Abstract
Bacterial L-form conversion, or existence without cell walls, is assumed a universal phenomenon in nature. An interesting aspect of this phenomenon is occurrence of L-forms in vaccine strains. Since BCG is currently a widely used and extensively studied live vaccine for tuberculosis, understanding L-form conversion of M. bovis BCG bacilli can provide new insight into behavior of BCG vaccine. In this respect, specific features, concerning the ability of BCG vaccine to produce viable filterable forms and L-forms, were studied by filtration and starvation stress experiments in vitro. The filterable forms obtained after filtration of BCG suspension, grew on Middlebrook 7H9 semisolid agar and formed typical “fried eggs” L-form colonies. Electron microscopy clearly demonstrated presence of L-form elements with size smaller than the size of bacterial filter pores of 0.2 µm in M. bovis BCG strains. Development of L-form subpopulation with typical morphological appearance of self-replicating cell wall-defective forms was observed after filtration, as well as after starvation stress. Specific DNA detection of pncA gene in derived L-form cultures from filterable and stressed BCG strains verified their identity as M. bovis BCG. In conclusion, the results confirm existence of filterable forms in commercial BCG vaccine, which are able to develop L-form population under appropriate conditions. L-form transformation of BCG bacilli displays a new intriguing aspect concerning exhibition of unusual features and atypical behavior of live BCG vaccine. Further research is requested to explore the influence of L-form phenomenon on BCG vaccine effects in vivo
Keywords: :
Introduction
BCG is a live attenuated strain of M. bovis, an organism closely related to M. tuberculosis and part of the M. tuberculosis complex.Citation1 After isolation of M. bovis by Nocard in 1902, the bacillus of Calmette and Guerin (BCG) was created by serial passages of M. bovis in a bile-containing medium at 3-weekly intervals for 13 y.Citation2 To date, BCG is the only vaccine currently licensed for prevention of tuberculosis. Since M. bovis BCG is an attenuated live strain, little is known about how long it can survive in vaccinated persons. Reports about detection and isolation of BCG bacilli from patients with AIDS many years after their vaccination,Citation3-Citation5 as well as studies on different aspects of BCG vaccine, give rise to difficult questions, considering how little is known, about the behavior and mechanisms, by which M. bovis BCG bacilli can persist in vivo for a long time. In our previous study, we found that M. bovis BCG can convert to cell wall deficient forms (L-forms) inside macrophages during infection in guinea pigs and that the L-conversion phenomenon enhanced their in vivo survival and persistence abilities significantly.Citation6 Essentially, bacterial L-form conversion, i.e., existence without rigid walls, is universal but difficultly recognized phenomenon in nature. The ability of bacteria to exist as populations of self-replicating forms with defective or entirely missing cell walls (L-forms) is considered an adaptive strategy of bacteria to survive and reproduce under unfavorable circumstances.Citation7-Citation9
It can be assumed that occurrence of L-forms in vaccine strains is probably happening and that this event may bring new unusual and unpredictable features and effects of live vaccines. Since BCG is the current widely used and extensively studied live vaccine for tuberculosis, it is important to understand L-form conversion of M. bovis BCG bacilli and its contribution to behavior of vaccine in experimental models.
Because filterability is one of the characteristics of the L-forms of bacteria, considerable interest has been shown in their capacity to pass filters, as was first demonstrated by Klieneberger-NobelCitation10 for the L-form of Streptobacillus moniliformis, and was later confirmed for the L-forms of other bacteria.Citation11-Citation15
In this study, we tried to emphasize on some specific features and important aspects concerning the ability of BCG vaccine to yield viable filterable and L-forms.
Results
Growth Characteristics
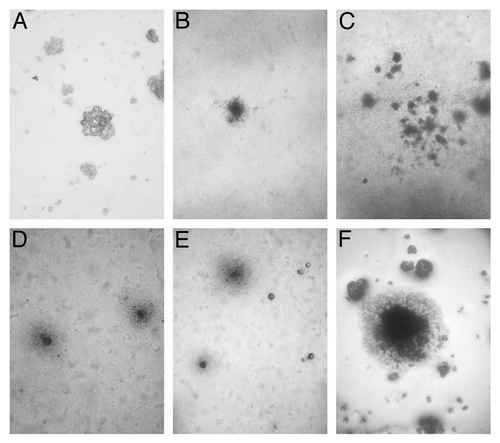
The filterable forms obtained after filtration of BCG suspension grew in Middlebrook 7H9 broth as visible small white snowflake-like particles. After sub-cultivation on Middlebrook 7H9 semisolid agar, BCG growth was observed by light microscopy. The morphology of developed L-form colonies is presented in . Typical “fried eggs” colonies of different sizes, sub-structural consistencies and period of forming were examined. The colonies grown from filterable BCG forms were reduced in size, in comparison to stress starved BCG. The L-form colonies that developed from filterable forms had granular substructure, formed dark centers ingrown into agar and were usually with smaller or missing peripheries ( and C). In contrast, the non-filtered starved BCG suspensions gave rise to larger colonies with typical L-form morphology, which were characterized by dense compact center and delicate periphery ().
Figure 1. Light microscopy of control BCG-D colony (A), and typical “fried eggs” L-form colonies grown on Middlebrook 7H9 semisolid agar: colonies of filterable forms developed from control BCG-BG suspension (B) and (C) and .colonies obtained after starvation of BCG-BG (D) and (E) and BCG-D cultures (F). Magnification: (C)–200x; (A), (B), (D), (E) and (F)-400x; (F)-800x.
Electron Microscopy
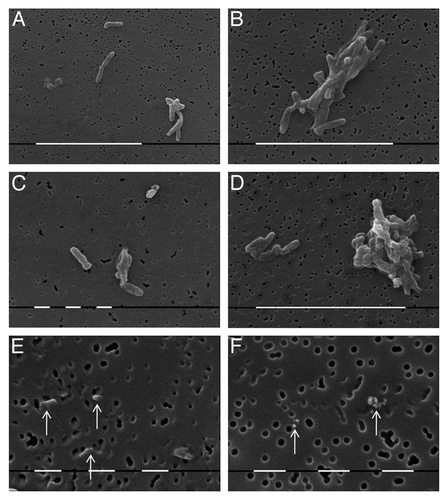
Scanning electron microscopy (SEM) of cultures obtained from control BCG suspension grown on Löwenstein-Jensen medium is presented in . The BCG bacilli of two strains (BCG-BG and BCG-D) showed morphology of typical rod bacilli arranged singly ( and C) or in irregular clusters ( and D). Of special interest was the observation of very small granules situated on membrane filters with size equal or smaller than the size of pores with diameter of 0.22 µm, which evidenced their ability to pass through bacterial filters ( and F).
Figure 2. SEM of cells from culture of control strains: BCG-BG- (A), (B) and (E); and BCG-D- (C), (D) and (F). Typical single rod shaped bacilli (A) and (C), bacterial clusters (B) and (D), and filterable forms which size is similar or smaller than pore size of bacterial filter (E) and (F), arrows. Bar (white lines) = 10µm (A), (B) and (D); 1 µm (C), (E) and (F)
Cell morphology of subpopulation developed after filtration of control BCG suspension showed a presence of many small granular forms and L-elementary bodies (). They were organized in irregular clusters () or were dispersed as single oval bodies of varied shape and size (). Newly formed small rod bacilli were recognized as well (). Presence of single filterable forms, smaller than the pores of filter membrane, was also observed ().
Figure 3. SEM of BCG subpopulation developed after filtration of control suspension: Clusters of granular forms and L-elementary bodies (A) and (B) or disperse single bodies of varied size (C) and (D), small rod shaped bacilli and filterable forms (E) and (F), arrows. Bar = 1µm (a, c, e); 10µm (b, d, f), Bar = 1µm
SEM of BCG cells after starvation stress is presented in . Existence of typical L-forms as spherical large bodies (), elongated forms () and giant mother cells () was observed. The sequential enlargement of these cells and disruption of their membranes was followed by release of many small elementary bodies and granules ().
Figure 4. SEM of BCG cultures after starvation stress: BCG-BG – (A), (E), (F) and BCG-D- (B), (C), (D). Typical L-form cells: spherical large bodies (A) and (B) giant “mother” cells (C) and (E), elongated forms (A) and (D), small elementary bodies and granules (C), (E) and (F). Bar = 100 µm (A); 10 µm (b, c, e, f); 1 µm (D)
Transmission electron microscopy (TEM) investigation of ultrathin cell sections clearly demonstrated the ultrastructure of L-form elements (). Cell morphology of control BCG culture showed typical bacillary morphology of dividing cells with cell envelope, electron dense ribosomal and transparent nucleoid zones (). After starvation stress, the cytoplasm of most cells was condensed in the cell center and the cell walls were separated (). Disruption of cell walls was followed by formation of protoplast rod-shaped or spherical forms (). Electron dense ribosome-rich elementary bodies were observed inside large, elongated L-forms (). This examination corresponds to the SEM observation of the BCG cultures after starvation (see ).
Figure 5. TEM of BCG-BG cell ultrathin sections (longitudinal and transversal) from: Control culture of dividing cells with cell walls and cytoplasm substructures of ribosomes and nucleoid (A) and (B); Stress starved culture- cells with separated cell walls (C), wall less bacterial cells (D), elongated L-forms with elementary bodies located inside (E) and single spherical L-bodies (F). Bar = 0.2µm
DNA –based Verification of Filterable and L-forms as M. bovis BCG
Specific DNA test (pnc A gene fragment amplification) was used for identification of filterable and L-forms as M. bovis BCG. For PCR analysis of M. bovis-specific pattern, we selected an amplification of a pnc A gene fragment, which evidenced that filterable forms and L-forms originated from M. bovis BCG (). The allele-specific PCR based method for distinguishing M. bovis from M. tuberculosis showed high discriminatory power between tested samples. The primer set designed for M. bovis amplified all M. bovis BCG strains (filterable and L-form cultures) except M. tuberculosis H37Rv (). On the other hand, the primer set designed for M. tuberculosis, gave a positive result only for M. tuberculosis H37Rv ().
Figure 6.pncA allele-specific PCR. Amplifications were performed with chromosomal DNA from M. bovis (3); M. tuberculosis H37Rv (4); M. bovis BCG-BG, L-form (5); M. bovis BCG-D, L-form (6); M. bovis BCG-BG, filtered culture (7) and M. bovis BCG-D, filtered culture (8). PCR controls with water were also included, lines 2 and 9. All samples in gel A were tested with primers specific for M. tuberculosis, whereas all samples in gel B - with primers specific for M. bovis. DNA ladder (100–1000bp) was used in line 1 as size marker in both gels.
Discussion
L-form conversion is assumed to be a general property of bacteria and as adaptive reaction to unfavorable environmental factors. Spontaneous growth of mycobacteria in wall-deficient state is believed to be, by some authors, as a natural phase of their life style.Citation7-Citation9 In this respect, occurrence of L-forms in BCG vaccine strains is a phenomenon of practical interest associated mostly with behavior and features of live BCG vaccine.
The current study demonstrates mechanisms for isolating BCG L-forms by filtration or inducing conversion by starvation, which could be used to determine the role of L-forms in bacterial persistence.
The results obtained from our filtration experiments refer to filterable L-forms, freely existing in BCG vaccine. SEM observations clearly demonstrated presence of elements with size smaller than the size of bacterial filter pores of 0.2 µm within the populations of both M. bovis BCG strains. Furthermore, development of cultures from filterable forms observed after filtration of control BCG vaccine suspensions confirmed that filterable bodies (with size of 0.22 μm at least) were able to regenerate and grow as L-form colonies. Studies on filterable and granular forms of bacteria have been conducted by many authors.Citation9-Citation15 It has been assumed that they are the smallest reproductive elements and play an important role in the life cycle of L-forms. The plasticity of the L-form elements may be a factor which determines the filterability of the bacterial L-forms. Filtration methods have been used to separate L-forms by other authors as well.Citation16,Citation17
L-forms of M. bovis BCG were produced in our study by starvation stress in vitro. Electron microscopy examinations demonstrated typical ultrastructural morphology of cell wall-deficient and variable sized L-bodies. Large spherical bodies, giant “mother” cells, small elementary bodies and granules, seen in cultures of BCG L-forms, suggest that these bodies undergo division processes. It is important to note that derived L-form cultures from filterable and stressed BCG strains are similar in morphology, ultrastructure and reproductive processes. Specific gene detection in derived L-form cultures from filterable and stressed BCG strains confirmed their identity as M. bovis BCG.
It is generally accepted that mycobacterial L-forms can occur spontaneously or they can be induced under suitable condition and manipulation of treatment.Citation9,Citation18-Citation20 The conversion to L-forms essentially is a population effect where many or most of surviving cells in the culture convert.Citation21 Filtration experiment with M. bovis BCG L-forms indicated that the smallest L-bodies were able to pass through bacterial filters and to form L-type colonies. Domingue suggests that small, electron dense L-bodies are capable of developing along several different routes depending on the stimulus received and they have the potential for unlimited growth and division.Citation22 On the other hand, the smallest filterable forms are considered as minimal reproductive cells, which can be formed from large L-bodies in all possible ways. It is believed that such filterable bodies contain a bacterial genome and minimal metabolic capability, but sufficient to initiate reproduction.Citation10,Citation22 Devadoss et al. found presence of filterable granular cocci and developmental stages of mycobacterial life cycle in commercially prepared BCG vaccines.Citation23 Detection of viable filterable forms in BCG vaccine which are able to reproduce as L-forms, seems an interesting fact in terms of evaluating vaccine characteristics. Although BCG effectiveness has been a subject of various scientific debates, possible influence of L-form phenomenon on vaccine effects has been never discussed. The importance of this phenomenon for live vaccines may be reflected in several directions concerning immunogenicity, persistence and safety. As far as cell wall deficiency facilitates the bacterial survival under unfavorable conditions, L-forms of different bacterial species have been shown to survive and persist for an extended period in vivo.Citation24-Citation27 Of all bacteria, L-forms predominate and are crucial to the survival of mycobacteria in vivo.·Citation6,Citation9,Citation20 Occurrence of M. bovis BCG L-forms inside macrophages has been observed during experimental infection in guinea pigs.Citation6 L-forms of BCG bacilli have been isolated from blood of persons vaccinated against TB with BCG vaccine.Citation28 While significant data evidently indicate the long-lasting persistence of L-forms in vivo,Citation6,Citation7,Citation9,Citation20 the possible influence of filterable and L-forms on immunogenicity of BCG vaccine is unclear and needs further research. It is likely that missing cell wall and antigen insufficiency in BCG L-forms may contribute to loss of immunogenicity and decrease of vaccine effectiveness. Data from experimental infections with L-forms demonstrated their atypical interactions with phagocyte cells, incomplete and ineffectual phagocytosis and long-lasting persistence. It may be suggested that prolonged non-specific irritation of the immune system by L-forms could become a reason for side effects of allergic or autoimmune origin.Citation24-Citation27 Having in mind all unusual properties of L-forms, the presence of viable filterable L-form elements in commercial BCG vaccine can be evaluated as risk factor for vaccine safety in view of possible unpredictable adverse events. This specific insight on properties of vaccine strains needs to be taken into account when developing of new viable mycobacterial vaccines. Further research is requested to explore the role of L-form phenomenon on BCG vaccine effects in vivo.
Materials and Methods
Bacterial Strains
Freeze-dried BCG vaccines manufactured at Statens Serum Institute, Copenhagen, Denmark (Mycobacterium bovis BCG-D) and at BB-NCIPD, Sofia Bulgaria (Mycobacterium bovis BCG-BG, strain Sofia SL222) were purchased. For reconstitution of both M. bovis BCG strains, the contents of 10 ampules from each vaccine (1 A/ 0.5 mg) was collected and diluted in 1ml of commercial diluent (Diluted Sauton SSI). The suspension was then inoculated onto Löwenstein-Jensen medium (LJ) and incubated at 37°C for 42 d.
Filtration experiment and culturing filterable forms
The contents of ten ampules of each vaccine were collected and diluted in 2 ml of Middlebrook 7H9 broth. The suspensions were filtrated through a sterile 25 mm syringe filter with pore size of 0.22µm (Fisherband, MCE) and incubated for two weeks at 37°C. After incubation, the broth cultures were centrifuged at 6000 rpm for 15 min. The resulting pellet was plated on semisolid medium, which was prepared from Middlebrook 7H9 broth (Difco) solidified with 0.8% (w/v) Bacto Agar (Difco) and incubated at 37°C for a week. The resulting growths and colonies were observed by light microscopy and identified by DNA-based identification tests. Experiments were repeated five times. Control experiments for sterile performance of experimental procedure were performed. Filtration of non-inoculated sterile broth did not give rise to any growth.
Production of M. bovis BCG L-forms by starvation stress
Biomass of 0.2 g harvested from fresh well developed on LJ culture of both M.bovis BCG strains (BCG-BG and BCG-D) was inoculated into 2ml of sterile saline and incubated at 37°C for 30 d. After incubation, the cell suspension was centrifugated at 4000 rpm for 20 min., the supernatant was removed, the sediment was re-suspended in 500 μl saline and plated on Middlebrook 7H9 semisolid medium.The Petri dishes were enveloped with parafilm and incubated at 37°C for one week. Production and isolation of L-forms from both M. bovis BCG strains was achieved through five serial passages of starved mycobacterial cultures at weekly intervals on Middlebrook 7H9 semisolid medium. At the fifth passage after starvation, the tested M.bovis BCG strains gave rise to L-form growth and typical L-form colonies with “fried egg” appearance.
Electron microscopy
Observations were performed on L-form cultures and cultures, developed from filterable forms of both M. bovis BCG strains (BCG-BG and BCG-D). Bacterial cultures were fixed with 4% (v/v) glutaraldehyde in 0.1 M cacodylate buffer with 4.5% w/v sucrose, pH 7.2 and post-fixed in 1% (w/v) osmium tetroxide in the same buffer at the room temperature for 2 h and dehydrated in serial ascending ethanol concentrations. For scanning electron microscopy (SEM), specimens were placed on membrane filters with pore size diameter of 0.22µm (Millichrom, Isopore), covered with 15–20 Å gold, visualized and photographed with scanning electron microscope Phillips SCM 515. For transmission electron microscopy (TEM), after dehydration in ethanol and propylene- oxide series, cell pellets were embedded in epoxy resin Epon-Araldite (Serva, Heidelberg, Germany). Resin blocks polymerised at 56 ◦C for 48 h. Ultrathin cell sections were made with crystal glass knives on a Reichert-Jung Ultracut Microtome and were stained with 5% (w/v) uranyl acetate in 70% (v/v) methanol and 0.4% (w/v) lead citrate. Observations were made with Zeiss 10C electron microscope at 60 kV.
Detection of specific DNA fragments in M. bovis BCG filterable and L-form cultures
Single colonies of mycobacterial L-form variants were picked up for genetic testing. Several precautions were taken to avoid contamination during the extraction procedure and in the PCR reactions. The extraction, PCR and post-PCR analyses were conducted in separate laminar boxes and rooms. Sterile aerosol protection filter tips were used to avoid cross-contamination. Two extraction blanks were always included in the same procedure and an additional PCR blank was included in each PCR reaction, containing no DNA template. Chromosomal DNA was isolated as described by Van Embden et al.Citation29
Amplification of pncA gene fragment
The PCR was performed as described previously.Citation30 The PCR primers for pncA used in this study were as follows: the forward primer, pncATB-1.2 (5′ATGCGGGCGTTGATCATCGTC-3′), complements bases 1 to 21 in the gene. The reverse primers, pncAMT-2 (5′-CGGTGTGCCGGAGAAGCGG-3′) and pncAMB-2 (5′-CGGTGTGCCGGAGAAGCCG-3′), complement bases 185 to 168. All DNA samples were subjected to two differential amplifications in separate tubes. Both reactions were performed with the same forward primer, pncATB-1.2, and one of the two discriminator primers, pncAMT-2 for control M. tuberculosis H37Rv or pncAMB-2 for M. bovis. The PCR master mix for M. tuberculosis contains 200 mM of each deoxynucleoside triphosphate, 25 pmol of each primer (pncATB-1.2 and pncAMT-2), 1.0 U of Taq-DNA polymerase (USB), 10 mM Tris hydrochloride (pH 8.3), 50 mM KCl, 2 mM MgCl and 2.5µl DNA template in a final volume of 25µl. The M. bovis-specific amplification reaction mixtures were identical to those of M. Tuberculosis, but contained primer pncAMB-2 instead of pncAMT-2. The cycling parameters were 95°C for 12 min, followed by 30 three-step cycles, including denaturation at 94°C for 1 min, annealing at 67°C for 1 min, and extension at 72°C for 1 min. The amplification products were analyzed by electrophoresis on a 2% agarose gel and visualized by ethidium bromide fluorescence. A unique amplification product of 185 bp (pncA) must be visualized in either M. tuberculosis or M. bovis reactions.
Acknowledgments
This work was supported by grant ID Nº 02/27 of the National Scientific Fund in Bulgaria. We thank Albena Cherneva for excellent technical assistance.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- von Reyn CF, Vuola JM. New vaccines for the prevention of tuberculosis. Clin Infect Dis 2002; 35:465 - 74; http://dx.doi.org/10.1086/341901; PMID: 12145732
- Hesseling AC, Rabie H, Marais BJ, Manders M, Lips M, Schaaf HS, et al. Bacille Calmette-Guérin vaccine-induced disease in HIV-infected and HIV-uninfected children. Clin Infect Dis 2006; 42:548 - 58; http://dx.doi.org/10.1086/499953; PMID: 16421800
- Armbruster C, Junker W, Vetter N, Jaksch G. Disseminated bacille Calmette-Guérin infection in an AIDS patient 30 years after BCG vaccination. J Infect Dis 1990; 162:1216; http://dx.doi.org/10.1093/infdis/162.5.1216; PMID: 2230251
- Smith E, Thybo S, Bennedsen J. Infection with Mycobacterium bovis in a patient with AIDS: a late complication of BCG vaccination. Scand J Infect Dis 1992; 24:109 - 10; http://dx.doi.org/10.3109/00365549209048409; PMID: 1589715
- Reynes J, Perez C, Lamaury I, Janbon F, Bertrand A. Bacille Calmette-Guérin adenitis 30 years after immunization in a patient with AIDS. J Infect Dis 1989; 160:727; http://dx.doi.org/10.1093/infdis/160.4.727; PMID: 2794567
- Markova N, Michailova L, Kussovski V, Jourdanova M. Formation of persisting cell wall deficient forms of Mycobacterium bovis BCG during interaction with peritoneal macrophages in guinea pigs. [eJBio] Electronic Journal of Biology 2008; 4:1 - 10
- Prozorovski SV, Kaz LN, Kagan GJ. Bacterial L-forms: Mechanisms of Formation, Structure, Role in Pathology. Moscow: Medicine Publishing; 1981.
- Domingue GJ. Cell-wall Deficient Bacteria: Basic Principles and Clinical Significance. Reading MA:Addison Wesley Publishing Co; 1982.
- Mattman LH. Cell wall Deficient Forms. Stealth Pathogens. 3rd ed. Boca Raton, FL, USA: CRC Press Inc; 2001.
- Klieneberger-Nobel E. Filterable forms of bacteria. Bacteriol Rev 1951; 15:77 - 103; PMID: 14847983
- Carrire L, Roux J, Mandin J. A propos du cycle L des bacteries: obtention de forms naines, viables et filtrables en milieu liquid. C R Seanc Soc Biol Fil 1954; 148:2050 - 2
- Tulasne R, Lavillaureix J. Filtration et ultrafiltration d’une souche de forme L fixées. C R Hebd Seances Acad Sci 1958; 246:3296 - 8; PMID: 13547568
- Dienes L, Madoff S. Development and growth of L forms of bacteria and PPLO on membrane filters. Proc Soc Exp Biol Med 1966; 121:334 - 9; PMID: 5295851
- Van Boven CPA, Ensering HL, Hijmans W. Size determination by the filtration method of reproductive elements of group A streptococcal L-forms. J Gen Microbiol 1968; 52:403 - 12
- Kohbata S, Emura S, Kadoya C. Filterable forms of Nocardia: a preferential site of infection in the mouse brain. Microbes Infect 2009; 11:744 - 52; http://dx.doi.org/10.1016/j.micinf.2009.04.013; PMID: 19376258
- Rada B. [Filterability of the L-phase and of the globular form of bacteria]. Zentralbl Bakteriol 1959; 176:85 - 90; PMID: 13854438
- Wittler RC. A filterable stage isolated from the L-form of Haemophilus pertussis. . 1954; 41
- Beran V, Havelkova M. J. Kaustova J, Dvorska L, Pavlik I: Cell wall deficient forms of mycobacteria: a review. Vet Med 2006; 51:365 - 89
- Michailova L, Kussovski V, Radoucheva T, Jordanova M, Berger W, Rinder H, et al. Morphological variability and cell-wall deficiency in Mycobacterium tuberculosis ‘heteroresistant’ strains. Int J Tuberc Lung Dis 2005; 9:907 - 14; PMID: 16104639
- Markova N, Michailova L, Jourdanova M, Kussovski V, Valcheva V, Mokrousov I, et al. Exhibition of persistent and drug-tolerant L-form habit of Mycobacterium tuberculosis during infection in rats. Cent Eur J Biol 2008; 3:407 - 16; http://dx.doi.org/10.2478/s11535-008-0032-7
- Madoff S. The bacterial L-Forms. Marcel Dekker, INC, New York, Basel, 1986.
- Domingue GJ. Demystifying pleomorphic forms in persistence and expression of disease: Are they bacteria, and is peptidoglycan the solution?. Discov Med 2010; 10:234 - 46; PMID: 20875345
- Devadoss PO, Klegerman ME, Groves MJ. A scanning electron microscopy study of mycobacterial development stages in commercial BCG vaccines. Curr Microbiol 1991; 22:247 - 52; http://dx.doi.org/10.1007/BF02092317
- Markova N, Michailova L, Vesselinova A, Kussovski V, Radoucheva T, Nikolova S, et al. Cell wall-deficient forms (L-forms) of Listeria monocytogenes in experimentally infected rats. Zentralbl Bakteriol 1997; 286:46 - 55; http://dx.doi.org/10.1016/S0934-8840(97)80074-6; PMID: 9241800
- Mihailova L, Markova N, Radoucheva T, Veljanov D, Radoevska S. Cell interactions of Listeria monocytogenes L forms and peritoneal exudative cells in rats. Can J Microbiol 1993; 39:1014 - 21; http://dx.doi.org/10.1139/m93-154; PMID: 8306205
- Michailova L, Markova N, Radoucheva T, Stoitsova S, Kussovski V, Jordanova M. Atypical behaviour and survival of Streptococcus pyogenes L forms during intraperitoneal infection in rats. FEMS Immunol Med Microbiol 2000; 28:55 - 65; http://dx.doi.org/10.1111/j.1574-695X.2000.tb01457.x; PMID: 10767608
- Michailova L, Kussovsky V, Radoucheva T, Jordanova M, Markova N. Persistence of Staphylococcus aureus L-form during experimental lung infection in rats. FEMS Microbiol Lett 2007; 268:88 - 97; http://dx.doi.org/10.1111/j.1574-6968.2006.00567.x; PMID: 17168999
- Xalabarder C. Electron microscopy of tubercle bacilli. Excerpta Med Sect XV Chest Dis 1958; 11: 467- 473. 36.
- van Embden JDA, Cave MD, Crawford JT, Dale JW, Eisenach KD, Gicquel B, et al. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol 1993; 31:406 - 9; PMID: 8381814
- Espinosa de los Monteros LE, Galán JC, Gutiérrez M, Samper S, García Marín JF, Martín C, et al. Allele-specific PCR method based on pncA and oxyR sequences for distinguishing Mycobacterium bovis from Mycobacterium tuberculosis: intraspecific M. bovis pncA sequence polymorphism. J Clin Microbiol 1998; 36:239 - 42; PMID: 9431955