Abstract
Sipuleucel-T (Provenge) (Sip-T) is first -in class as a therapeutic autologous vaccine approved for the treatment of men with asymptomatic or minimally symptomatic castrate-resistant metastatic prostate cancer. This product is the culmination of decades of basic immunological and prostate cancer investigations and 13 y of clinical trial investigations. Sip-T represents a paradigm shift in cancer therapeutics and represents the first approved autologous therapeutic cancer vaccine, which has demonstrated a survival benefit. The potential benefit of this product is the excellent risk to benefit ratio, which will allow for the combination of this approach with other more toxic therapies. The favorable risk to benefit will also afford the opportunity for trials investigating this product earlier in the disease state and in combination with local therapies. The ability to target more localized or lower volume disease will maximize the therapeutic benefit over a longer period of time. The novelty of the platform of this approach could be used to treat any cancer with a tumor-specific cell surface target. The main product of Sip-T is the re-infusion of a patient’s antigen presenting cells from leukapheresis after ex-vivo exposure to a chimeric protein of human GM-CSF and PAP. In metastatic CRPC patients three infusions of these activated cells over a month lead to statistically significant 4.1 mo increase in median survival and a 22.5% reduction in risk of death. The main side effect from this re-infusion of activated immune cells is a “flu-like” syndrome that includes chills, fatigue, fevers, back pain, nausea, joints aches and headaches in decreasing order of frequency. Immune monitoring during the clinical trials also demonstrated a specific cellular and antibody immune response, suggesting the proposed mechanism of adoptive immunotherapy to PAP was behind this survival benefit. This product also serves as a proof of principle for targeted immunotherapy for others cancers with defined cell surface markers. In summary, the approval of Sip-T based on a survival benefit and very tolerable safety profile will 1) enhance our ability to care for men with advanced prostate cancer, 2) allow for further investigations of this approach in combination with others therapies with different mechanisms of action and non-overlapping toxicities, and 3) allow further investigations earlier in the course of the disease.
Prostate Cancer
In 2012, Prostate cancer is expected to claim 28,170 lives and interfere with the lives of 224,00 men in the United States.Citation1 In 2008, the world estimate was 918,000 new diagnosis and 258,000 deaths.Citation2 The protracted timeline from diagnosis to death suggests an even greater prevalence of men living with prostate cancer between diagnosis and death. Lethal prostate cancer is both metastatic and castrate-resistant. The timeline from diagnosis to death can span decadesCitation3 making prostate cancer an excellent target for adoptive immunotherapy. The presence of several prostate-specific targets such as prostate-specific antigen (PSA), prostatic acid phosphatase (PAP), and prostate-specific membrane antigen (PSMA) also make prostate cancer a prime target for adoptive immune approaches. Each of these prostate-specific targets have been used as serum markersCitation4 over the past several decades. The current product, Sip-T, exploits the presence of a target, such as PAP, on the surface of 95% of prostate cancer cells.
Eventually, up to 60% of men diagnosed with prostate cancer relapse despite localized treatments such as surgery, radiation, and thermal therapies. These men develop locally advanced or distant disease in need of a more systemic therapy. The ability to detect small amounts of residual disease with serum marker PSA may allow an adoptive immune strategy to target smaller tumor burden with increased benefit. The mainstay of systemic therapy over the past half a century is based on the Nobel Prize work of Huggins and Hodges who identified the critical link between androgens and prostate cancer growth. Based on this observation, androgen deprivation therapy has improved survival in men with advanced prostate cancer for decades. Default use of hormonal blockade has been recently challenged due to some of the collateral damage to men by creating a hypogonadal state and has been linked to the metabolic syndrome and the associated health risks.Citation5 Fortunately, from an oncologic point of view, most men will initially respond to intermittent or continuous hormonal blockade.
Sip-T provides an additional therapy for men facing the terminal form of prostate cancer which was previously limited to second-line hormonal manipulations and docetaxel based chemotherapy. Recently, additional therapies are becoming available targeting this same patient population as described below. This adoptive immune approach has the potential to be combined with other approaches with different mechanisms of action (i.e., cytotoxic, microtubule inhibition, steroidal synthesis inhibition, and androgen receptor blockade) to maximize the therapeutic benefit and minimize the side effects to improve both the quantity and quality of life.
Background of Product Development
This product combines functional Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) and PAP (PA2024) with a patient's own leukapheresis product ex vivo to create APC8015 or Sip-T. PA2024 consists of functional human PAP linked by its carboxy terminus by a Gly-Ser linker to the N-terminus of functional human GM-CSF. PAP was the initial serum tumor marker for prostate cancer(PCA), being present on approximately 95% of PCA tumor cells. PAP has been the proposed target for PCA for decades.Citation4 GM-CSF is an immune growth and maturation factor, which has been heavily investigated in other vaccine models.Citation6 GM-CSF has been shown immune responses and tumor growth suppression in animal models. GM-CSF has also been used in clinical immunotherapy trials for the past several decades. Sip-T combines a patient ‘s immature immune cells, prostate-specific target and immune activation, resulting in an infusion of activated immune cells to a prostate-specific target, PAP.
Preclinical Studies and Proposed Mechanism of Action
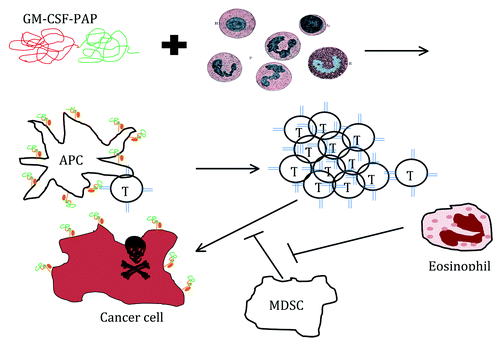
Preclinical studiesCitation7 have demonstrated the ability to target self-antigens on rat prostate tissue without generating severe autoimmune complications. It is clear that the most potent immune activator is the specific cell-mediated immune response. The proposed mechanism of Sip-T is based on our current understanding of the immunological response and the components of the final product. The immunology behind the design of Sip-T therapy is elegantly simple, targeting the very basic of adaptive immune T cell immune function that was elucidated largely in the early 1980s. In order for T cells to function, they must encounter cognate antigen on an activated antigen-presenting cell (APC). One of GM-CSF's pleiotropic effects is exerting potent growth and differentiation effects on APCs. By making a fusion protein (PA2024) of human PAP and human GM-CSF, this prostate-exclusive antigen is specifically delivered to APC elicited by GM-CSF and is a powerful way to ensure any stimulated APC will also be presenting PAP peptides to T cells specific for it. A patient’s leukapheresis cells that have been treated with GM-CSF/PAP are then re-infused. The theory is that the APC loaded with PAP peptide will activate PAP-specific T cells in the body, which will mobilize an army of effectors anti-prostate cancer T cells.
In the immune surveillance subset of clinical trial patients, a robust anti-PAP and anti-PA2024 T-Cell activation and antibody responses were generated. Interestingly, patients' T cell responses to PAP did not directly correlate with survival, possibly due to insufficient statistical power in trials conducted to date to adequately address this secondary objective. So what is the mechanism of Sipuleucel-T therapy? It currently remains unknown. Several theories are possible to explain the development of a PAP specific T-cell response without a survival benefit. However, a distinct possibility lies in the presence of myeloid-derived suppressor cells (MDSC) within the patient’s leukapheresis product. These cells comprise a heterogeneous population of immature monocytic and granulocytic cells, which can potently inhibit productive T cell activation and have the power to quench robust active T cell responses. MDSC have been detected in most types of human and rodent model cancers, and are thought to play an integral part in tumor progression. While MDSC have been reported in untreated prostate cancer patients,Citation8 there are no reports of MDSC status in Sip-T eligible patients. As GM-CSF is also a potent growth cytokine for MDSC, Sip-T therapy could also mobilize MDSC which, when returned to the patient, prevent T cell activation or inhibit any anti-PAP T cell responses that develop. An attractive argument for the lack of T cell correlation is that a subset of patients who developed anti-PAP T cells in the periphery; possessed tumor resident MDSC that inhibited the activity of stimulated anti-PAP T cells in situ.
Two aspects of the immune system that correlated more closely with outcome were the development of anti-PAP IgG/IgM and eosinophilia. While IgG and IgM could induce antibody-dependent cellular cytotoxicity, opsonization for tumoricidal macrophages, or complement-mediated lysis, there has been no data offered to support this. Eosinophilia has been observed in many human cancersCitation9,Citation10 and as a positive prognostic indicator in some,Citation11-Citation16 and rodent models show eosinophilic anti-tumor activity.Citation17 However, their role in the clinical tumor microenvironment is quite unclear. Eosinophilia has been associated with IL-2 therapy in animal models and clinical trials of patients with metastatic kidney cancer.Citation18 Eosinophilia per se may not be the reason for extended survival, but may indicate a perturbation in the granulocytic compartment that is inhibitory to the development or function of MDSC (). To date it is still unclear how Sip-T modulates the immune system and elucidation of the mechanism(s) may provide additional opportunities to enhance this exciting therapeutic and further favorably manipulate the immune system.
Figure 1. GM-CSF/PAP fusion protein is incubated with patients' WBC for APC activation and loading with PAP peptide. Cultured APC are infused to patient which then mobilize anti-PAP T cells. When these cells recognize a prostate tumor with PAP peptide, they deliver a lethal hit. MDSC can block this activity and render an immune response ineffective. Perhaps conditions favorable for eosinophil accumulation prevent MDSC generation or function.
Release Assays and Final Characterization of Product
The FDA approval of the final product is for a minimum of 50 million CD54 positive cells in 250 mL of lactated Ringer's solution. The release criteria include negative Gram Stain, endotoxin assay, and an in process sterility with 2-d incubation. Final 7-d sterility is released after infusion has occurred and the physician will be notified and specific antimicrobial would be recommended post infusion. Careful patient identification is verified from leukapheresis to infusion. The ex vivo exposure of PA2024 to the leukapheresis product must occur in a FDA approved GLP facility with stringent rules regarding infusion time from preparation.
Clinical Studies
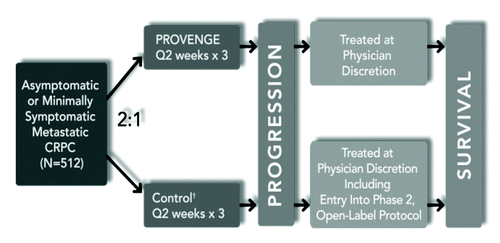
A series of clinical trials initially started in 1997 with FDA Approval based on the results of the pivotal IMPACT (D9902B) trial in 2010.() A series of Phase I/II trials were initiated. Thirteen patients with castrate resistant and metastatic prostate cancer were treated with APC8015 and then pulsed with injections of PA2024 at the Mayo Clinc.Citation19 As a BETA study it demonstrated both biologic efficacy and a safe toxicity assessment with 3/13 with greater than 50% declines in PSA and limited mild fevers, chills, myalgia, pain and fatigue, respectively. A Phase II trial at the Mayo treated 21 patients with 2 APC8015 infusions followed by 3 monthly PA2024 injections.Citation20 This Phase II trial again demonstrated a similar safety profile and less PSA declines, but did demonstrate a complete radiographic response lasting more than 4 y in one patient. In 1997 -1999 sequential Phase I and II trials at the University of California at San Francisco confirmed the desired biologic response and expected safety profile.Citation21 In this study 31 men were enrolled. The phase I trial included 12 heavily pretreated men who received increasing doses of APC8015 from 0.2 x 10^9 to 2.0 x 10^9 nucleated cells. Again, a safe toxicity assessment was seen but only 20% of the men demonstrated a PSA decline of greater than 25%.
In 2000, the common measure by both clinicians and the FDA was PSA response. Based on limited PSA response but strong immunological evidence and an excellent toxicity assessment the first Phase III D9901 trial using the current protocol of three APC8015 infusions in one month was initiated in 2000 with time to progression (TTP) as the main endpoint. The TTP endpoint was chosen since PSA was not deemed reliable with this treatment approach and survival endpoints are difficult to achieve in a suitable time frame. This studyCitation22 completed enrollment of 127 patients in 2005. This trial failed to demonstrate a statistical difference in the primary endpoint of TTP, but did demonstrate a benefit in TTP in a subset of men with Gleason Scores less than or equal to 7 out of 10. Once again a consistent safe toxicity profile was experienced. More importantly, the secondary endpoint of overall survival revealed at three years, 34% vs 11% of patients were alive comparing treatment to control, respectively. The median survival benefit for the treatment group was 4.5 mo (25.9% vs. 21.4%). A companion phase III trial, D9902A, enrolled 98 men with a primary endpoint of TTP and demonstrated similar results.
Using the same treatment protocol and changing the primary endpoint to overall survival the pivotal Phase III trial D9902B or IMPACT trial enrolled 512 men and demonstrated improved overall survival with median survival improved by 25.8% vs. 21.7% and maintained the safety profile.Citation23 The main adverse events in the integrated safety data with treatment arm vs. placebo were chills (53.1 vs. 10.8), fatigue (41.1% vs. 34.7%), fever (31.3% vs. 9.6%), back pain (29.6% vs. 28.7%), nausea (21.5% vs. 14.9%), joint ache (19.6% vs. 20.5%) and headache (18.1% vs. 6.6%).
A series of ongoing analysis and active trials (IMPACT, ProACT, OpenACT, NeoAct) and proposed trials combining Sip-T with other approved therapeutics continue to provide supportive immunologic information and continued safety data. Continued analysis of the data from these trials should allow clinicians to expand the range of patients treated and the combinations of therapy utilized to maximize the therapeutic benefit of this agent while minimizing the side effect profile of these newer agents.
Regulatory Issues
The approval of Sip-T was eventually based on the survival benefit of the IMPACT trial and the integrated safety profile of the other trials outlined above. Being the first autologous therapeutic vaccine provides challenges for the FDA and Dendreon. Dendreon submitted to a Biologics License Application (BLA) and in January of 2007 the FDA assigned that BLA to priority review status. Priority Review Status is given to products with an ability to enhance the safety or efficacy of a treatment of a lethal disease. In March of 2007 the advisory committee to the FDA recommended approval by a majority vote based on the efficacy and safety data available. Despite this recommendation, the FDA in May of 2007 decided that evidence was still insufficient for approval. On April 29, 2010, after the completion of the IMPACT trial the FDA approved Sip-T for men with minimally symptomatic or asymptomatic castrate-resistant metastatic prostate cancer.
Public Health Issues
The development of a therapeutic adoptive immunotherapy for prostate cancer has demonstrated improved survival in men with advanced prostate cancer. It is clear in a specific cohort that this improved survival is secondary to a direct immune response to PAP. The ability to generate a specific immune response the a target on 95% of prostate cancer cells begs the question of whether this approach could be used much earlier in the disease. OthersCitation24 have argued that the placebo group was negatively advantaged by the three leukapheresis procedures and the survival benefit realized was artificial. A trial targeting patients at high risk for recurrence after prostatectomy has not matured but interim analysis has demonstrated improved outcomes in patients receiving a period of hormonal blockade with or without Sip-T. The true benefit of this novel therapy to men with PCA will only be realized by continued investigation of this product including rigorous immune correlation with responses. These ongoing and future investigations will allow clinicians and patients to make educated decision about use of this product.
Commercial Issues
Establishing a personalized therapeutic vaccine presented new challenges during commercialization. Initially, Dendreon, Inc. used a corporate production facility to generate and test the product for clinical trials. During the D9901 trial, a 12-station production facility in New Jersey handled the clinical trial volume. This facility was increased to 48 workstations and approved for use 3/10/11 and the Los Angeles production facility came online with 36 stations was approved on 6/29/11. An additional facility in Atlanta, Georgia is awaiting approval and will further increase capacity. Due to the personalized nature of this product, the ramp up after approval was initially limited to 50 centers around the country. Currently there are close to 500 approved infusion sites.
Advantage and Disadvantage Relative to Other Approaches
Prior to approval of Sip-T, the treatment of castrate resistant prostate cancer relied on additional methods of targeting the androgen pathway. Secondary hormonal manipulation with antiandrogens (e.g., Flutamide (Eulexin), Bicalutamide (Casodex), Nilutamide (Nilandron)), block activation of the androgen receptor and are associated with additional treatment effect over LHRH agonists (e.g., Leuprolide acetate (Viadur, Eligard, Lupron),Goserelin acetate (Zoladex), Triptorelin (Trelstar) or LHRH antagonist (e.g., degarelix acetate (Firmagon)). Additional benefit can be derived by removal of these agents known as an anti-androgen withdrawal syndrome. Super anti-androgens such as MDV3100,Citation25 which have a 5-fold stronger binding affinity over bicalutamide,Citation26 have clinically demonstrated significant added benefit in survival, and is approaching approval.
Ketoconazole, an approved antifungal agent inhibits steroidal genesis has been a successful means of secondary hormonal manipulation in combination with corticosteroid replacement. On April 28, 2011 the FDA approved an oral agent abiraterone acetate (Zytiga) which inhibits CYP17A1 enzyme and therefore inhibits the conversion of pregnenolone and progesterone to dehydroepiandrosterone (DHEA) and androstenedione, respectively. Both DHEA and androstenedione are precursors of testosterone. The international multicenter trial that lead to approval enrolled 1195 men who received abiraterone acetate plus prednisone had a median overall survival duration of 14.8 mo, compared with 10.9 mo for patients receiving prednisone plus placebo. The most common adverse events observed were fluid retention (30.5% vs. 22.3%), hypokalemia (17.1% vs. 8.4%) with grade 3/4 hypokalemia (3.8% vs. 0.8%) and grade 3/4 hypertension (1.3% vs. 0.3%), liver function test abnormalities (10.4% vs. 8.1%), cardiac problems (12.5% vs. 9.4)%.Citation27,Citation28Abiraterone acetate (Zytiga) has been approved in post-docetaxel metastatic CRPC patients, but ongoing trials suggest activity in chemotherapy naïve patients. Abiraterone phase III trial results in the chemotherapy naïve population are expected in 2012.
Prior to the approvals of Sip-T and abiraterone acetate (Zytiga), docetaxel chemotherapy was the next treatment in men with castrate-resistant, metastatic PCA who failed second line hormonal manipulations. Shortly after the approval of Sipuleucel-T a second-line chemotherapy was approved on June 17, 2010 with cabazitaxel (Jevtana). Cabazitaxel is a microtubular inhibitor approved for men after failed docetaxel therapy. A phase 3 trial demonstrated a statistically significant 30% reduction in risk of death compared with mitoxantrone and prednisone with a median survival (15.1 vs 12.7). Median survival benefits have been demonstrated with each of these approaches.()
Table 1. Comparison of Treatments for Castrate Resistant Metastatic Prostate Cancer
Summary
The second decade of the 21st century appears to be a very exciting time in therapeutic development for advanced prostate cancer and cancer vaccine development. The prior decades of preclinical and clinical research have lead to the approval of Sip-T as the first autologous therapeutic cancer vaccine. This new paradigm in cancer therapeutics has already established a basis for extending this approach to other cancers and should continue to provide insights into the immune response to cancer allowing additional discoveries. Furthermore, other highly effective non-immunologic approaches to prostate cancer treatment have been approved and if combined with Sip-T may synergize in effectiveness, providing tremendous benefit to prostate cancer patients.
| List of Abbreviations | = | PAP, Prostate Acid Phosphatase |
| GM-CSF | = | Granulocyte-Macrophage Colony Stimulating Factor |
| PA2024 | = | PAP-GM-CSF Chimeric Protein |
| APC8015 | = | Activated Antigen Presenting Cells |
| PCA | = | Prostate Cancer |
| IMPACT | = | IMmunotherapy for Prostate AdenoCarcinoma Treatment |
| APC | = | antigen-presenting cell |
| Sipuleucel-T | = | (Provenge)- Sip-T or APC8015 |
| PSA | = | prostate-specific antigen |
| PSMA | = | prostate-specific membrane antigen |
Acknowledgments
The authors would like to thank the patients and their families for participating in clinic trials that lead to novel therapies being approved. Additionally, we would like to thank Caroline Gardner for her editorial expertise.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62:10 - 29; http://dx.doi.org/10.3322/caac.20138; PMID: 22237781
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127:2893 - 917; http://dx.doi.org/10.1002/ijc.25516; PMID: 21351269
- Pound CR, Brawer MK, Partin AW. Evaluation and treatment of men with biochemical prostate-specific antigen recurrence following definitive therapy for clinically localized prostate cancer. Rev Urol 2001; 3:72 - 84; PMID: 16985694
- Killian CS, Emrich LJ, Vargas FP, Yang N, Wang MC, Priore RL, et al. Relative reliability of five serially measured markers for prognosis of progression in prostate cancer. J Natl Cancer Inst 1986; 76:179 - 85; PMID: 2418245
- Grossmann M, Zajac JD. Management of side effects of androgen deprivation therapy. [x.] Endocrinol Metab Clin North Am 2011; 40:655 - 71, x; http://dx.doi.org/10.1016/j.ecl.2011.05.004; PMID: 21889727
- Sanda MG, Ayyagari SR, Jaffee EM, Epstein JI, Clift SL, Cohen LK, et al. Demonstration of a rational strategy for human prostate cancer gene therapy. J Urol 1994; 151:622 - 8; PMID: 8308972
- Fong L, Ruegg CL, Brockstedt D, Engleman EG, Laus R. Induction of tissue-specific autoimmune prostatitis with prostatic acid phosphatase immunization: implications for immunotherapy of prostate cancer. J Immunol 1997; 159:3113 - 7; PMID: 9317107
- Vuk-Pavlović S, Bulur PA, Lin Y, Qin R, Szumlanski CL, Zhao X, et al. Immunosuppressive CD14+HLA-DRlow/- monocytes in prostate cancer. Prostate 2010; 70:443 - 55; PMID: 19902470
- Lowe D, Jorizzo J, Hutt MS. Tumour-associated eosinophilia: a review. J Clin Pathol 1981; 34:1343 - 8; http://dx.doi.org/10.1136/jcp.34.12.1343; PMID: 7035499
- Samoszuk M. Eosinophils and human cancer. Histol Histopathol 1997; 12:807 - 12; PMID: 9225164
- Nielsen HJ, Hansen U, Christensen IJ, Reimert CM, Brünner N, Moesgaard F. Independent prognostic value of eosinophil and mast cell infiltration in colorectal cancer tissue. J Pathol 1999; 189:487 - 95; http://dx.doi.org/10.1002/(SICI)1096-9896(199912)189:4<487::AID-PATH484>3.0.CO;2-I; PMID: 10629548
- Dorta RG, Landman G, Kowalski LP, Lauris JR, Latorre MR, Oliveira DT. Tumour-associated tissue eosinophilia as a prognostic factor in oral squamous cell carcinomas. Histopathology 2002; 41:152 - 7; http://dx.doi.org/10.1046/j.1365-2559.2002.01437.x; PMID: 12147093
- Ownby HE, Roi LD, Isenberg RR, Brennan MJ. Peripheral lymphocyte and eosinophil counts as indicators of prognosis in primary breast cancer. Cancer 1983; 52:126 - 30; http://dx.doi.org/10.1002/1097-0142(19830701)52:1<126::AID-CNCR2820520123>3.0.CO;2-Y; PMID: 6850535
- Goldsmith MM, Belchis DA, Cresson DH, Merritt WD 3rd, Askin FB. The importance of the eosinophil in head and neck cancer. Otolaryngol Head Neck Surg 1992; 106:27 - 33; PMID: 1734363
- Dalal BI, Das KC, Dutta TK, Malakar K. Local and systemic eosinophilia in patients with carcinoma of the uterine cervix undergoing radiation therapy: correlation with radiation response. Clinical oncology (Royal College of Radiologists (Great Britain)) 1992;4:18-21.
- Bethwaite PB, Holloway LJ, Yeong ML, Thornton A. Effect of tumour associated tissue eosinophilia on survival of women with stage IB carcinoma of the uterine cervix. J Clin Pathol 1993; 46:1016 - 20; http://dx.doi.org/10.1136/jcp.46.11.1016; PMID: 8254087
- Mattes J, Hulett M, Xie W, Hogan S, Rothenberg ME, Foster P, et al. Immunotherapy of cytotoxic T cell-resistant tumors by T helper 2 cells: an eotaxin and STAT6-dependent process. J Exp Med 2003; 197:387 - 93; http://dx.doi.org/10.1084/jem.20021683; PMID: 12566422
- van Haelst Pisani C, Kovach JS, Kita H, Leiferman KM, Gleich GJ, Silver JE, et al. Administration of interleukin-2 (IL-2) results in increased plasma concentrations of IL-5 and eosinophilia in patients with cancer. Blood 1991; 78:1538 - 44; PMID: 1884020
- Burch PA, Breen JK, Buckner JC, Gastineau DA, Kaur JA, Laus RL, et al. Priming tissue-specific cellular immunity in a phase I trial of autologous dendritic cells for prostate cancer. Clin Cancer Res 2000; 6:2175 - 82; PMID: 10873066
- Burch PA, Croghan GA, Gastineau DA, Jones LA, Kaur JS, Kylstra JW, et al. Immunotherapy (APC8015, Provenge) targeting prostatic acid phosphatase can induce durable remission of metastatic androgen-independent prostate cancer: a Phase 2 trial. Prostate 2004; 60:197 - 204; http://dx.doi.org/10.1002/pros.20040; PMID: 15176049
- Small EJ, Fratesi P, Reese DM, Strang G, Laus R, Peshwa MV, et al. Immunotherapy of hormone-refractory prostate cancer with antigen-loaded dendritic cells. J Clin Oncol 2000; 18:3894 - 903; PMID: 11099318
- Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, Valone FH, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol 2006; 24:3089 - 94; http://dx.doi.org/10.1200/JCO.2005.04.5252; PMID: 16809734
- Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al, IMPACT Study Investigators. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010; 363:411 - 22; http://dx.doi.org/10.1056/NEJMoa1001294; PMID: 20818862
- Huber ML, Haynes L, Parker C, Iversen P. Interdisciplinary critique of sipuleucel-T as immunotherapy in castration-resistant prostate cancer. J Natl Cancer Inst 2012; 104:273 - 9; http://dx.doi.org/10.1093/jnci/djr514; PMID: 22232132
- Scher HI, Beer TM, Higano CS, Anand A, Taplin ME, Efstathiou E, et al, Prostate Cancer Foundation/Department of Defense Prostate Cancer Clinical Trials Consortium. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1-2 study. Lancet 2010; 375:1437 - 46; http://dx.doi.org/10.1016/S0140-6736(10)60172-9; PMID: 20398925
- Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 2009; 324:787 - 90; http://dx.doi.org/10.1126/science.1168175; PMID: 19359544
- Pal SK, Sartor O. Phase III data for abiraterone in an evolving landscape for castration-resistant prostate cancer. Maturitas 2011; 68:103 - 5; http://dx.doi.org/10.1016/j.maturitas.2010.10.009; PMID: 21093995
- Martin JM, Supiot S, Berthold DR. Pharmacotherapeutic management of locally advanced prostate cancer: current status. Drugs 2011; 71:1019 - 41; http://dx.doi.org/10.2165/11591500-000000000-00000; PMID: 21668040