Abstract
We prospectively compared serum antibody levels of 5 Streptococcus pneumoniae (Spn) proteins: PcpA PhtD, PhtE Ply and LytB associated with nasopharyngeal (NP) colonization and acute otitis media (AOM) infection in a cohort of 6–30 mo old children. Antigen-specific antibody titers were determined by ELISA. A total of 731 visits among 168 children were studied. There were 301 Spn NP colonization episodes documented in 109 (65%) children and 42 Spn AOM episodes in 34 (20%) children. IgG antibody titers to the 5 proteins were significantly different among children over time (p < 0.001), with a rank order as follows: PcpA > PhtE = PhtD > Ply > LytB Characterization of IgG and IgM acute and convalescent serum antibody levels of Spn AOM infection showed the kinetics of the response differed among children, with the same rank order of antibody levels over time. Individual data showed that some children responded to AOM with an antibody increase to one or more of these Spn proteins but some children failed to respond. We conclude that antibody levels to Spn proteins PcpA PhtD, PhtE, Ply and LytB, all rise over time in children age 6 to 30 mo following natural exposure to Spn after NP colonization and AOM; however, there were significant differences in quantity of antibody elicited among these potential vaccine antigens.
Introduction
Streptococcus pneumoniae (Spn) is a frequent cause of episodic and recurrent acute otitis media (AOM) in children in the United States.Citation1-Citation3 AOM and all respiratory bacterial infections begin pathogenesis with nasopharynx (NP) colonization. However, colonization is mostly asymptomatic; only when the condition of the host is altered, Spn may invade the middle ear, causing AOM.
A vaccine against Spn based on the organism’s proteins is an area of current research as it is anticipated that new serotypes will likely emerge under the selection pressure of vaccination with pneumococcal polysaccharide conjugates.Citation4 Several Spn proteins have been eliminated as vaccine candidates due to surface epitope heterogeneity, variable expression or other characteristics. Desirable Spn candidate antigens should be conserved among strains and immunogenic in children and adults. In the work reported here, we studied five Spn protein candidates: PhtD, the detoxified pneumolysin derivative, PlyD1, and a truncated form of LytB, PcpA and PhtE. PhtD and PhtE are pneumococcal histidine triad (Pht) proteins D and E with possible complement binding/degrading/interaction activity.Citation5 LytB is a pneumococcal choline binding protein that is a cell wall hydrolaseCitation6 and PcpA is a choline binding surface protein that elicits protection against pneumococcal infection in an animal model.Citation7,Citation8 Pneumolysin (Ply) has a wide range of cytotoxic and inhibitory effects on host tissue and immune cells.Citation9 In this study, we used the pneumolysin derivative, PlyD1, which has three point mutations that do not interfere with anti-pneumolysin antibody responses.
For Spn vaccine development it is useful to know whether a target antigen is immunogenic in the human host in the age time frame when vaccination is anticipated to be given. This study sought an answer to that question by measurement of antibodies elicited in young children after natural Spn exposure such as after asymptomatic NP colonization and after a local infection such as AOM. To our knowledge, this is the first study to prospectively compare the natural antibodies elicited to 5 Spn proteins simultaneously in a cohort of children 6–30 mo of age during NP colonization and AOM. The comparisons of interest we report here include: 1. Changes in the levels of PhtD, LytB, PcpA, PhtE and Ply-specific IgG antibodies in children as they increased in age from 6 to 30 mo of age; 2. Changes in antibody levels following detected colonization of the NP with Spn and AOM infection by Spn; 3. Characterization of IgG and IgM in acute and convalescent serum following Spn AOM; and 4. Variations in individual antibody repertoire and responses in the AOM vaccine target age of children.
Results
NP colonization and AOM events. A total of 731 visits among 168 children were studied. There were 301 Spn NP colonization episodes documented in 109 (65%) children and 42 Spn AOM episodes in 34 (20%) children. Because the study design called for NP/OP sampling at 7 specific times separated by 3 to 6 mo, some Spn colonization events were not detected by culture but most likely occurred as reflected in significant rises in specific antibody to one or more of the Spn antigens studied.
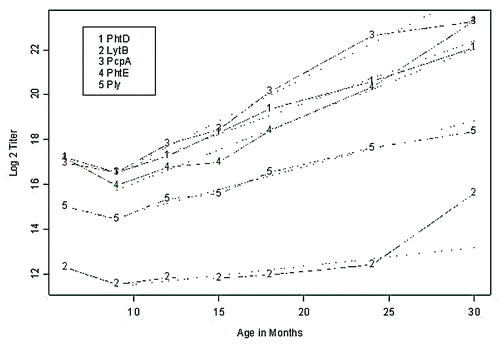
Natural acquisition of serum antibody to proteins (PhtD, LytB, PcpA, PhtE and Ply) over time. shows the geometric mean end point titers of serum antibodies to PhtD, LytB, PcpA, PhtE and Ply at 6, 9, 12, 15, 18, 24, and 30 mo of age corresponding to visits 1–7 for the study cohort. A decrease between 6 to 9 mo is evident, whereas a linear gradient is observed between 9 to 30 mo. Estimated slopes (log 2 titer / month) were 0.28 (SE = 0.02), 0.08 (SE = 0.02), 0.38 (SE = 0.03), 0.30 (SE = 0.02), 0.20 (SE = 0.02) for PhtD, LytB, PcpA, PhtE and Ply, respectively. At 6 mo, no significant difference was detected between PhtD, PcpA and PhtE antibody levels, while significant differences (p < 0.05) were detected between Ply and LytB, and between these and the remaining 3 proteins. A similar conclusion holds at 9 mo. At 15 mo and higher, all pairs of antibody levels to the specific proteins are significantly different (p < 0.05). Overall, IgG antibody titers to the 5 proteins were significantly different among the study children: PcpA > PhtD = PhtE > Ply > LytB (p < 0.001).
Figure 1. Serum IgG antibody levels to Spn proteins PhtD, LytB, PcpA, PhtE and Ply in healthy children increased with age. Natural acquisiton of serum IgG antibodies to Spn proteins PhtD, LytB, PcpA, PhtE and Ply. Plots of the geometric mean titers (end point titer) during 7 sampling visits at 6, 9, 12, 15, 18, 24 and 30 mo of age. The y axis is changed to log2 scale. The numbers of sera included at each time point were 107, 88, 65, 61, 55, 44, and 6. A linear regression fit from 9 to 30 mo is superimposed.
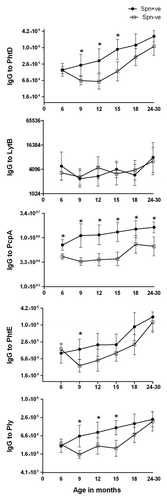
Comparison of serum antibody levels to PhtD, LytB, PcpA, PhtE and Ply in children Spn NP colonized vs. non-colonized children, at various ages. We compared the level of antibody to PhtD, LytB, PcpA, PhtE and Ply in 21, 21, 18, 23, 15 and 24 children who were culture-positive for Spn at age 6, 9, 15, 18, and 24–30 mo respectively with 71, 62, 45, 39, 34 and 27 children of the same age who were not culture-positive. The patterns were significantly different for the 5 proteins (). For PhtD, a significant difference was shown at age 9, 12, and 15 mo. For LytB, no significant difference was seen between those with detected colonization and those without detected colonization at any age. For PcpA, the differences between children with detected colonization and those without was significantly different at all the ages (p value < 0.001). For PhtE, a significant difference was identified between children with detected colonization and those without detected colonization at 9 mo of age. For Ply, a significant difference was identified between children with detected colonization and those without detected colonization at age 9, 12 and 15 mo. Variation in antibody quantity to each of the studied proteins was noted in both colonized and non-colonized groups and contributed to the absence of significant differences among groups for some time points. Overall, NP colonization was associated with variably higher IgG antibody levels to PcpA > PhtE > PhtD > Ply > LytB (p < 0.001).
Figure 2. Comparison of serum IgG antibody GMTs to Spn proteins PhtD, LytB, PcpA, PhtE and Ply in NP colonized (•Spn+ve) and non-colonized (□ Spn-ve) healthy children from 6 mo to 30 mo of age. To better represent the data, y axis is changed to log2 scale. Mann-Whitney test was used to compare the colonized vs. non-colonized children at particular visits for all the proteins. The * sign indicates that the difference in colonized vs uncolonized children were statistically significant: for PhtD at visit 9, 12 and 15 mo with p < 0.001; for PcpA at all the visits (p < 0.001), for PhtE at 9 mo with p < 0.05 and for Ply at 9, 12 and 15 mo with p < 0.005.
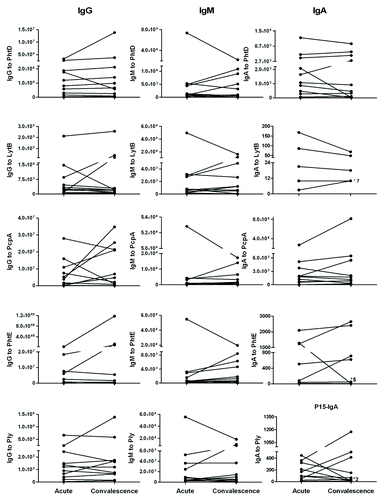
Paired Acute and Convalescent Serum IgG, IgM and IgA antibody levels to Spn proteins in children with AOM. The geometric mean titers of IgG, IgM and IgA antibody at acute and convalescence sera of AOM children is shown in . In individual responses of children to all five proteins before and after an AOM are shown. When we examined the individual responses for the 11 paired sera available we observed that 5 (50%) of 10 children showed a rising IgM and to a lesser extent IgG antibody level, suggesting a primary antibody response. Four (40%) of 10 children showed a rising IgG and to a lesser extent IgM antibody level suggesting a secondary antibody response. One (10%) of 10 children had no change in antibody level between acute and convalescent sera suggesting the absence of an antibody response. Serum IgA antibody levels were largely unchanged comparing acute to convalescent sera for all 5 proteins. No protein had a significant difference in the frequency of rises in IgA among the children. Among these 11 children, 5 children have AOM caused by serotype 19A, 3 of serotype 11, one of serotype 4, one child of serotype 21 or 39 and one of both serotype 15 and 19A. We did not observe any correlation of between serotype and antibody titers to the studied Spn proteins
Table 1. Comparison of serum IgG, IgM and IgA antibody to Spn proteins PhtD, LytB, PcpA, PhtE and Ply in the acute and convalescence stage of AOM
Discussion
Progress in the development of a serotype-independent, protein-based Spn vaccine to prevent AOM is hampered by gaps in knowledge of the immune response to vaccine candidates. Here we have characterized the serum antibody response to 5 Spn proteins mounted by children who experienced Spn NP colonization and AOM. We found a rise in the serum levels of PhtD, LytB, PcpA, PhtE and Ply-specific IgG antibodies in children as they increased in age from 6 to 30 mo of age. The rank order of antibody concentration was highest to PcpA followed by PhtE, then PhtD, then Ply and last LytB. Changes in serum antibody levels following detected NP colonization without progression to AOM and NP colonization associated with AOM caused by Spn were of a similar magnitude and rank order. Examination of specific IgG and IgM antibody responses with AOM showed that some children experienced a primary response, others a secondary response and others an absent response (no change in acute vs. convalescence antibody titer) to the studied Spn proteins in this age group of children.
Antibody responses in serum have been studied previously following NP colonization in children for Spn protein antigens PhtD, CbpA, LytC, PsaA, PspA and Ply.Citation12-Citation14 In those studies, similar to ours, the gradual acquisition of serum antibody in children over time was observed, Spn exposure resulted in higher serum antibody titers and rises in antibody titers had different kinetics for different antigens studied. For example, Holmlund et al.Citation12 studied the serum antibody concentrations to PspA, PsaA, Ply, PhtD, CbpA and LytC in pregnant women, cord blood samples and young children from 6 weeks to 10 mo of age. The children had NP cultures obtained at the time of the serum samples to detect Spn colonization events. They found similar GMCs for all proteins in the cord blood and maternal samples. In the young children, the GMCs for PhtD, CbpA, LytC, PspA and Ply decreased until 18 weeks of age and started to increase after that age suggesting that the infant’s own antibody production started to rise at 4 to 5 mo of age. The increase in antibody by the young children did not approach the levels of the adult mothers by the age of 10 mo. Unlike the other pneumococcal protein antigens, the GMCs of anti-PsaA increased significantly as the children aged and reached the GMC of the mothers by 3.5 mo of age. The increase in antibody concentration in young infants was found to be associated with Spn NP colonization events but followed different kinetics depending upon the Spn antigen, similar to our study.
In a study with similar design to ours, Simell et al.Citation13 evaluated the development of serum anti-CbpA and –PhtD in young children at 6, 12, 18 and 24 mo of age in relation to Spn exposure and obtained MEF at the time of AOM to determine etiology. Serum samples from mothers were obtained at the infant’s 2-mo visit. They found the GMCs of serum anti-CbpA and –PhtD decreased between 6 and 12 mo of age and started to increase thereafter. Despite the increase in serum antibody the levels in 24 mo olds were well below that of the adult mothers. Again Simell et al.Citation13 showed that the development of GMCs to Spn antigens was strongly associated with NP or MEF cultures positive for Spn. Children with prior cultures positive for Spn had significantly higher GMCs as compared with children without prior cultures positive for Spn. In addition to the above findings, Simell et al.Citation13 looked at the GMCs for CbpA and PhtD at 12 and 18 mo of age and the relationship to the subsequent development of Spn AOM in the following 6 mo. Children with higher serum anti-CbpA showed a trend toward lower rates of Spn AOM during the subsequent 6 mo.
Our group previously reported that NP colonization was an immunizing event in children relative to NTHi outer membrane proteins D, P6 and OMP26.Citation10,Citation15 When we examined differences between antibody levels in children who had Spn NP colonization detected at various time points between 6 and 30 mo of age, we did observe greater increases in serum antibody to PhtD, PcpA, PhtE and Ply but not LytB compared with children who did not have detected Spn NP colonization. We interpret those findings to indicate that NP colonization was an immunizing event. However, even among the children who did not have Spn NP colonization detected, rises in serum antibody were noted. Probably NP colonization by Spn occurred between study visits without detection due to NP sampling frequency. The significant difference in antibody level among detected NP colonized compared with undetected colonized children beginning at age 6 mo for PcpA and for PhtD, PhtE and Ply several months later provides evidence that NP colonization with Spn is associated with stimulation of serum antibody to proteins expressed by the bacteria at the target age for an AOM vaccine.
When we examined the isotypes of serum antibody in acute and convalescent sera surrounding an AOM for the subset of children where we had paired serum, we found variable IgG and IgM antibody levels. The repertoire of antibody detected was consistent with primary, secondary and absent immune response with AOM. In earlier work, Samukawa et al.Citation16 studied the immune response to Spn surface protein A (PspA) in the sera of various age groups in the general population. In the first 2 y of life they found comparable amounts of IgG and IgM serum antibodies to PspA whereas in adults IgG predominated. In contrast, Virolainen et al.Citation17 evaluated serum antibodies in children with AOM to Ply. Eight of 10 children experienced a seroconversion in Ply antibody levels at a median age of 20 mo old but all were of the IgA class only. Rapola et al.Citation18 studied the serum antibody response to Ply and pneumococal surface adhesion A (PsaA) in children with AOM age 2 mo to 2 y. Antibody levels were compared among three groups: Spn NP colonized, Spn AOM, and neither colonized nor AOM infections due to Spn. At the time of the sampling, children with NP colonization had the highest anti-Ply and PsaA antibody levels, children with a current AOM were next highest, then children with no current but a past history of Spn NP colonization or AOM lower. Lowest were those with no current or previous documented history of Spn colonization or AOM. Wide variations in antibody levels were measured. Age of the child (as in our study) and pre-existing antibody level were identified as important covariates in predicting an antibody response to NP colonization.
In the course of a prospective study still in progress, we have described serum antibody responses to NTHi protein P6, OMP26 and protein D in healthy children after NP colonization and with infrequent (AOM 10), the influence of breastfeeding on the frequency of AOM and on serum antibody levels to NTHi vaccine candidate protein P6,Citation15 and serum antibody responses to NTHi protein P6, OMP26 and protein D in otitis prone children.Citation19
Our study has limitations. The difficulty in obtaining blood from young children repetitively between 6 and 30 mo of age is considerable, particularly when coupled with additional blood drawing for acute and convalescent levels surrounding an infection such as AOM. Therefore, despite best efforts, we did not obtain blood samples from every child at every visit as designed. This created windows of missing data that we addressed as we could statistically. The analysis of acute and convalescent IgG and IgM antibody levels points to the complexity in defining an acute and convalescent time point for AOM infection, and even more so for NP colonization when sampling is spaced by several months as in this study. The proteins we studied are known to be properly folded up to pH 9.0 as determined by biophysical methods and immunization of animals eliciting antibodies that react with intact bacteria in an antibody binding assay with detection by flow cytometry and providing protection against S. pneumoniae challenge. However, we cannot be certain that the adsorption of the proteins to a solid phase at pH 9.4 has not affected the structure of the proteins.
This paper adds to a body of literature evaluating our prospectively enrolled, longitudinal study cohort, with a focus on Spn vaccine antigens. For Spn we have previously described antibody responses to PcpA PhtD, PhtE Ply and LytB in non-otitis prone vs. otitis prone children.Citation20 We have shown that PcpA is an adhesin of Spn that facilitates adherence to human NP and lung epithelia and that human antibody to PcpA can function to reduce adherence of Spn to NP cells.Citation21 We have clearly established the role of PhtD and PhtE in Spn adherence to human upper and lower airway epithelial cells and that human antibody directed to either PhtD or PhtE causes a direct effect of blockage of adhesion of Spn to the human epithelial cells.Citation22 The importance of demonstrating acquisition of serum antibody in a pediatric cohort at an age when vaccination is most likely to occur is paramount to encourage further study. In that regard our observations are strongly supportive of the potential of PcpA, PhtE, PhtD and Ply to be incorporated in pneumococcal vaccines against Spn infection in children.
Methods
General design. This report includes data for the time span June, 2006 to December, 2009 from children enrolled in a prospective study supported by the National Institutes of Deafness and Communication Disorders as previously described.Citation10 Healthy children without previous episodes of AOM were enrolled from a middle class, suburban socio-demographic pediatric practice in Rochester, NY (Legacy Pediatrics). Healthy children had serum, NP and oropharyngeal (OP) cultures and NP wash samples obtained seven times, every 3–6 mo, between 6 and 30 mo of age (at age 6, 9, 12, 15, 18, 24, and 30 mo). Since the samples were collected over time, not all children completed their participation at the time when this analysis was performed. In addition, if a child developed symptoms compatible with AOM, they were examined by validated otoscopist pediatricians with pneumatic otoscopy and if middle ear infection was suspected a tympanocentesis was performed to confirm the diagnosis. At the time of the acute AOM diagnosis and three weeks later acute and convalescent serum, NP and OP cultures and NP wash samples were obtained. All children had received pneumococcal conjugate vaccine containing 7 serotypes at age 2, 4, 6, and 15 mo of age. The study was approved by the University of Rochester and Rochester General Hospital Research Subjects Review Board and written informed consent was obtained for participation and all procedures.
Sample collection. NP, OP, middle ear fluid (MEF) sampling was as previously described.Citation11
Microbiology. Bacteria were isolated as previously described.Citation11
Detection of protein-specific antibodies byELISA. Protein- specific antibody titers were determined by ELISA using purified recombinant proteins (provided by sanofi pasteur). The proteins were stored in a neutral pH buffer. Under these conditions, the proteins are properly folded, as evidenced by: i) Biophysical methods, such as DSC, fluorescence, CD and FTIR, and ii) Immunization of animals elicited antibodies that react with intact bacteria in an antibody binding assay with detection by flow cytometry and provided protection against S. pneumoniae challenge. The 96-well Nunc-Immulon-4 plates were coated with 0.25 μg-0.5 μg/ml of individual protein antigens (100μl/well) in coating buffer (bicarbonate, [pH 9.4]) and incubated overnight at 4°C. After washing the plates were blocked with 3% skim milk at 37°C for 1h (200 μl per well). After five washes, 100 μl of serum at a starting dilution of 1:100 (in PBS-3% skim milk) was added to the wells and diluted serially 2 fold. The mixture was incubated at room temperature for 1 h. followed by the addition of affinity purified goat anti-human IgG, IgM or IgA antibody conjugated to hoarseradish-peroxidase (Bethyl Laboratories, Inc., Montgomery, TX) as a secondary antibody. The reaction products were developed with TMB Microwell Peroxidase Substrate System (KPL, Gaithersburg, MD), stopped by the addition of 1.0 molar phosphoric acid and read by an automated ELISA reader using a 450-nm filter. Results are reported as end-point titers. An in-house positive control serum (mixture of human sera) was run on each plate to control for plate-to-plate variability. The inter-test coefficient of variation was ≤ 30%.
Statistics. In general, significance levels of comparisons of titer levels was performed using a paired two-sample procedure for pairwise comparisons, or a Friedman's test for multiple treatments, using the subject as blocking variable. In cases where the block design is significantly incomplete, a bootstrap procedure using subjects for replicates was employed. Titer levels were first transformed by a base 2 logarithm.
| Abbreviations: | ||
| Acute Otitis Media | = | AOM |
| Nasopharyngeal | = | NP |
| Oropharyngeal | = | OP |
Acknowledgments
This study was supported by sanofi pasteur and NIH NIDCD RO1 08671. Arthur Chang assisted in preparation of the figures. We thank Sally Thomas, LPN, CCRC, the nurses and staff of Legacy Pediatrics and the collaborating pediatricians from Sunrise Pediatrics, Westfall Pediatrics, Lewis Pediatrics and Long Pond Pediatrics and the parents who consented and the children who participated in this long and challenging study.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Casey JR, Pichichero ME. Changes in frequency and pathogens causing acute otitis media in 1995-2003. Pediatr Infect Dis J 2004; 23:824 - 8; http://dx.doi.org/10.1097/01.inf.0000136871.51792.19; PMID: 15361720
- Block SL, Hedrick J, Harrison CJ, Tyler R, Smith A, Findlay R, et al. Community-wide vaccination with the heptavalent pneumococcal conjugate significantly alters the microbiology of acute otitis media. Pediatr Infect Dis J 2004; 23:829 - 33; http://dx.doi.org/10.1097/01.inf.0000136868.91756.80; PMID: 15361721
- Casey JR, Adlowitz DG, Pichichero ME. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J 2010; 29:304 - 9; PMID: 19935445
- Pichichero ME, Casey JR. Emergence of a multiresistant serotype 19A pneumococcal strain not included in the 7-valent conjugate vaccine as an otopathogen in children. JAMA 2007; 298:1772 - 8; http://dx.doi.org/10.1001/jama.298.15.1772; PMID: 17940232
- Adamou JE, Heinrichs JH, Erwin AL, Walsh W, Gayle T, Dormitzer M, et al. Identification and characterization of a novel family of pneumococcal proteins that are protective against sepsis. Infect Immun 2001; 69:949 - 58; http://dx.doi.org/10.1128/IAI.69.2.949-958.2001; PMID: 11159990
- García P, González MP, García E, López R, García JL. LytB, a novel pneumococcal murein hydrolase essential for cell separation. Mol Microbiol 1999; 31:1275 - 81; http://dx.doi.org/10.1046/j.1365-2958.1999.01238.x; PMID: 10096093
- Glover DT, Hollingshead SK, Briles DE. Streptococcus pneumoniae surface protein PcpA elicits protection against lung infection and fatal sepsis. Infect Immun 2008; 76:2767 - 76; http://dx.doi.org/10.1128/IAI.01126-07; PMID: 18391008
- Hava DL, Camilli A. Large-scale identification of serotype 4 Streptococcus pneumoniae virulence factors. Mol Microbiol 2002; 45:1389 - 406; PMID: 12207705
- Marriott HM, Mitchell TJ, Dockrell DH. Pneumolysin: a double-edged sword during the host-pathogen interaction. Curr Mol Med 2008; 8:497 - 509; http://dx.doi.org/10.2174/156652408785747924; PMID: 18781957
- Pichichero ME, Kaur R, Casey JR, Sabirov A, Khan N, Almudevar A. Serum Antibody Response to Haemophilus influenzae Outer Membrane Protein P6, OMP26 and Protein D After Nasopharyngeal Colonization and Acute Otitis Media. Vaccine 2010; 28:7184 - 92; http://dx.doi.org/10.1016/j.vaccine.2010.08.063; PMID: 20800701
- http://spneumoniae.mlst.net/misc/info.asp
- Holmlund E, Quiambao B, Ollgren J, Nohynek H, Käyhty H. Development of natural antibodies to pneumococcal surface protein A, pneumococcal surface adhesin A and pneumolysin in Filipino pregnant women and their infants in relation to pneumococcal carriage. Vaccine 2006; 24:57 - 65; http://dx.doi.org/10.1016/j.vaccine.2005.07.055; PMID: 16115703
- Simell B, Ahokas P, Lahdenkari M, Poolman J, Henckaerts I, Kilpi TM, et al. Pneumococcal carriage and acute otitis media induce serum antibodies to pneumococcal surface proteins CbpA and PhtD in children. Vaccine 2009; 27:4615 - 21; http://dx.doi.org/10.1016/j.vaccine.2009.05.071; PMID: 19524618
- Holmlund E, Quiambao B, Ollgren J, Jaakkola T, Neyt C, Poolman J, et al. Antibodies to pneumococcal proteins PhtD, CbpA, and LytC in Filipino pregnant women and their infants in relation to pneumococcal carriage. Clin Vaccine Immunol 2009; 16:916 - 23; http://dx.doi.org/10.1128/CVI.00050-09; PMID: 19403781
- Sabirov A, Casey JR, Murphy TF, Pichichero ME. Breast-feeding is associated with a reduced frequency of acute otitis media and high serum antibody levels against NTHi and outer membrane protein vaccine antigen candidate P6. Pediatr Res 2009; 66:565 - 70; http://dx.doi.org/10.1203/PDR.0b013e3181b4f8a6; PMID: 19581824
- Samukawa T, Yamanaka N, Hollingshead S, Klingman K, Faden H. Immune responses to specific antigens of Streptococcus pneumoniae and Moraxella catarrhalis in the respiratory tract. Infect Immun 2000; 68:1569 - 73; http://dx.doi.org/10.1128/IAI.68.3.1569-1573.2000; PMID: 10678976
- Virolainen A, Jero J, Chattopadhyay P, Karma P, Eskola J, Leinonen M. Comparison of serum antibodies to pneumolysin with those to pneumococcal capsular polysaccharides in children with acute otitis media. Pediatr Infect Dis J 1996; 15:128 - 33; http://dx.doi.org/10.1097/00006454-199602000-00008; PMID: 8822285
- Rapola S, Kilpi T, Lahdenkari M, Mäkelä PH, Käyhty H. Antibody response to the pneumococcal proteins pneumococcal surface adhesin A and pneumolysin in children with acute otitis media. Pediatr Infect Dis J 2001; 20:482 - 7; http://dx.doi.org/10.1097/00006454-200105000-00003; PMID: 11368104
- Kaur R, Casey JR, Pichichero ME. Serum Antibody Response to Three Non-typeable Haemophilus influenzae Outer Membrane Proteins During Nasopharyngeal Colonization and Acute Otitis Media in Otitis Prone and Non-Prone Children. Vaccine 2011; 29:1023 - 8; http://dx.doi.org/10.1016/j.vaccine.2010.11.055; PMID: 21129398
- Kaur R, Casey JR, Pichichero ME. Serum antibody response to five Streptococcus pneumoniae proteins during acute otitis media in otitis-prone and non-otitis-prone children. Pediatr Infect Dis J 2011; 30:645 - 50; http://dx.doi.org/10.1097/INF.0b013e31821c2d8b; PMID: 21487325
- Khan MN, Sharma S, Filkins L, Pichichero ME. PcpA of Streptococcus pneumoniae is a putative adhesin that elicits functional antibodies in humans. Microbes Infect 2012; in review
- Khan MN, Pichichero ME. PhtD and PhtE of Streptococcus pneumoniae are adhesins that elicit functional antibodies in humans. FEMS Immunology 2012; In press