials in middle and low income countries.
Results
Study population
Nine hundred healthy, HIV-seronegative study participants were enrolled at 21 collaborating research centers (CRCs) in 11 countries on 4 continents (). The publications resulting from these studies are referenced in the table.
Table 1. Number of volunteers enrolled by region and collaborating research center
Demographic characteristics
There were significant differences in the distribution of gender, age, race and BMI (all p < 0.0001) between the four regions (). Overall, 383 (42.6%) females and 517 (57.4%) males were enrolled. There was a significantly higher proportion of male participants in Africa (66.4%) compared with Europe (50.3%), US (49.2%) or India (53.2%). Overall, the highest proportion of participants was between 18–25 y of age and African CRCs enrolled a higher proportion of individuals between 18–25 y of age than other centers. The highest proportion of participants > 46 y (24.8%) was enrolled in Europe. The majority of volunteers had a BMI between 18.5 and 24.9. More than 25% of US volunteers had a BMI over 30, and approximately 13% of Indian volunteers had a BMI below 18.5. The median BMI for volunteers in the US was significantly higher than the median BMI for other regions.
Table 2. Demographics
Terminations and altered vaccination schedules by region
Eight hundred sixty-two (96%) participants completed all study visits on the planned schedule. The percentage of participants who completed the studies in Africa (97.5%) and India (98.4%) was higher than the rate in Europe (93.9%) and the US (93.9%). Thirty-eight (4%) volunteers terminated their participation early [Europe: n = 19 (6%), US: n = 8 (6%), Africa: n = 10 (3%), India: n = 1 (2%)]. The most common reasons were loss to follow up (n = 14) and withdrawal of consent (n = 10). Three fatal SAEs unrelated to study vaccine and one HIV infection resulted in early terminations. (Table SA). No other SAEs resulted in early terminations.
Seven hundred fifteen (79%) participants completed their vaccination schedule per protocol. Reasons for altered vaccination schedules (n = 185) included investigator/study decision (n = 13), pregnancy (n = 7), volunteer refusal (n = 6), pre-existing undiagnosed and other illnesses (n = 7), AEs (n = 4), missed vaccination visit (n = 2); in addition, a brief regulatory “hold” was imposed on two studies due to a preclinical finding in experiments with a related but different vaccine, resulting in the largest number of missed visit windows [n = 128/185 (69%)]. (Table SB)
Unsolicited adverse events ( and )
Table 3. Severity and relationship of reported AEs*, overall and by region
Table 4. Logistic regression analysis of experiencing moderate or above AE
There was no significant difference in the rate of unsolicited AEs beyond 28 d post-vaccination between placebo and vaccine recipients (data not shown); hence, they were combined for all the analyses. In total, 2134 AEs were reported by 76% (686/900) of participants. The overall rate of adverse events was 3.78, 2.70, 2.38 and 2.37 per person-year for Africa, Europe, US and India, respectively. The respective rates for moderate or greater AEs were 1.06, 0.82, 0.62 and 0.27 per person-year.
AEs by severity, relationship and age group, overall and by region ( and )
Figure 1. Region and age group were evaluated in a multivariate logistic regression model as potential predictors of experiencing moderate or above AE (see ). The covariates were evaluated in a multivariate model if the corresponding p value was less than 0.1 from the univariate model. The numbers above each bar indicates the number of volunteers in that group.
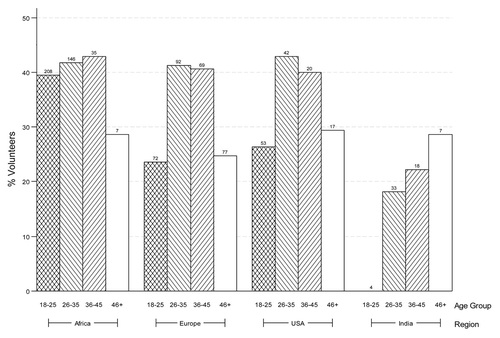
Overall, 73% (n = 1548) of the AEs were mild, 24% (n = 519) moderate and 3% (n = 67) severe or very severe. 97% (n = 2078) of the AEs were assessed by the investigator as unrelated or unlikely related to study product. Overall, the proportion of participants with any AEs was higher in Africa (83%) compared with Europe (71%), US (74%) or India (65%) (p = 0.0001).
In univariate models
Region was significantly associated with experiencing moderate or greater AEs (p = 0.01) while age group was marginally significant (p = 0.06). Body Mass Index (p = 0.48) and gender (p = 0.50) were not significantly associated with moderate or greater AE in univariate analysis, and therefore were not included in the multivariate model ().
Multivariate logistic regression analyses
Both region (p = 0.005) and age group (p = 0.03) were significantly associated with experiencing moderate or greater AEs. Africa (40%, p = 0.001), Europe (33%, p = 0.01) and US (34%, p = 0.02) had significantly higher proportions of volunteers with moderate or greater AEs compared with India (19%); the rate in Africa was not significantly different from the rate in Europe (p = 0.06) or US (p = 0.21). Compared with age groups 18–25 (34%) and > 46 (26%), age groups 26–35 (39%, p = 0.03 and 0.048, respectively) and 36–45 (39%, p = 0.047 and 0.04, respectively) had significantly higher proportions of volunteers with moderate or greater AEs.
Frequency of moderate or greater AEs by MedDRA system organ class (SOC). ()
Table 5. Moderate or greater AEs by MedDRA SOC level summary*
Moderate or greater AEs (n = 586) by frequency of their SOC, and the percentage of all AEs reported in each region are shown in . For Infections and Infestations (42% of all moderate or greater events), the rate was significantly lower in India (4.9%) compared with Africa (13.1%), Europe (10.6%) and US (9.4%) (p = 0.02). The differences between Africa, Europe and US were not significant. Upper respiratory tract infection was the most common clinical diagnosis.
For gastrointestinal disorders (8.5% of all moderate or greater AEs), there was a statistically significant difference between regions (p = 0.004): Europe: 3.8%, Africa: 2.2%, US: 0.4%, India: 0%. Dyspepsia, diarrhea and peptic ulcer were the most common single diagnoses. general disorders/administration site disorders (e.g., cold/flu-like symptoms) and injury/poisoning/procedural complications (e.g., fractures, soft tissue injuries and strains/sprains) each accounted for 6% of moderate or greater AEs and musculoskeletal/connective tissue disorders for 5.6%. There were no statistically significant differences between regions for these AEs. The remaining classifications were too infrequent to examine statistically for regional differences.
Laboratory abnormalities
Overall, laboratory abnormalities accounted for less than 5% of AEs reported; 41% of these were of moderate or greater severity. They were classified under the following three SOC: (1) blood and lymphatic system disorders; (2) investigations and (3) hepatobiliary disorders. Most laboratory abnormalities were isolated, judged clinically not significant and resolved spontaneously. There was no consistent pattern and, overall, no significant regional difference in the rate of moderate or greater laboratory abnormalities. The most common abnormalities were (1) decreased absolute neutrophil count; (2) increased bilirubin level; (3) increased alanine aminotransferase (ALT) level; (4) decreased hemoglobin level; and (5) decreased platelet count. (Table SC)
Serious AEs. ()
Table 6. SAEs (beyond 28 d) by MedDRA SOC*
Forty-five serious adverse events (SAEs) were reported in the specified period. None were considered definitely, probably or possibly related to study product. The percentage of volunteers with SAEs was similar among the four age groups (p = 0.14); it was significantly higher for India (11%) compared with Africa (5%), Europe (4%) or US (2%) (p = 0.04). Hospitalization was the most common reason for an event being serious. Most common SAE diagnoses in were infectious diseases. Three deaths occurred (one suicide each in the US and in Europe, one viral encephalitis in Africa). (Table SD)
Concomitant medications
The most commonly prescribed medications were analgesics/non-steroidal anti-inflammatory drugs (NSAID) followed by antibiotics/anti-infectives/antifungals.
Discussion
Over the past decade, in Africa and India, IAVI has undertaken a significant effort to develop capacity to conduct clinical trials with preventive HIV vaccine candidates. The study vaccines were safe and well-tolerated.Citation2-Citation13 A common notion was that early phase clinical trials should be done in industrialized countries, in part due to concerns about co-morbidity and compromised health status of individuals residing in middle- or low-income countries. This report demonstrates that carefully selected individuals recruited into Phase 1 and 2A trials in sub-Saharan countries and in India have a similar health status to study participants in Europe and the US. Follow-up and compliance were equally good in Africa and India as in the US and in Europe.
The premise of this analysis is that by excluding the 28 d post-vaccination, only AEs representing background morbidity would be included in the analysis; this premise is supported by the lack of significant difference between the rates of AEs in vaccine and placebo recipients. In addition, no patterns of unexpected or late AEs due to vaccines were identified in any of these studies.Citation2-Citation13
The most common AEs reported in each of the regions were infectious diseases. Although overall, the proportion of participants with AEs was higher in Africa, this was mostly attributable to infectious diseases that were short-lived, easily manageable and not related to respective study vaccines. Infectious diseases, such as influenza, other viral infections or malaria, can mimic severe reactions to vaccination, and diligence is required in all settings to exclude such confounding factors.
In the absence of laboratory reference ranges for African and Indian populations, reference ranges derived from Caucasian populations were used in these trials. IAVI recently sponsored a study in several African countries to establish in healthy individuals local reference ranges of laboratory parameters,Citation14 and other studies of this type have been published for African populations.Citation15 Although, in the studies reported here, laboratory abnormalities accounted only for a small percentage of all AEs, most of the mild abnormalities would have been considered within normal limits, had local reference ranges been used. Nevertheless, it is important to be cognizant, especially, of anemia in women, since setting exclusion criteria according to standard US or European values may make enrolment of healthy women in low- or middle-income country settings difficult.
This report has several limitations. Although event evaluation criteria, data collection methods and investigator training were largely standardized, the level of investigator experience differed between CRCs. Cultural perceptions about clinical events and medical care also differ between the regions. Hence, both medical practitioners and study volunteers in different regions may judge similar events somewhat differently. This variation was kept to the minimum by use of a standard table for defining and grading severity of events, routine reviews and discussions between site investigators and IAVI teams, and careful review by Safety Monitoring Boards or Committees. Using our data, it was not possible to compare by continent the volunteers who were screened out, as recruitment methods differed substantially, and data on medical history or examination were not collected in sufficient detail, or in a standardized way, across all studies.
The number of CRCs participating and number of participants enrolled is greatest in Africa and Europe, and least in India; thus, the data from Africa and Europe may be the most robust.
Another potential limitation is the possibility that AEs causally related to study vaccines could manifest more than 28 d post-vaccination. However, this is unlikely an important factor, as the overall rates of events between vaccinated and placebo recipients did not differ, no pattern of related AEs emerged in any study, and most study vaccines caused reactogenicity only within the first few days after vaccination.Citation2-Citation13 A number of individuals did not receive all vaccinations per protocol, however the changes in vaccination schedules are unlikely to affect the conclusions, because the most were due to an administrative delay in vaccination rather than failure to deliver the requisite number of vaccinations.
Overall Conclusion
Concerns that Phase I and II studies in Africa and India will be confounded by background morbidity in carefully selected, healthy participants are not warranted. Our data suggest that background morbidity, as described by unsolicited AEs reported by clinical trial participants from different geographic regions, is generally similar and within acceptable limits for these healthy individuals at low risk of HIV infection on four continents. When investigational vaccines are studied in the regions for which they are ultimately intended, data on both safety and immunogenicity are potentially more relevant than data from dissimilar populations. Furthermore, the studies build capacity and expertise in clinical and laboratory teams and regulatory bodies.Citation16 In addition, study volunteers can benefit from trial participation through regular medical checkups, better access to health care and clinic referrals as needed, family planning, HIV counseling and testing. Conduct of such studies under the auspices of the US FDA or European authorities, as well as the responsible national authorities in low- or middle-income countries, helps to ensure that the data are accepted worldwide for registration purposes.
As evidenced by this study and others, the volunteers enrolled in clinical trials provide a rich potential source of epidemiologic information. Volunteers enrolled in Phase 1 vs. efficacy studies of preventive vaccines or other interventions for HIV and other infectious diseases may have different health status from low-risk volunteers, and volunteers may differ from the general population, whether in industrialized or less-developed countries. A coordinated effort, by investigators and sponsors of multiple larger clinical trials, to plan for analysis of pooled data on baseline health status and events over time could provide useful information on the prevalence and incidence of other infectious and non-infectious diseases to guide future health interventions.
Materials and Methods
Study population
Healthy, HIV-seronegative adults were enrolled into clinical trials with different HIV-1 candidate vaccines. Eligible participants, between 18 and 60 y of age, provided written informed consent, were willing to undergo HIV testing and receive results. Sexually active participants agreed to use effective contraceptive methods for at least 4 mo after the last vaccination. All potential participants were screened for acute and chronic diseases through medical history and physical examination. Routine laboratory parameters included complete blood count and differential, clinical chemistry (ALT/AST, creatinine) and urinalysis. Exclusion criteria included chronic medical conditions, clinically significant laboratory abnormalities, prevalent HIV-1 or HIV-2 infection, risk behavior for HIV acquisition, positive Hepatitis B surface antigen or Hepatitis C antibody, active untreated syphilis, pregnancy or lactation, clinical signs of active tuberculosis, and recent receipt of blood transfusion or blood products.
Study designs
Sixteen Phase 1 and 2A preventive HIV vaccine trials were double-blind, randomized, and placebo-controlled; three were small open trials. Follow-up for safety varied between 12 and 18 mo after enrolment, but only the first 12 mo are included in this report for uniformity. The study vaccines were based on DNA plasmids or were replication-incompetent vectors, such as modified vaccinia Ankara, adeno-associated virus serotype 2 or adenovirus serotype 5. ()
Approvals
All study protocols were approved by the respective institutional, national and international ethical, scientific and regulatory authorities. Studies were conducted according to ICH-GCP guidelines and accepted ethical guidelines. All participants provided written informed consent after a thorough discussion of risks, benefits and procedures.
Study procedures/clinical evaluations
Health status of study participants was monitored by medical history, physical examination and routine laboratory parameters. At scheduled clinic visits, participants were asked to report adverse events they experienced since the previous scheduled visit, and they were encouraged to contact the clinic if they became ill. Spontaneously reported (unsolicited) AEs were graded for severity using standard criteria predefined in the respective protocols and the DAIDS Table for Grading the Severity of Adult and Pediatric Adverse Events,Citation17 and assessed for relationship to study product. Laboratory abnormalities were reported as AEs, if severe, serious or judged clinically significant. (Categories for severity: mild = grade 1, moderate = grade 2, severe = grade 3, very severe or potentially life-threatening = grade 4. Categories for relationship: definitely, probably, possibly, probably not/unlikely, not related). All AEs were followed until resolution or stabilization. Investigators, study physicians and nurses received specific training on safety reporting. Throughout the study period, medical study personnel were accessible either in the respective clinic or outside working hours by cell phone for investigation of any complaint or clinical event, as well as for care, treatment and referrals, as appropriate.
Safety data were reviewed regularly by independent Safety Review Boards/Data and Safety Monitoring Boards. All AEs were coded to a Preferred Term (PT) and assigned to a System Organ class (SOC) by MedDRA (Medical Dictionary for Regulatory Activities) software. Coding was reviewed by physicians at IAVI and the Statistical and Data Center.
Statistical considerations
The main outcomes of interest were the proportion of volunteers with moderate or greater AE and the proportion of volunteers with any AE. To avoid potential confounding effects of vaccine-related events, solicited reactogenicity and other AEs occurring within 28 d after any administration were excluded. We postulate that AEs occurring distant from vaccination are likely indicative of background morbidity. Additional analyses included the frequency of AEs and the proportion of volunteers with SAE. For each region, the overall rate of adverse events per person-year was calculated as the total number of adverse events in that region divided by the total duration of follow-up in years, after excluding the 28-d post-vaccination periods.
All statistical comparisons (except the frequency) of AEs were based on the maximum severity per participant recorded at clinic visits. Comparisons of categorical and continuous factors were conducted using the Fisher’s exact test and Wilcoxon rank-sum test, respectively. A two-sided p-value of less than 0.05 was considered to be statistically significant. Statistical analyses were performed using SAS version 9.2, Cary, NC.
Geographic region (Europe, Africa, USA and India), Body Mass Index (BMI), gender and age group were evaluated in univariate and multivariate logistic regression models as potential predictors of experiencing moderate or greater AEs. The covariates were evaluated in a multivariate model if the corresponding p value was less than 0.1 in the univariate model.
Additional material
Download Zip (247.7 KB)Acknowledgments
We thank all the study participants, the study physicians and research teams at the collaborating research centers for their outstanding work and dedication, and to the respective Safety Review Boards for overseeing the studies. We also thank the IAVI Clinical Program Managers (Wendy Komaroff, Jennifer Lehrman, Helen Thomson, Dani Vooijs), the Data Management group (Carl Verlinde, Sarah Yates) the IAVI study monitors and Sabrina Welsh. Dr Gwynneth Stevens and Paramesh Chetty oversaw safety laboratory accreditation and quality assurance in Africa and India. We extend our thanks also to Drs Elizabeth Adams and Matt Price for careful review of the manuscript.
Funding
All studies were sponsored by the International AIDS Vaccine Initiative and funded by its donors, including the US Agency for International Development (USAID Cooperative Agreement Number GPO-A-00–06–00006–00), the Governments of Canada, Denmark, Ireland, The Netherlands, Norway, Sweden, the UK, the Basque Autonomous Government, the European Union and the Bill and Melinda Gates Foundation.
The contents of this manuscript are the responsibility of IAVI and do not necessarily reflect the views of USAID or the US government.
For more information, see www.iavi.org.
Disclosure of Potential Conflicts of Interest
None of the co-authors reports any potential conflict of interest that might influence their scientific judgment and interfere with their objective assessment of this manuscript.
The EMMES Corporation is a Contract Research Organization, to which IAVI subcontracted data coordination, management and analysis. The EMMES Corporation played no role as funder.
Financial Disclosure
IAVI is a non-profit organization.
Supplemental Materials
Supplemental materials may be found here: www.landesbioscience.com/journals/vaccines/article/19454
References
- Millennium Development Goals for Health: What Will It Take to Accelerate Progress? Wagstaff A, Claeson M, Hecht RM, Gottret P, Fang Q. http://www.ncbi.nlm.nih.gov/pubmed?term=%22Jamison%20DT%22%5BEditor%5D&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVAbstract Disease Control Priorities in Developing Countries. 2nd edition. Washington (DC): World Bank; 2006. Chapter 9.
- Cebere I, Dorrell L, McShane H, Simmons A, McCormack S, Schmidt C, et al. Phase I clinical trial safety of DNA- and modified virus Ankara-vectored human immunodeficiency virus type 1 (HIV-1) vaccines administered alone and in a prime-boost regime to healthy HIV-1-uninfected volunteers. Vaccine 2006; 24:417 - 25; http://dx.doi.org/10.1016/j.vaccine.2005.08.041; PMID: 16176847
- Peters BS, Jaoko W, Vardas E, Panayotakopoulos G, Fast P, Schmidt C, et al. Studies of a prophylactic HIV-1 vaccine candidate based on modified vaccinia virus Ankara (MVA) with and without DNA priming: effects of dosage and route on safety and immunogenicity. Vaccine 2007; 25:2120 - 7; http://dx.doi.org/10.1016/j.vaccine.2006.11.016; PMID: 17250931
- Guimarães-Walker A, Mackie N, McCormack S, Hanke T, Schmidt C, Gilmour J, et al, IAVI-006 Study Group. Lessons from IAVI-006, a phase I clinical trial to evaluate the safety and immunogenicity of the pTHr.HIVA DNA and MVA.HIVA vaccines in a prime-boost strategy to induce HIV-1 specific T-cell responses in healthy volunteers. Vaccine 2008; 26:6671 - 7; http://dx.doi.org/10.1016/j.vaccine.2008.09.016; PMID: 18812202
- Jaoko W, Nakwagala FN, Anzala O, Manyonyi GO, Birungi J, Nanvubya A, et al. Safety and immunogenicity of recombinant low-dosage HIV-1 A vaccine candidates vectored by plasmid pTHr DNA or modified vaccinia virus Ankara (MVA) in humans in East Africa. Vaccine 2008; 26:2788 - 95; http://dx.doi.org/10.1016/j.vaccine.2008.02.071; PMID: 18440674
- Goonetilleke N, Moore S, Dally L, Winstone N, Cebere I, Mahmoud A, et al. Induction of multifunctional human immunodeficiency virus type 1 (HIV-1)-specific T cells capable of proliferation in healthy subjects by using a prime-boost regimen of DNA- and modified vaccinia virus Ankara-vectored vaccines expressing HIV-1 Gag coupled to CD8+ T-cell epitopes. J Virol 2006; 80:4717 - 28; http://dx.doi.org/10.1128/JVI.80.10.4717-4728.2006; PMID: 16641265
- Mehendale S, van Lunzen J, Clumeck N, Rockstroh J, Vets E, Johnson PR, et al. A phase 1 study to evaluate the safety and immunogenicity of a recombinant HIV type 1 subtype C adeno-associated virus vaccine. AIDS Res Hum Retroviruses 2008; 24:873 - 80; http://dx.doi.org/10.1089/aid.2007.0292; PMID: 18544020
- Ramanathan VD, Kumar M, Mahalingam J, Sathyamoorthy P, Narayanan PR, Solomon S, et al. A Phase 1 study to evaluate the safety and immunogenicity of a recombinant HIV type 1 subtype C-modified vaccinia Ankara virus vaccine candidate in Indian volunteers. AIDS Res Hum Retroviruses 2009; 25:1107 - 16; http://dx.doi.org/10.1089/aid.2009.0096; PMID: 19943789
- Vasan S, Schlesinger SJ, Chen Z, Hurley A, Lombardo A, Than S, et al. Phase 1 safety and immunogenicity evaluation of ADMVA, a multigenic, modified vaccinia Ankara-HIV-1 B’/C candidate vaccine. PLoS One 2010; 5:e8816; http://dx.doi.org/10.1371/journal.pone.0008816; PMID: 20111599
- Vasan S, Schlesinger SJ, Huang Y, Hurley A, Lombardo A, Chen Z, et al. Phase 1 safety and immunogenicity evaluation of ADVAX, a multigenic, DNA-based clade C/B’ HIV-1 candidate vaccine. PLoS One 2010; 5:e8617; http://dx.doi.org/10.1371/journal.pone.0008617; PMID: 20111582
- Vardas E, Kaleebu P, Bekker LG, Hoosen A, Chomba E, Johnson PR, et al. A Phase 2 Study to Evaluate the Safety and Immunogenicity of a Recombinant HIV-1 Vaccine Based on Adeno-Associated Virus. AIDS RHR 2010; 26:933 - 42; PMID: 20666584
- Jaoko W, Karita E, Kayitenkore K, Omosa-Manyonyi G, Allen S, Than S, et al. Safety and immunogenicity study of Multiclade HIV-1 adenoviral vector vaccine alone or as boost following a multiclade HIV-1 DNA vaccine in Africa. PLoS One 2010; 5:e12873; http://dx.doi.org/10.1371/journal.pone.0012873; PMID: 20877623
- Vasan S, Hurley A, Schlesinger SJ, Hannaman D, Gardiner DF, Dugin DP, et al. In vivo electroporation enhances the immunogenicity of an HIV-1 DNA vaccine candidate in healthy volunteers. PLoS One 2011; 6:e19252; http://dx.doi.org/10.1371/journal.pone.0019252; PMID: 21603651
- Karita E, Ketter N, Price MA, Kayitenkore K, Kaleebu P, Nanvubya A, et al. CLSI-derived hematology and biochemistry reference intervals for healthy adults in eastern and southern Africa. PLoS One 2009; 4:e4401; http://dx.doi.org/10.1371/journal.pone.0004401; PMID: 19197365
- Lugada ES, Mermin J, Kaharuza F, Ulvestad E, Were W, Langeland N, et al. Population-based hematologic and immunologic reference values for a healthy Ugandan population. Clin Diagn Lab Immunol 2004; 11:29 - 34; PMID: 14715541
- Van den Broeck J, Robinson AKL. Towards research equity -- challenges of safety monitoring during clinical trials in resource-limited settings. West Indian Med J 2007; 56:163 - 5; http://dx.doi.org/10.1590/S0043-31442007000200011; PMID: 17910148
- DAIDS Table for Grading the Severity of Adult and Pediatric Adverse Events. . www.rsc-tech.com