Abstract
Background
Adult and elderly subjects previously immunized with cell culture-derived (CCIV; Optaflu®) or egg-derived (TIV; Agrippal®) trivalent influenza vaccines were enrolled in two extension studies (E1 and E2) to evaluate safety and immunogenicity after revaccination with CCIV/TIV alone or in combination with concomitant pneumococcal vaccine (PV).
Methods
Adults and elderly subjects (n = 2609) were randomized 1:1 in E1 and allocated 3:1 in E2 to receive CCIV/TIV. In E2, a subset of elderly subjects was randomized to receive CCIV/TIV, with or without PV. Adverse reactions were monitored for six months and immunogenicity was assessed by hemagglutination inhibition (HI) assay using CHMP criteria.
Results
Overall, the safety profile of both vaccines was similar, no serious adverse events related to either vaccine occurred. Mild or moderate pain was the most commonly reported reaction. Reactogenicity was slightly higher in elderly subjects receiving CCIV/TIV concomitantly with PV [46% vs. 37%; p = non-significant (NS)]. Both vaccines met CHMP licensure criteria for adults and elderly subjects. With concomitant CCIV and PV, all three CHMP criteria were met for A/H1N1 and A/H3N2, whereas the B strain only met seroprotection and GMR criteria.
Conclusions
Safety and immunogenicity of CCIV was not influenced by the type of vaccine received previously or by concomitant PV administration.
Background
Influenza is a major global health concern, particularly in high-risk groups such as the elderly, causing considerable morbidity and mortality.Citation1-Citation3Vaccination is the most effective method of preventing seasonal influenza and reducing influenza-associated morbidity and mortality.Citation4
Conventionally influenza vaccines are developed in embryonated hens’ eggs, but limitations associated with the use of eggs include long lead times,4 growth constraints for some strains.Citation5,Citation6 and limited scope for scale-up of the manufacturing process.Citation7 To address these limitations, the World Health Organization (WHO) has recommended development of alternative systems for influenza virus cultivation, specifically mammalian cell culture.Citation8
Several cell lines have been investigated as substrates for the manufacture of influenza vaccine, including Madin–Darby canine kidney (MDCK), PER.C6®, and Vero cells.Citation9-Citation11In comparison with other cell lines such as Vero cells, MDCK cells are more sensitive in the isolation of influenza virus strains A .Citation12 The MDCK cell line successfully supports influenza virus growth and is the most well-defined and accepted cell substrate to grow influenza viruses in large quantities. Furthermore, a MDCK cell culture-derived vaccines has demonstrated a good safety profile, was immunogenic according to regulatory authorities’ criteria, and was as efficacious as a licensed egg-based vaccineCitation10,Citation13-Citation18.
Novartis Vaccines has developed several influenza vaccines using MDCK cell culture including a trivalent seasonal subunit vaccine, Optaflu®, and an A/H1N1v pandemic vaccine, Celtura™ as well as a candidate H5N1.Citation19 In June 2007, the European Medicines Agency (EMA) approved the cell culture-derived influenza trivalent subunit vaccine (CCIV; Optaflu) for use in adults.Citation10,Citation14The safety, immunogenicity and efficacyCitation18 of this CCIV has been demonstrated in several clinical trials in adult and elderly subjects in Europe and the US.Citation13,Citation15-Citation17
As use of CCIV increases in routine medical practice, people may receive different influenza vaccines produced using cells or eggs, across different influenza seasons. In 2004–2005 a phase III trial demonstrated CCIV was safe, immunogenic and non-inferior to the licensed egg-derived trivalent subunit vaccine (TIV; Agrippal®).Citation17 In this paper we report two extension studies from that trial conducted to evaluate the long-term safety and immunogenicity of CCIV when administered alone or in combination with a 23-valent polysaccharide pneumococcal vaccine (PV, sanofi pasteur) in adult or elderly subjects who previously received CCIV or TIV in the parent study.Citation17 As influenza and pneumococcal vaccines are routinely recommended for older adults and they often are given together, we also examined the reactogenicity of concomitant administration of influenza and PV vaccine and its impact on the influenza antibody response.
Results
Subjects
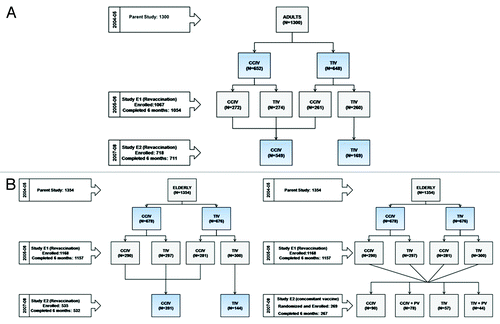
A total of 2235 participants were enrolled and randomized into study E1, of whom 2211 (1054 adults and 1157 elderly) participants completed the study according to protocol ( and B). Of these, 1522 participants were enrolled in study E2 and allocated to two different safety assessment groups - the revaccination group (1253) and the concomitant vaccination with PV group (269). Of these, 1243 (adults 711 and elderly 532) and 267 (elderly) participants, respectively, completed the study according to the protocol ( and B).
Figure 1. (A) Flow of adult subjects across study E1 and E2. (B) Flow of elderly subjects across study E1 and E2.
Demographic and baseline characteristics were generally similar among vaccine groups in both studies (). All the participants were Caucasians.
Table 2. Demographic characteristics across studies E1 and E2
Safety and reactogenicity
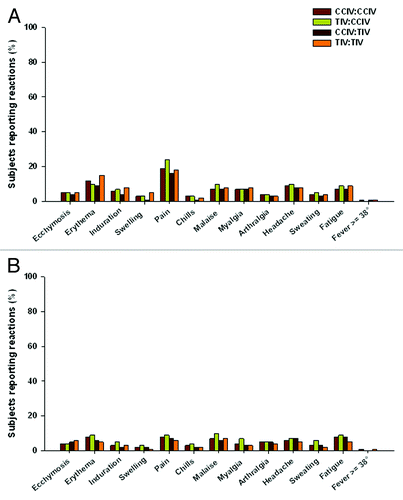
Observed safety and reactogenicity profiles in both studies were generally similar across the individual vaccine groups (–). In both studies, CCIV and TIV influenza vaccines were well tolerated and their reactogenicity profiles were not affected by the type of vaccine received during the previous influenza seasons.
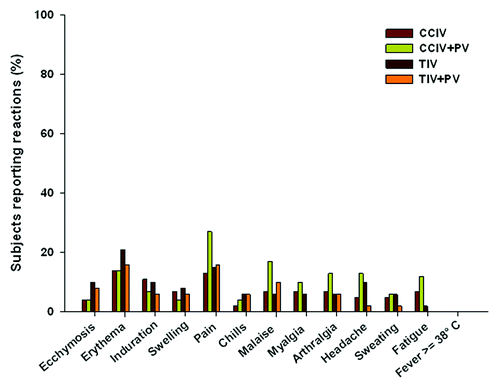
Figure 4. Local and systemic reactions in elderly subjects – study E2 (randomized concomitant vaccine group).
Weeks 1–3 (revaccination—with influenza vaccine): In both studies the percentages of subjects reporting local and systemic reactions were similar between vaccine groups of the same age, with elderly subjects reporting a lower frequency of reactions than adults ( and). Injection site pain was the most commonly reported local reaction in adults and elderly subjects in both studies; however, the percentage of elderly subjects experiencing pain was lower than in adults. In both studies the majority of pain was mild to moderate in both age groups, severe pain incidence being < 1% . The percentage of subjects reporting pain was similar in CCIV and TIV groups in E1 (adults: CCIV 22%; TIV 17% p = non-significant (NS) and elderly: CCIV 8%; TIV 7% p = NS) and in adults in E2 (CCIV 29%; TIV 30%, p = NS), but significantly higher with CCIV (16%) in elderly subjects in E2, than with TIV (8%, p = 0.026). Headache, fatigue, malaise, and myalgia were the most commonly reported systemic reactions in adults and elderly subjects for both vaccines in both studies. Most systemic reactions were mild or moderate in severity and of short duration. Generally, systemic reactions peaked between 6 h to 2 d and subsided between 3 to 7 d in both age groups in both studies. Overall, the frequency of subjects reporting one or more unsolicited AE was similar for both vaccine groups in both studies: E1, adults: CCIV 9%; TIV 7% (p = NS) and elderly: CCIV 8%; TIV 6%, (p = NS) and E2, adults: CCIV 6%; TIV 9% (p = NS) and elderly: CCIV 6%; TIV 8% (p = NS). The number of reported serious adverse events (SAEs) during the first three weeks was low in both studies: E1 (1 SAE in CCIV adult group; and 3 SAEs each in CCIV and TIV elderly group) and E2 (2 SAEs by 1 adult subject in CCIV group; and 2 SAEs one each from CCIV and TIV elderly group). No reported SAEs were considered to be related to the study vaccine.
Figure 3. Local and systemic reactions in adult (A) and elderly (B) subjects – study E2 (un-randomized revaccination group).
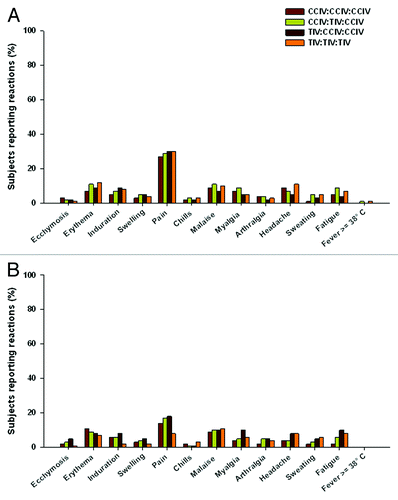
6 mo follow-up (Revaccination—with influenza vaccine): No possibly or probably related AEs or SAEs were experienced during the 3 week to 6 mo safety follow-up period for either study. All AEs reported during this time period were unrelated to the study vaccine and were common or expected illnesses for this population, with incidence rates balanced between groups. During the 6 mo follow-up period the percentage of SAEs were similar for vaccine groups in E1 (adults: CCIV 2%; TIV 1% p = NS and elderly: CCIV 4%; TIV 5% p = NS) and E2 (adults: CCIV 3%; TIV 3% p = NS and elderly: CCIV 6%; TIV 4% p = NS). Two deaths in the TIV elderly group and three deaths in the CCIV group (2 elderly, one adult) were reported during study E1 and four deaths during E2, two each from the CCIV and TIV elderly groups, none of these deaths being assessed as vaccine related.
Weeks 1–3 (Concomitant influenza with PV— E2 elderly subset): In the randomized subset of elderly subjects incidences of solicited local and systemic reactions were significantly higher (p = 0.024) in subjects receiving concomitant CCIV+PV compared with TIV+PV (). Mild to moderate pain was the most common local reaction. The highest percentage of subjects experiencing pain was reported by the group receiving concomitant CCIV+PV vaccines (CCIV 13%; CCIV+PV 27%; TIV 15%; TIV+PV 16%, p = NS). The overall percentages of subjects reporting unsolicited AEs were similar across vaccine groups (CCIV 14%; CCIV+PV 12%; TIV 10%; TIV+PV 8%, p = NS). No SAEs were reported.
6 mo follow-up (Concomitant influenza with PV – E2 elderly subset): The rate of SAEs was slightly higher in the concomitant vaccine groups (CCIV 4%; CCIV+PV 6%; TIV 2%; TIV+PV 12% p = NS). None of the events reported in the four vaccine groups were judged as possibly/probably related to the vaccines and no deaths were reported.
Immunogenicity
Study E1: (Revaccination—with influenza vaccine): In study E1, immunogenicity was assessed in a subset of 483 subjects (adults 239 and elderly 244) wherein, 120 adults received CCIV (CCIV:CCIV 60; TIV:CCIV 60) and 119 received TIV (CCIV:TIV 60; TIV:TIV 59); 122 elderly subjects received CCIV (CCIV:CCIV 61; TIV:CCIV 61) and 122 received TIV (CCIV:TIV 61; TIV:TIV 61). In adult subjects, the CHMP seroprotection criterion was met by both vaccines for all three strains (). In both vaccine groups the GMR and seroconversion/significant increase criteria were only met by the H3N2 strain, to which the population had not been exposed during the parent study (A/H3N2/Fujian/411/2002-like). GMR criterion for the B strain was met by the CCIV group only. In elderly subjects all three CHMP criteria were met by all three strains ().
Table 3. Immunogenicity across different vaccine groups in study E1 and E2
Study E2 – (Revaccination/Concomitant influenza with PV): In study E2, immunogenicity was assessed in a subset of the per protocol (PP) population including 118 adults (CCIV 95; TIV 23) and 151 randomized elderly who received CCIV (44); CCIV + PV (45); TIV (33); or TIV+PV (29).
Adults: The seroprotection criterion was met in both vaccination groups for A/H1N1 and A/H3N2, but not for the B strain. GMR and seroconversion criteria were met by all three strains in the CCIV group, but only by A/H1N1 in the TIV group ().
Elderly: In the pooled CCIV group, all three CHMP criteria were met by strains A/H1N1 and A/H3N2, only seroprotection and GMT increase were met by the B strain. In the pooled TIV groups, all three CHMP criteria were met for the A/H1N1 and A/H3N2 strains and only the GMR criterion was met by the B strain ().
Discussion
Influenza vaccination is recommended annually for older adults in most countries and, in the United States, for all persons of any age. As cell culture-derived and egg-derived influenza vaccines will increasingly be distributed side by side, it is likely that over the course of several influenza seasons, individuals will be immunized with different products, produced using both egg-based (TIV) and cell culture-based (CCIV) technologies. The immunogenicity and reactogenicity profiles of the various available seasonal influenza vaccine formulations are similar,Citation13,Citation15-Citation17 but the implications of their alternate use over time have not been investigated. Little information is available on the effect of co-immunization with PV which is often administered at the same time as influenza vaccine in the elderly.
Overall, the present studies show that revaccination with CCIV and TIV in adults or elderly was equally well tolerated, with similar reactogenicity profiles for each age group. Safety and reactogenicity were not influenced by the type of vaccine received in previous influenza seasons, although higher reactogenicity rates were observed with concomitant administration of PV. These results are comparable with the reactogenicity profiles reported in the parent study, demonstrating that revaccination does not impact on tolerability of CCIV or TIV. The increase in reactogenicity seen on co-administration with PV is comparable to that reported in other similar studiesCitation20-Citation22. Concomitant administration had no impact on the severity of the observed adverse reactions, and no impact on the antibody response to the influenza viral antigens.
These studies show no significant negative impact on either safety or immunogenicity following the alternate use of egg- and cell culture-derived vaccines over three influenza seasons. Additionally, both CCIV and TIV can safely be concomitantly administered to the elderly with 23 valent polysaccharide pneumococcal vaccine. Although influenza vaccine responses were not impaired by coadministration with PV, the impact on antibody responses to the pneumococcal antigens was not studied. As 13v pneumococcal conjugate vaccine (PCV13) will be used increasingly in older adults, additional studies of concomitant influenza vaccines coadministered with PCV13 will be needed.
Methods
Study design
The original phase III randomized study was conducted to compare the immunogenicity and reactogenicity profiles of CCIV and TIV in 1300 adult (18–60 y) and 1354 elderly (≥ 61 y) subjects during the 2004–2005 influenza season at five centers in Poland.Citation17 Following approval by the relevant ethics committees, and with the written informed consent of all participants, two extension studies (E1 and E2) were conducted in those subjects who participated in the parent study (). All studies were performed in accordance with the Declaration of Helsinki and Good Clinical Practice (GCP).
In study E1 (2005–2006; randomized, observer blind) the safety and immunogenicity (in a subset) of CCIV and TIV were assessed after revaccinating adult and elderly subjects with either the same or the alternate type of vaccine than the parent study.
In study E2 (2007–2008; non-randomized, single blind) adult and elderly subjects were revaccinated with either TIV (if randomized in parent or E1 study to TIV) or CCIV (all other subjects) to assess safety and immunogenicity (subset of adults only).
In addition, a subset of subjects from E2 elderly group was randomized to receive CCIV or TIV alone or concomitantly with polysaccharide pneumococcal vaccine (PV) and was assessed for safety and immunogenicity.
Study population
Healthy adult (18–60 y of age) and elderly (≥ 61 y) subjects who had completed the parent study were assessed for eligibility to participate in each extension study. Those with a history of hypersensitivity to study vaccine components; impaired or altered immune function (E1); laboratory-confirmed influenza 6 mo prior to first visit in E1; acute respiratory disease, fever within 5 d of enrollment; vaccination against influenza during the 6 mo before first visit in E1; pregnant/breastfeeding females; or participation in another clinical trial in the 90 d prior to first visit in either extension study as well as participation in another trial for the entire period of extension studies, were excluded.
Randomization
Using randomization lists provided by the study sponsor, participants were randomly assigned to different study groups. In study E1, subjects who received CCIV or TIV in the parent study were randomized 1:1 to receive either CCIV or TIV, giving four vaccination groups in each age group (Group 1 - CCIV:CCIV; Group 2 - CCIV:TIV; Group 3 - TIV:CCIV; Group 4 - TIV:TIV) ( and B).
In the E2 study, in order to evaluate revaccination, subjects were allocated 3:1 to receive CCIV or TIV. Subjects who participated in both parent and E1 studies and who had received at least one dose of CCIV were allocated to receive CCIV, while those who received 2 doses of TIV were allocated to receive TIV. Subjects who participated only in the parent study and received or were planned to receive CCIV in study E1 were allocated CCIV. Those who received TIV in the parent study and were planned to receive TIV in study E1 were allocated TIV resulting in four vaccination groups for each age group (Group 1 - CCIV:CCIV:CCIV; Group 2 - CCIV:TIV:CCIV; Group 3 - TIV:CCIV:CCIV; Group 4 - TIV:TIV:TIV) ( and B). To evaluate concomitant vaccine administration of PV with CCIV or TIV, a subset of elderly subjects aged 65 y and above (n = 240) were randomized in 5:5:3:3 ratio to four vaccine groups, Group 1 - CCIV; Group 2 - CCIV+PV; Group 3 -TIV; and Group 4 - TIV+PV ()
Vaccines
Both subunit influenza vaccines used in these studies were produced by Novartis Vaccines and Diagnostics. The investigational CCIV (Optaflu® Novartis Vaccines, Marburg, Germany) was produced in MDCK cells and the licensed influenza control vaccine (Agrippal® Novartis Vaccines, Siena, Italy) was produced in eggs. Each 0.5 mL dose of vaccine contained 15 µg of viral hemagglutinin (HA) for each of the three WHO recommended virus strains for the 2005–2006 (E1) and 2007–2008 (E2) Northern hemisphere influenza seasons (). The polysaccharide pneumococcal vaccine (Pneumo 23, sanofi pasteur, Lyon, France) contained 25 µg of each of the 23 pneumococcal serotypes.
Table 1. Vaccine Strains composition
CCIV and TIV (0.5 mL) were administered by intramuscular injection in the deltoid region, preferably in the subject’s non-dominant arm. When given concomitantly, PV was injected intramuscularly (0.5 mL) in the deltoid region of the other arm.
Safety monitoring
Each participant maintained a daily diary card for 22 d after vaccination. For the first 7 d, participants were asked to record occurrences of solicited local and systemic reactions, other indicators of reactogenicity (axillary temperature, the use of analgesic or antipyretic medication, the impact of vaccination reactions on daily activities), and any other adverse events (AEs). AEs were recorded up to 3 weeks after vaccination. Serious AEs or those AEs resulting in premature withdrawal from the study or requiring a physician’s visit (E1) were recorded up to 6 mo after vaccination.
Immunogenicity assessment
10 mL blood samples were collected pre- and post-vaccination (day 22) in the immunogenicity subset of both studies. Serum antibodies against A/H1NI, A/H3N2, and B influenza-virus strains were assessed using the hemagglutination inhibition (HI) test using egg-derived vaccine antigens, and expressed as the reciprocal of the highest dilution giving total inhibition of hemagglutination.Citation23 Immunogenicity was evaluated using the European Union Committee for Medicinal Products for Human Use (CHMP) criteria: for seroprotection, an HI titer ≥ 40 in > 70% of adult and > 60% of elderly participants; for geometric mean ratio (GMR) of titers, a postvaccination increase of > 2.5 in adult and > 2.0 in elderly participants; and for seroconversion, a change in HI titer from < 10 to ≥ 40 or a ≥ 4-fold increase in HI titer in > 40% of adult and > 30% of elderly participantsCitation18
Statistical analysis
Data analysis was performed by Novartis Vaccines Biostatistics and Clinical Data Management using SAS® software Version 8.2 (SAS Institute, Cary, NC) based on a predefined analysis plan developed by Novartis Vaccines.
There was no statistical null hypothesis associated with the safety and immunogenicity objectives in either study. For the E1 study, sample size was not based on power calculation whereas for study E2 there was a sample size calculation for the immunogenicity analysis in a subset of concomitant vaccine evaluation. For the randomized elderly subset the sample size was chosen to demonstrate non-inferiority. Assuming a standard deviation of 0.65 (calculated as the upper limit of the 90% CI of the standard deviation) 360 evaluable subjects, 180 subjects in each vaccine group (Influenza vaccine + concomitant vaccine group and Influenza vaccine administered alone group) were sufficient to demonstrate the non-inferiority objective with a power of 95% (98% for each single strain; one sided α = 0.025). The criterion to assess non-inferiority being that the lower limit of the 2-sided 95% confidence interval (CI) for the ratio of the post-vaccination GMTs between the influenza vaccine concomitantly administered with pneumococcal vaccine groups and influenza vaccine alone groups is greater than 0.5.
The sample size for the immunogenicity subsets of each vaccine group was in compliance with the sample size requirements of the current CHMP guideline for influenza vaccines (CPMP/BWP/214/96). All immunogenicity analyses were performed descriptively, by means of calculating 95% confidence intervals (CI) around measures of immunogenicity of the two vaccines.
Acknowledgments
The authors would like to thank the study investigators Dr. Ryszard Konior, Dr. Jolanta Guzik, Dr. Maria Świeboda and Dr. Marian Patrzałek. Editorial assistance was provided by Dr Jamie Stirling and Dr.Pinki Rajeev, Novartis Vaccines and Diagnostics. Responsibility for opinions, conclusions, and interpretation of data lies with the authors.
Trial registration number
NCT00306527 (E1); NCT00579345 (E2)
Funding
Both studies were sponsored by Novartis Vaccines and Diagnostics.
Financial disclosure and conflict of interest
Agnieszka Szymczakiewicz-Multanowska has received research funding from Novartis Vaccines and was working at Monipol at the time of the study; Maria Lattanzi, Allen Izu, MariaVittoria Sparacio, Csaba Kovacs, and Nicola Groth are employees of Novartis Vaccines and Diagnostics. Daniela Casula was an employee of Novartis Vaccines and Diagnostics at the time of the study.
Previous presentation
Long-term safety of a cell-derived influenza subunit vaccine after revaccination in healthy adult and elderly subjects. A Multanowska, M Lattanzi. Influenza Vaccines for the World, Cannes, France, 27–30 April 2009.
References
- Influenza vaccines. Wkly Epidemiol Rec 2005; 80:279 - 87; PMID: 16171031
- Fiore AE, Shay DK, Broder K, Iskander JK, Uyeki TM, Mootrey G, et al, Centers for Disease Control and Prevention (CDC), Advisory Committee on Immunization Practices (ACIP). Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep 2008; 57:RR-7 1 - 60; PMID: 18685555
- Monto AS, Ansaldi F, Aspinall R, McElhaney JE, Montaño LF, Nichol KL, et al. Influenza control in the 21st century: Optimizing protection of older adults. Vaccine 2009; 27:5043 - 53; http://dx.doi.org/10.1016/j.vaccine.2009.06.032; PMID: 19559118
- Bridges CB, Katz JM, Levandowski RA and NJ C. Inactivated influenza vaccines. 5 ed. Philadelphia: Saunders, 2008 (Plotkin SA, Orenstein WA and PA O, eds. Vaccines)
- Ulmer JB, Valley U, Rappuoli R. Vaccine manufacturing: challenges and solutions. Nat Biotechnol 2006; 24:1377 - 83; http://dx.doi.org/10.1038/nbt1261; PMID: 17093488
- Wright PF. Vaccine preparedness--are we ready for the next influenza pandemic?. N Engl J Med 2008; 358:2540 - 3; http://dx.doi.org/10.1056/NEJMp0803650; PMID: 18550873
- Mabrouk T, Ellis RW. Influenza vaccine technologies and the use of the cell-culture process (cell-culture influenza vaccine). Dev Biol (Basel) 2002; 110:125 - 34; PMID: 12477315
- (WHO) WHO. Influenza. Fact sheet No. 211. 2003. Available at: http://www.who.int/mediacentre/factsheets/fs211/en/. Accessed 5 august 2010.
- Belshe RB. Translational research on vaccines: influenza as an example. Clin Pharmacol Ther 2007; 82:745 - 9; http://dx.doi.org/10.1038/sj.clpt.6100419; PMID: 17971813
- Halperin SA, Smith B, Mabrouk T, Germain M, Trépanier P, Hassell T, et al. Safety and immunogenicity of a trivalent, inactivated, mammalian cell culture-derived influenza vaccine in healthy adults, seniors, and children. Vaccine 2002; 20:1240 - 7; http://dx.doi.org/10.1016/S0264-410X(01)00428-5; PMID: 11803087
- Kistner O, Barrett PN, Mundt W, Reiter M, Schober-Bendixen S, Dorner F. Development of a mammalian cell (Vero) derived candidate influenza virus vaccine. Vaccine 1998; 16:960 - 8; http://dx.doi.org/10.1016/S0264-410X(97)00301-0; PMID: 9682344
- Glezen WP. Emerging infections: pandemic influenza. Epidemiol Rev 1996; 18:64 - 76; PMID: 8877331
- Ambrozaitis A, Groth N, Bugarini R, Sparacio V, Podda A, Lattanzi M. A novel mammalian cell-culture technique for consistent production of a well-tolerated and immunogenic trivalent subunit influenza vaccine. Vaccine 2009; 27:6022 - 9; http://dx.doi.org/10.1016/j.vaccine.2009.07.083; PMID: 19666152
- Doroshenko A, Halperin SA. Trivalent MDCK cell culture-derived influenza vaccine Optaflu (Novartis Vaccines). Expert Rev Vaccines 2009; 8:679 - 88; http://dx.doi.org/10.1586/erv.09.31; PMID: 19485748
- Groth N, Montomoli E, Gentile C, Manini I, Bugarini R, Podda A. Safety, tolerability and immunogenicity of a mammalian cell-culture-derived influenza vaccine: a sequential Phase I and Phase II clinical trial. Vaccine 2009; 27:786 - 91; http://dx.doi.org/10.1016/j.vaccine.2008.11.003; PMID: 19027046
- Reisinger KS, Block SL, Izu A, Groth N, Holmes SJ. Subunit influenza vaccines produced from cell culture or in embryonated chicken eggs: comparison of safety, reactogenicity, and immunogenicity. J Infect Dis 2009; 200:849 - 57; http://dx.doi.org/10.1086/605506; PMID: 19673652
- Szymczakiewicz-Multanowska A, Groth N, Bugarini R, Lattanzi M, Casula D, Hilbert A, et al. Safety and immunogenicity of a novel influenza subunit vaccine produced in mammalian cell culture. J Infect Dis 2009; 200:841 - 8; http://dx.doi.org/10.1086/605505; PMID: 19673651
- Frey S, Vesikari T, Szymczakiewicz-Multanowska A, Lattanzi M, Izu A, Groth N, et al. Clinical efficacy of cell culture–derived and egg‐derived inactivated subunit influenza vaccines in healthy adults. Clin Infect Dis 2010; 51:997 - 1004; http://dx.doi.org/10.1086/656578; PMID: 20868284
- Keitel W, Groth N, Lattanzi M, Praus M, Hilbert AK, Borkowski A, et al. Dose ranging of adjuvant and antigen in a cell culture H5N1 influenza vaccine: safety and immunogenicity of a phase 1/2 clinical trial. Vaccine 2010; 28:840 - 8; http://dx.doi.org/10.1016/j.vaccine.2009.10.019; PMID: 19835829
- Perucchini E, Consonni S, Sandrini MC, Bergamaschini L, Vergani C. Adverse reactions to influenza vaccine alone or with pneumococcal vaccine in the elderly. [No abstract available.] J Am Geriatr Soc 2004; 52:1219 - 20; http://dx.doi.org/10.1111/j.1532-5415.2004.52327_5.x; PMID: 15209671
- Grilli G, Fuiano L, Biasio LR, Pregliasco F, Plebani A, Leibovitz M, et al. Simultaneous influenza and pneumococcal vaccination in elderly individuals. Eur J Epidemiol 1997; 13:287 - 91; http://dx.doi.org/10.1023/A:1007398606807; PMID: 9258527
- De Stefano F, Richard A, Goodman MD, et al. Simultaneous Administration of influenza and pneumococcal vaccines. JAMA 1982; 247:2551 - 2554; http://dx.doi.org/10.1001/jama.1982.03320430055032
- Committee for Proprietary Medicinal Products (CPMP). Note for guidance on harmonisation of requirements for influenza vaccines. CPMP/BWP/214/96. 12 March, 1997. Available at http://www.emea.europa.eu/pdfs/human/bwp/021496en.pdf. Accessed 5 august 2010.