Abstract
In this open labeled phase 1 clinical trial with enterovirus 71 (EV71) vaccine (ClinicalTrials.gov number: NCT01267903) performed in Donghai County, Jiangsu Province, China, in January 2011. A total of 100 healthy participants, stratified by age (40 adults aged 16–22 y and 60 children aged 6–15 y), were enrolled from volunteers and sequentially received EV71 vaccines of 160U (only for children), 320U, or 640U on day 0 and 28, in a manner of dose escalation. All the participants were followed for 28 d after each shot. During the study period, 37 participants reported at least one injection-site or systemic adverse reaction. No case of grade 3 adverse reaction or serious adverse event (SAE) was observed. Also no dose-related increase in reaction rate was noticed. Pain at injection-site and fever were the most frequently reported local and systematic reaction, respectively. The studied EV71 vaccines demonstrated acceptable tolerability and no anti-nuclear antibody (ANA) seropositive was detected pre or post vaccinations in participants. Also, no clinically significant abnormal change for the liver or kidney function indexes was found. In the according-to-protocol cohort for immunogenicity, it was observed one dose of EV71 vaccine elicited good immune response in the participants, especially for the ones with sero-positive baseline. No obvious dose-response relationship for immunogenicity was found. This study was co-founded by Beijing Vigoo Biological Co., LTD and Chinese governmental grants (2008BAI69B01 and 2009ZX10004–806).
Introduction
Enterovirus 71 (EV71) is a viral pathogen within the Picornaviridae family and causes diseases in humans with manifestations such as hand, foot and mouth disease (HFMD), herpangina, aseptic meningitis, encephalitis, and pulmonary edema.Citation1 While EV71 can infect all ages, it more commonly and severely infects children between 6 mo to 3 y of age.Citation2 EV71-infected children can develop severe neurological complications that lead to rapid clinical deterioration and death. Since 1997, there have been numerous outbreaks caused by EV71 in the Southeast Asia and Pacific region, including Malaysia, Singapore, Taiwan, Japan, South Korea, Vietnam and mainland ChinaCitation3–Citation8, resulting in hundreds of thousands of cases. In China, the reported incidence of HFMD cases escalated significantly from 2007 to 2009. The year 2007 experienced 83,344 reported cases with 17 deaths. In 2008, when HFMD became a notifiable disease, the number of reported cases increased to 488,955 with 126 fatalities.Citation8 Currently no effective antiviral drugs are available against severe EV71 infection.Citation9 Thus, the only means to prevent EV71 infection is through hand washing or avoidance of contact between infected and susceptible individuals, which with limited efficacy.Citation2 Consequently, the development of safe and effective EV71 vaccines was in an urgent need.
Recently, several EV71 candidate vaccines have been successfully developed and evaluated in animal modelsCitation10and further study in human are expected. In January 2011, a formaldehyde-inactivated, alum-adjuvanted EV71 vaccine (genotype C4) developed by Beijing Vigoo Biological Co., LTD (Beijing, China) was approved to undergo a phase 1 clinical trial, with the aim of evaluating the tolerability of the new vaccine in healthy adults and children. Here we presented the results.
Results
Study Participants
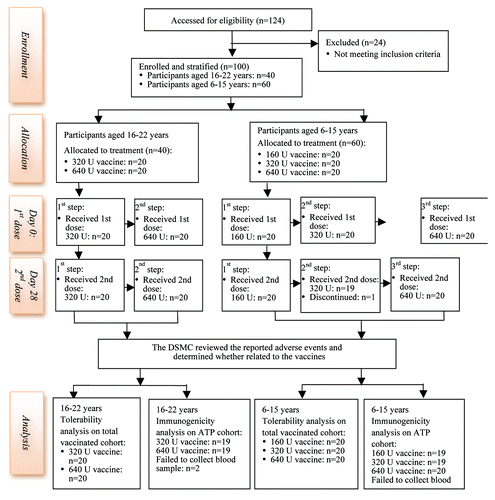
We enrolled 40 adults (20 females and 20 males) aged 16 to 22 y with an average age of 17.4 y and 60 children (26 girls and 34 boys) aged 6 to 15 y with an average age of 9.0 y. All participants were of Chinese heritage, received at least one dose of allocated dosage of EV71 vaccine and were included in tolerability analysis. One participant voluntarily withdrew after the observation period of first dose and did not receive the second dose. The serum samples were obtained from all participants at day 0, 28 and 96 participants at day 56 (). Therefore, 96 participants were included in the according-to-protocol (ATP) cohort for immunogenic analysis.
Figure 1. Trial Profile. The reasons why 24 participants excluded are listed in Table S1, available with the full text of this article. One participant received the first dose of vaccine but did not receive the second dose, because he requested to withdraw from the study.
Tolerability
All the 100 participants returned their diary cards after the first dose and 99 after the second dose. A total of 37 (37.0%) participants reported at least one injection-site or systemic adverse reaction (). All the reported adverse reactions were mild or moderate. No serious adverse event (SAE) was observed. Significant higher reaction incidence rate was found after the first dose than after the second dose with 31 participants versus14 participants (p = 0.0236). Pain at injection-site was the most frequently reported local symptom, followed by pruritus (). Fever was the most commonly reported systemic symptom with the incidence rate of 35.0–55.0% in the 16–22 y group and of 10.0–20.0% in the 6–15 y group. Other symptoms were of relatively low incidence rate. No participants reported immediate allergic reaction within 30 min after vaccination.
Table 1. The distribution of adverse reactions reported after vaccination with EV71 vaccines in different treatment groups, according to age groups
Table 2. The distribution of injection-site reactions and systematic reactions reported after vaccinations with EV71 vaccines in different treatment groups, according to age groups
During the follow-up period, a total of 24 participants (24%, 95%CI: 16.0–33.6) reported adverse events unrelated to vaccination according to the judgment of Data and Safety Monitoring Committee (DSMC). These adverse events included fever, nasosinusitis, chilblain, infection of the upper respiratory tract, dizzy, headache, stomachache, tenosynovitis, traumatism and so on. Most of them were clearly diagnosed or had other causes rather than vaccinations. The 24 participants were evenly distributed among treatment groups both in adults (p = 0.2881) and children (p = 0.9079).
All the serum samples were tested for ANA, none of which were found positive before or after the vaccinations. Liver and kidney function test showed that one case with elevated alanine aminotransferase (ALT) over five times and two cases with positive occult blood in urine 3 d after vaccinations. The participants or their parents were informed about the results and a further comprehensive kidney or liver function examination was suggested. However, these abnormal changes were proved to be transient and no relevant symptoms associated with liver or kidney disorders were found.
Immunogenicity
Of the 38 adults and 58 children who completed the study, 27 (70.1%) adults and 53 (91.4%) children were seropositive (i.e., neutralizing antibody titer > = 1:8) at baseline, with the pre-vaccination geometric mean titers (GMTs) of 43.8 (95%CI: 23.3–82.4) in adults and 119.8 (95%CI: 74.4–192.9) in children, respectively. Twenty-eight days after first shot, all the participants were seropositive and over 80% were seroconverted (). One dose of EV71 vaccine elicited good immune response with the GMTs increased to 2701.8 (1248.4–5847.4) in adults, and 4642.2 (3248.5–6633.7) in children. However, the GMT of the children dropped significantly 28 d after receipt of the second dose.
Table 3. GMTs /sericonversion rates for anti-EV71 in the participants received the EV71 vaccines, on day 28 after first or second dose (ATP cohort for immunogenicity)
Considering the baseline neutralizing antibody (NT) titer may impede vaccine effectiveness and confound the interpretation of vaccine-induced responses, a sub-analysis was performed according to the baseline NT titers of the participants. In the 16 participants with the seronegative baseline, one dose of EV71 vaccine elicited moderate antibody response for both seroconversion rate and GMT, and which were significantly boosted by the second dose. For the seropositive participants, obvious antibody response with GMTs and seroconversion rates were elicited by the first dose while no significant boosting response was observed after the second. The overall dose-response relationship for immunogenicity could hardly been told from the current data (Table S2).
Discussion
Since its first emergence in the United States in 1969, EV71has been recognized as one of the two dominant pathogens causing epidemics of HFMD worldwide.Citation11 Though most cases of HFMD were mild and self-limited, outbreaks of HFMD caused by EV71 can present with high incidence of neurological complications, leading to severe and fatal cases.Citation12 Sporadic EV71 cases were common in the Chinese mainland. From 1998 to 2009, there have been annual reports of morbidity and mortality caused by EV71 infection. Effective vaccine is considered a top priority to control EV71 outbreaks.Citation13
The preclinical study showed that NT induced by inactivated EV71 vaccine can protect mice against lethal challenge of EV71 virus.Citation14,Citation15 Two studies in Taiwan had demonstrated that passive immunization was able to protect against lethal EV71 challenge in new born mice.Citation1,Citation16 Those results showed that humoral immunity played a key role in protecting against EV71-caused disease. Therefore, it is valid to use neutralizing antibody to reflect the immunogenicity of the study vaccine.
This phase I trial was the first EV71 vaccine clinical trial ever conducted in mainland China, in line with local criteria for vaccine licensure by the Chinese State Food and Drug Administration (SFDA). No comparator group was included in this trial, because the comparator group may not be able to exclude confounding factors due to the low sample size, instead of increasing the number of participants and funding expenses. The experimental EV71 vaccine demonstrated an acceptable tolerability profile. The most frequently reported injection-site and systematic reactions were pain and fever, respectively. Though this is an open label study conducted at a single center with a small sample size and no control group, the incidence rates of adverse reactions in this study were in the same range with those observed with other enterovirus vaccines such as hepatitis A vaccine.Citation17,Citation18 No unexpected adverse reactions, SAE and fatal cases were reported. Additionally, no clinically relevant changes were observed in blood biochemical and urine routine parameters. All these results showed that the experimental EV71 vaccine was well tolerated and had good safety profile in adults and children.
The baseline NT titer showed that most adults and children enrolled in this study were seropositive to EV71. Therefore one dose of vaccine induced significant immune response in these participants, especially for the pre-seropositive ones. The second dose may not necessary in this population. Although, the sub-analysis results showed that two doses could elicit better immune response for the pre-seronegative participants. Epidemiology study showed that HFMD incidence was higher in infants less than 5 y old, especially in infants less than 3 y old.Citation19,Citation20 The population aged 5 y or more often had preexisting NT antibody, rarely developed clinical manifestations after EV71 infection.Citation21 Therefore the participants in this study were not the target populations of EV71 vaccine. But according to the licensure requirement issued by China SFDA, the phase I clinical trial with biologicals should be conducted in adults first, and then in children and infants sequentially. The results in this study warranted further study in infants.
As the most phase 1 clinical trials, one of the limitations of this trail was the small sample size. Although the data in showed a trend toward lower reactogenicity with higher antigen content, we cannot get a conclusion like that yet, due to the small sample size. The distribution with frequency of the reaction in different treatment group may be a possible artifact, with the wild rages of 95% confidence intervals, overlapping with each other. Besides, the dose-response relationship for immunogenicity of this vaccine was expected to be demonstrated in further study. Currently, a large-scale, double blind, randomized, controlled phase 2 clinical trial is ongoing in China to further evaluate the immunogenicity and safety of the EV71 candidate vaccine.
In conclusion, this study indicated that the inactivated, alum-adjuvanted EV71 vaccine appeared to be well tolerated and immunogenic in Chinese adults aged 16 to 22 y and children aged 6–15 y.
Methods
Study Design and Participants
This is an open-label parallel-group phase 1 clinical trial performed in January, 2011, in Donghai County, Jiangsu Province, China. The study was designed and conducted by Jiangsu Provincial Center for Disease Control and Prevention (JSCDC). A total of 100 participants were enrolled from volunteers in local communities, with 40 adults aged 16 to 22 y and 60 children aged 6 to 15 y. Eligible participants were healthy male or female aged 6 to 22 y without any of the following conditions: acute febrile disease; history of HFMD or EV71 vaccination; bleeding disorder; history of hematologic, hepatic, renal, cardiac or respiratory disease; immunodeficiency; treatment with cytotoxic or immunosuppressive drugs within the past six months; receipt of blood products within the past three months; receipt of any attenuated alive vaccine or any other investigational medicines within the past one month; and being unable to comply with the study schedule. Each participant was screened via both medical history inquiry and physical examination to meet eligible criteria. Females were required to have a negative urine pregnancy test before inclusion and prior to each vaccination. The written informed consent was obtained from all participants. For participants below the legal age of consent (i.e., < 18 y old), written informed consent was obtained from the participants and his/her parents or legally acceptable representatives. The trial was approved by the Chinese SFDA. Institutional review board approval was obtained from the ethics committee of the JSCDC. The trial was conducted according to the Good Clinical Practice (GCP), in compliance with the Declaration of Helsinki.
Vaccine
The inactivated EV71 vaccine was developed by Beijing Vigoo Biological Co., LTD, using EV71 strain FY (genotype C4) as virus seed, which was grown in Vero cell using serum-free medium. The cultivated virus was harvested, filtrated and concentrated by ultrafiltration. The concentrated suspension was purified, then inactivated by formaldehyde and adsorbed onto aluminum hydroxide. The investigational vaccines contained 160U, 320U, or 640U antigen with 0.25μg, 0.5μg, or 1.0μg of protein, respectively. Each 0.5-mL vaccine dose contained 0.18mg of alum.
Procedure
The trial was conducted in two stages. The 40 adults aged 16 to 22 y were first recruited, and sequentially received vaccine in a manner of dose escalation, beginning with lower dosage of 320U in the first 20 adults, and then 640U in the other 20 adults. An independent DSMC was set up to review the safety data and responsible for the judgment of whether a reaction was associated with the vaccinations. The dose escalation was allowed only when no vaccination-related SAE were observed for one week after vaccination with lower dosage. After all the 40 adults received the allocated vaccines, the study would go to the second stage, where 60 children aged from 6 to 15 y were recruited, and sequentially received vaccine of 160U, 320U, 640U in the same manner. There were 20 participants in each dosage group within each age strata.
The study vaccine was administered intramuscularly in deltoid region on day 0 and day 28. Participants were observed closely for at least 30 min for immediate adverse events after each dosing, with medical treatment readily available in case of a rare anaphylactic reaction. Then the participants or their guardians were given diary cards to record injection-site and systemic reactions to the vaccines administered, as well as recording daily temperatures for the first 7 d after receipt of the vaccines. Two extra visits were performed by the study staffs on day 3 and 7 after each vaccination to determine if there had been any SAEs since vaccination and to remind them to record any reactions including SAEs and concomitant medication completely and properly on the diary cards. Participants or their guardians were also instructed to record any adverse events recorded in the diary card that are ongoing after day 7 up to day 28. The diary cards were returned to the sites at visits scheduled on day 28 after each vaccination.
Adverse reactions were graded according to the scale issued by Chinese SFDA.Citation22 Anti-nuclear antibodies (ANAs) were tested to show the safety profile by indirect immunofluorescence assay in all the participants at day 0, 28, and 56. In children, ALT, creatinine (Cr), urinary protein, leukocyturia and hematuria were tested to observe any abnormal change in liver and kidney function 3 d after each vaccination. Blood samples collected on day 0, 28, 56 were also used to determine the neutralizing antibody (NT) response as exploratory analysis.
Statistical Analysis
Because this was a phase 1 clinical trial, the pre-specified sample size of 20 in each treatment group was in accordance with the requirement of Chinese SFDA, not based on power calculation.
Tolerability analysis was performed on the total vaccinated cohort. All evaluable participants who received two doses and for whom with data concerning immunogenicity endpoint measures available were included in immunogenic according-to-protocol (ATP) analysis.
The primary endpoint was tolerability regarding the frequency and intensity of injection-site and systemic adverse reactions, and SAEs after vaccinations. ANAs status and any abnormal changes in live and kidney function were also presented to show the safety profile. The secondary endpoints were immunogenicity including seroconversion rates and the NT GMTs after the second dose. NT titer was log-transformed to fit normal distribution for the calculation of GMTs and the NT titer below 1:8 was allocated a value of 1:4. Seroconversion was defined as a subject with NT of less than 1:8 before vaccination and 1:32 or more after vaccination, or with NT of 1:8 or more before vaccination and at least 4-fold increase after vaccination. The analysis on tolerability and immunogenicity endpoints was primarily descriptive, with 95% CI estimated when appropriate. All statistical analyses were using SAS 9.1 (SAS Institute Inc., Cary, NC, USA). No interim analysis was expected in this study.
| Abbreviations: | ||
| ALT | = | alanine aminotransferase |
| ANA | = | anti-nuclear antibody |
| ATP | = | according-to-protocol |
| CI | = | confidence interval |
| Cr | = | creatinine |
| DSMC | = | data and safety monitoring committee |
| EV71 | = | enterovirus 71 |
| GCP | = | good clinical practice |
| GMT | = | geometric mean titers |
| HFMD | = | hand, foot and mouth disease |
| JSCDC | = | Jiangsu provincial center for disease control and prevention |
| NT | = | neutralizing antibody |
| SAE | = | serious adverse event |
| SFDA | = | state food and drug administration |
Additional material
Download Zip (91 KB)Acknowledgments
This study was co-founded by Beijing Vigoo Biological Co., LTD and Chinese governmental grants (2008BAI69B01 and 2009ZX10004–806). We thank the following investigators, from Donghai county Center for Disease Control and Prevention, contributed to the trial: Y.Wang, H.-Y.Pang, B.Sun, T.-H.Wu, B.-Y.Xie, Q.-K.Wang, J.Zhang, S.-M.Hu, Z.-J.Ma, X.-Y.Qin, D.-J.Lu, H.-Z.Lu, Y.-L.Zhang, L.-H.Liu, J.-P.Tian, B.-Y.Yang, J.-F.Liu, B.-J.Yin, J.Cheng, K.-S.Zhang, Z.-S.Zhou, L.Chen,M.Lv, S.-R.Li, S.-Y.Li, L.Ge, H.-R.Liu, H.-X.Yan, S.-Q. Sun. We thank the following members from National Vaccine and Serum Institute for suggestion on clinical trial in this study: Dr. K. Zhao and Dr. W.-Y. Ze; The Shenzhengshi Ying He Yuan Medical Technology Development CO. LTD for monitoring the study.
Supplemental Materials
Supplemental Materials
Supplemental materials can be found at: www.landesbioscience.com/journals/vaccines/article/19521
Disclosure of Potential Conflicts of Interest
Xiu-Ling Li, Yun-Tao Zhang are employees of Beijing Vigoo Biological Co., LTD. The other authors declare that they have no conflicts of interest.
Financial Disclosure
This study was co-founded by Beijing Vigoo Biological Co., LTD and Chinese governmental grants (2008BAI69B01 and 2009ZX10004–806).
Role of the Funding Source
The study was co-sponsored by governmental grants (2008BAI69B01 and 2009ZX10004–806) and Beijing Vigoo Biological Co., LTD, which was involved in trial design, not involved in data collection, analysis, interpretation, and report writing. The corresponding authors were responsible for submitting the manuscript for publication and had full access to the data.
Ethics Committee Approval
Institutional review board approvals were obtained from the ethics committee of the Jiangsu Provincial Center for Disease Control and Prevention (JSCDC). This study was performed in accordance with the GCP and Helsinki Declaration of 1975.
References
- Foo DG, Alonso S, Chow VT, Poh CL. Passive protection against lethal enterovirus 71 infection in newborn mice by neutralizing antibodies elicited by a synthetic peptide. Microbes Infect 2007; 9:1299 - 306; http://dx.doi.org/10.1016/j.micinf.2007.06.002; PMID: 17890123
- McMinn PC. An overview of the evolution of enterovirus 71 and its clinical and public health significance. FEMS Microbiol Rev 2002; 26:91 - 107; http://dx.doi.org/10.1111/j.1574-6976.2002.tb00601.x; PMID: 12007645
- Jee YM, Cheon DS, Kim K, Cho JH, Chung YS, Lee J, et al. Genetic analysis of the VP1 region of human enterovirus 71 strains isolated in Korea during 2000. Arch Virol 2003; 148:1735 - 46; http://dx.doi.org/10.1007/s00705-003-0133-6; PMID: 14505086
- Chong CY, Chan KP, Shah VA, Ng WY, Lau G, Teo TE, et al. Hand, foot and mouth disease in Singapore: a comparison of fatal and non-fatal cases. Acta paediatr 2003; 92:1163 - 9
- Lee MS, Lin TY, Chiang PS, Li WC, Luo ST, Tsao KC, et al. An investigation of epidemic enterovirus 71 infection in Taiwan, 2008: clinical, virologic, and serologic features. Pediatr Infect Dis J 2010; 29:1030 - 4; PMID: 20543760
- Fujimoto T, Chikahira M, Yoshida S, Ebira H, Hasegawa A, Totsuka A, et al. Outbreak of central nervous system disease associated with hand, foot, and mouth disease in Japan during the summer of 2000: detection and molecular epidemiology of enterovirus 71. Microbiol Immunol 2002; 46:621 - 7; PMID: 12437029
- Chan LG, Parashar UD, Lye MS, Ong FG, Zaki SR, Alexander JP, et al, For the Outbreak Study Group. Deaths of children during an outbreak of hand, foot, and mouth disease in sarawak, malaysia: clinical and pathological characteristics of the disease. Clin Infect Dis 2000; 31:678 - 83; http://dx.doi.org/10.1086/314032; PMID: 11017815
- Zhu Q, Hao Y, Ma J, Yu S, Wang Y. Surveillance of hand, foot, and mouth disease in mainland China (2008-2009). Biomed Environ Sci 2011; 24:349 - 56; PMID: 22108323
- Lee BY, Wateska AR, Bailey RR, Tai JH, Bacon KM, Smith KJ. Forecasting the economic value of an Enterovirus 71 (EV71) vaccine. Vaccine 2010; 28:7731 - 6; http://dx.doi.org/10.1016/j.vaccine.2010.09.065; PMID: 20923711
- Ong KC, Devi S, Cardosa MJ, Wong KT. Formaldehyde-inactivated whole-virus vaccine protects a murine model of enterovirus 71 encephalomyelitis against disease. J Virol 2010; 84:661 - 5; http://dx.doi.org/10.1128/JVI.00999-09; PMID: 19864378
- Puenpa J, Theamboonlers A, Korkong S, Linsuwanon P, Thongmee C, Chatproedprai S, et al. Molecular characterization and complete genome analysis of human enterovirus 71 and coxsackievirus A16 from children with hand, foot and mouth disease in Thailand during 2008-2011. Arch Virol 2011; 156:2007 - 13; http://dx.doi.org/10.1007/s00705-011-1098-5; PMID: 21898187
- Chang LY, Lin TY, Hsu KH, Huang YC, Lin KL, Hsueh C, et al. Clinical features and risk factors of pulmonary oedema after enterovirus-71-related hand, foot, and mouth disease. Lancet 1999; 354:1682 - 6; http://dx.doi.org/10.1016/S0140-6736(99)04434-7; PMID: 10568570
- Lin YC, Wu CN, Shih SR, Ho MS. Characterization of a Vero cell-adapted virulent strain of enterovirus 71 suitable for use as a vaccine candidate. Vaccine 2002; 20:2485 - 93; http://dx.doi.org/10.1016/S0264-410X(02)00182-2; PMID: 12057603
- Xu J, Qian Y, Wang S, Serrano JM, Li W, Huang Z, et al. EV71: an emerging infectious disease vaccine target in the Far East?. Vaccine 2010; 28:3516 - 21; http://dx.doi.org/10.1016/j.vaccine.2010.03.003; PMID: 20304038
- Liu CC, Guo MS, Lin FH, Hsiao KN, Chang KH, Chou AH, et al. Purification and characterization of enterovirus 71 viral particles produced from vero cells grown in a serum-free microcarrier bioreactor system. PLoS One 2011; 6:e20005; http://dx.doi.org/10.1371/journal.pone.0020005; PMID: 21603631
- Wu TC, Wang YF, Lee YP, Wang JR, Liu CC, Wang SM, et al. Immunity to avirulent enterovirus 71 and coxsackie A16 virus protects against enterovirus 71 infection in mice. J Virol 2007; 81:10310 - 5; http://dx.doi.org/10.1128/JVI.00372-07; PMID: 17626076
- Fisch A, Cadilhac P, Vidor E, Prazuck T, Dublanchet A, Lafaix C. Immunogenicity and safety of a new inactivated hepatitis A vaccine: a clinical trial with comparison of administration route. Vaccine 1996; 14:1132 - 6; http://dx.doi.org/10.1016/0264-410X(96)00044-8; PMID: 8911009
- Wiedermann G, Ambrosch F, Kollaritsch H, Hofmann H, Kunz C, D’Hondt E, et al. Safety and immunogenicity of an inactivated hepatitis A candidate vaccine in healthy adult volunteers. Vaccine 1990; 8:581 - 4; http://dx.doi.org/10.1016/0264-410X(90)90013-C; PMID: 1965077
- Wang JF, Guo YS, Christakos G, Yang WZ, Liao YL, Li ZJ, et al. Hand, foot and mouth disease: spatiotemporal transmission and climate. Int J Health Geogr 2011; 10:25; http://dx.doi.org/10.1186/1476-072X-10-25; PMID: 21466689
- Choi CS, Choi YJ, Choi UY, Han JW, Jeong DC, Kim HH, et al. Clinical manifestations of CNS infections caused by enterovirus type 71. Korean J Pediatr 2011; 54:11 - 6; http://dx.doi.org/10.3345/kjp.2011.54.1.11; PMID: 21359055
- Le VT, Phan TQ, Do QH, Nguyen BH, Lam QB, Bach VC, et al. Viral etiology of encephalitis in children in southern Vietnam: results of a one-year prospective descriptive study. PLoS Negl Trop Dis 2010; 4:e854; http://dx.doi.org/10.1371/journal.pntd.0000854; PMID: 21049060
- The standard guidelines for adverse reactions grading of Vaccine clinical trials. http:// www. sda. gov.cn/WS01/CL0844/9350_5.html., 2005.