Abstract
Neisseria meningitidis serogroup B (MnB) is a significant cause of invasive meningococcal disease, but no broadly protective vaccine is yet approved. We assessed the safety and immunogenicity of a bivalent MnB vaccine composed of lipidated subfamily A and B variants of recombinant LP2086 (rLP2086, also known as factor H binding protein, fHBP). Forty-eight adults, ages 18–40 y, were randomized to receive 60, 120 or 200 μg of the bivalent rLP2086 vaccine or control at 0, 2 and 6 mo. Immunogenicity was assessed by rLP2086-specific immunoglobulin G (IgG) geometric mean titers for subfamily A and B proteins. Safety was determined by laboratory assessments of blood and urine and by reporting of solicited and unsolicited adverse events (AEs). The bivalent rLP2086 vaccine elicited high IgG titers following the second and third vaccination at all dose levels. In each of the four study arms, 11 of the 12 participating subjects reported ≥ 1 AE, and no serious AEs were reported. Local and systemic reactions were mainly mild to moderate. Laboratory abnormalities (including increased sodium, decreased neutrophils, and proteinuria) were not associated with clinical events and were not considered to be related to the study vaccine. Vaccinations were generally well-tolerated. Strong IgG antibody responses and the absence of clinically significant laboratory abnormalities support further development of the bivalent rLP2086 vaccine (www.clinicaltrials.gov; identifier: NCT00879814).
Introduction
Neisseria meningitidis is a Gram-negative commensal bacterium commonly found in the human nasopharynx. Carriage rates vary by age and setting and may be as high as 24% in young adults.Citation1 Invasive meningococcal disease (IMD) is often difficult to differentiate from other infectious diseases and can progress to fulminant disease and death within as little as 24 h.Citation2 Even with the availability of antibiotics, up to 14% of IMD cases result in death, and those who survive often suffer from serious morbidities, including cognitive difficulties, deafness and visual or behavioral problems.Citation3,Citation4
Among the 12 capsular serogroups of N. meningitidis, 5 serogroups (A, B, C, W-135 and Y) are responsible for most cases of IMD.Citation5-Citation7 Capsular polysaccharide conjugate vaccines have been developed and successfully used against all prevalent invasive serogroups except meningococcal serogroup B (MnB).Citation8 Within 2 y of the implementation of a national meningococcal serogroup C (MnC) conjugate vaccine program in the UK in 1999, the incidence of MnC disease in children and adolescents ages 19 and under had dropped by 80%.Citation9 By 2009 only 1% of IMD cases in England and Wales were caused by MnC.Citation10 Similar successes have been observed following conjugate MnC vaccine programs in Australia and the Netherlands.Citation11,Citation12 Following the success of these meningococcal vaccine programs, the majority of IMD cases in these countries are now caused by MnB.Citation13,Citation14
Because the MnB capsular polysaccharide polysialic acid is also present in human neuronal tissue, vaccines based on MnB polysaccharides provide poor immunogenic responses and approaches to overcome this have failed.Citation15,Citation16 An alternate approach has been the development of vaccines based on outer membrane vesicles, which have been used to combat epidemic MnB outbreaks.Citation5 These vaccines induce antibodies directed primarily against the hypervariable surface-exposed protein porin A (PorA).Citation17,Citation18 Outer membrane vesicle based vaccines generally offer effective protection against MnB strains carrying vaccine-homologous PorA variants.Citation18,Citation19 Consequently, these vaccines provide protection against epidemics caused by MnB strains carrying identical PorA variants, but are less effective against endemic disease-causing strains, which often express different PorA variants.
An ideal protein target would induce bactericidal antibodies against a broad range of MnB strains and have limited genetic variability. Meningococcal factor H-binding protein (fHBP, also known as lipoprotein 2086 or LP2086) is a surface expressed lipoprotein that downregulates complement-mediated bacterial lysis by binding human factor H.Citation20,Citation21 Importantly, the gene encoding LP2086 has been identified in virtually all MnB strains tested, and LP2086 has been shown to elicit bactericidal antibodies against a broad range of MnB strains.Citation22,Citation23 Epidemiological studies of LP2086 sequences have shown that this antigen segregates into 2 subfamilies (A and B).Citation22,Citation24 The amino acid sequence of LP2086 is also highly conserved, with > 83% identity within subfamilies and 60–75% identity between subfamilies A and B.Citation22,Citation24 A vaccine consisting of subfamily A and B LP2086 proteins is predicted to provide broad coverage against disease-causing MnB isolates representing diverse LP2086 variants, multilocus sequence typing (MLST) variants and PorA subtypes.Citation23
This study assessed the safety, immunogenicity and tolerability of 60, 120 and 200 μg dose levels of the bivalent recombinant LP2086 (rLP2086) vaccine in healthy adults.
Results
Subjects
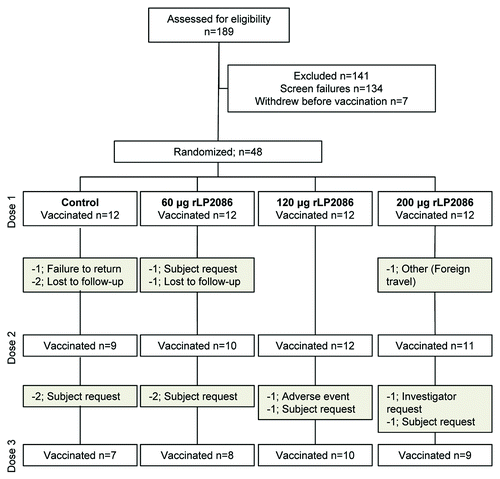
Forty-eight subjects were randomized into 4 groups of 12 to receive 60, 120 or 200 μg of the final formulation of the bivalent rLP2086 vaccine or a Tdap vaccine (tetanus toxoid, diphtheria toxoid and acellular pertussis)/placebo (). The mean age of the study population was 28.8 y (range, 18–40 y), and most participants were white (79.2%) and female (60.4%) (). A total of 14 subjects withdrew following randomization (). One subject in the 120 μg dose group withdrew because of an AE (mild gastritis) reported 173 d after the second injection; this event was not considered to be related to vaccine administration. One subject withdrew at the investigator’s request because of travel to a foreign country for an undetermined period of time. One subject was found to be participating in another investigational study and was withdrawn at the investigator’s request. One subject who was unable to complete the study visits requested withdrawal. This subject (120 μg; post–dose 2) reported a fever of 39.0°C that was considered by investigators to be related to the study vaccine. The proportion of subjects who received all 3 study injections was similar among the control group and each of the rLP2086 dose groups.
Table 1. Patient demographic characteristicsa
Immunogenicity
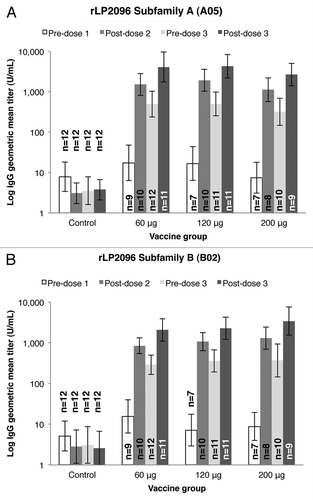
At each dose level, the rLP2086 vaccine elicited a robust increase in immunoglobulin G (IgG) geometric mean titers (GMTs) specific for both subfamily A and B LP2086 proteins (). Although differences in the magnitude of the GMTs did not appear to differ at the different vaccine dose levels, GMTs tended to be higher upon subsequent vaccinations.
Figure 2. Immunoglobulin G (IgG) geometric mean titers (GMT) specific for LP2086 A and B subfamilies. Anti-rLP2086 IgG antibody binding to the rLP2086 subfamily A (panel A) and subfamily B (panel B) proteins after immunization at three dose levels of rLP2086 or control is shown. GMTs are plotted on a logarithmic scale and shown with 95% confidence intervals (CI). n = Number of subjects with available data.
Local reactions
Individual frequencies of local reactions in each dose group were generally higher than those in the control group (). One subject at the 200 μg dose level reported fever following dose 3, but information about local reactions or systemic events was not transmitted via e-diary. Most subjects in each vaccine group reported either mild or moderate local reactions. When they were reported, severe local reactions occurred following 120 or 200 μg rLP2086 vaccinations. Local reactions were not reported by any subjects in the control group after doses 2 and 3. The most commonly reported local reaction in this study was pain. Two subjects reported severe pain after dose 2: 1 subject in the 120 μg group and 1 subject in the 200 μg group. One subject in the 120 μg dose group reported severe induration (swelling), but it did not interfere with daily activity. One subject in the 120 μg dose group reported severe erythema (redness) after dose 2, and 1 subject in the same group reported severe erythema after dose 3. One subject in the 200 μg dose group reported severe erythema after dose 3. Most local reactions resolved in 1–3 d. The longest duration reported for any local reaction was 14 d (pain and induration after the second injection, occurring in 1 subject at the 200 µg dose level).
Table 2. Local reactions reported within 7 days of each immunization by dose
Systemic events
The total and individual frequencies of systemic events were generally higher in the rLP2086 vaccine groups than in the control group (). Most of the events were mild or moderate. One subject in the 60 µg dose group reported a severe headache, and 4 subjects in the 200 µg dose group reported a total of five severe events (headache n = 1, muscle pain n = 2, nausea n = 1 and fatigue n = 1). Generally, systemic events resolved 1–3 d after vaccination. The longest duration of any systemic event was 14 d (fatigue); this was reported by a participant in the 200 µg dose group after the second injection. Two subjects receiving the 120 μg dose and 3 receiving the 200 μg dose reported fever within 7 d of vaccination (). All fevers resolved within 1 d.
Table 3. Systemic events reported within 7 days of each immunization by dose
Table 4. Subjects reporting fever within 7 days of each immunization by dose
Adverse events
AEs reported in this study were generally comparable between the active control group and each rLP2086 vaccine group. The most commonly reported AEs were mild laboratory abnormalities, which were recorded in all groups. There were 7 incidents of severe AEs in this study; all were laboratory abnormalities and included increased potassium (n = 3), increased sodium (n = 1), decreased neutrophils (n = 2) and proteinuria (n = 1). All severe AEs except 1 (decreased neutrophils) resolved without intervention. There was no consistent pattern of laboratory abnormalities, nor did abnormalities worsen with additional doses of the vaccine. Overall, laboratory abnormalities were intermittent and resolved without intervention. None of the laboratory abnormalities were considered related to the rLP2086 vaccine.
The next most commonly reported AE was upper respiratory tract infection, reported in 16.7% (n = 2) of participants in the 120 µg and 200 µg groups and 0% of participants in the 60 μg group [vs 8.3% (n = 1) in the control group]. Overall, 6 occurrences of vaccine-related AEs of mild severity were identified in 5 subjects. These included injection site pruritus (itching, n = 1, 60 µg; n = 1, 200 µg), injection site rash (n = 1, 120 µg), induration (n = 1, 120 µg; n = 1, 200 µg), and throat irritation (n = 1, 200 µg). All of the vaccine-related AEs resolved, and no AE was associated with an underlying diagnosis. There were no serious adverse events (SAEs), and no subjects died during this study.
Discussion
This phase 1 study demonstrates an acceptable safety profile for the bivalent rLP2086 vaccine in healthy adults. The frequency of local events was generally similar among the Tdap control (tetanus, diphtheria and acellular pertussis) and bivalent rLP2086 vaccine groups after the first dose with respect to pain at the injection site and induration. Systemic events were slightly more frequent for each of the vaccine groups after the first dose when compared with Tdap controls. Upon subsequent immunizations (when the control participants received saline placebo), local and systemic events were more common in the vaccine groups but did not appear to increase in frequency or severity when compared with the first vaccination. In addition, there were no SAEs.
These results should be interpreted with certain study limitations in mind. As a phase 1 clinical trial, the number of enrolled participants was not powered for statistical comparisons among the study arms. This study was designed to establish the safety profile of the final formulation of the rLP2086 vaccine in an adult population, and it was the first to assess possible laboratory abnormalities. Mild laboratory abnormalities were the most common AEs in this study and were observed with a similar frequency among vaccine and control groups. Any laboratory abnormality, no matter the significance, was reported as an AE. Although statistical comparisons were not performed, there was no discernible pattern of abnormalities at increasing rLP2086 vaccine dose levels or upon subsequent vaccinations. None of the laboratory abnormalities reported as AEs were considered related to the rLP2086 vaccine, and there were no sequelae to any reported AEs.
Immunogenicity results showed high serum IgG responses to rLP2086 vaccine-homologous subfamily A and B proteins at all rLP2086 dose levels. IgG titers in all rLP2086-vaccinated subjects remained higher than control groups for at least 4 mo after the second vaccination, suggesting that the vaccine elicits good priming for a durable response. It is interesting to note that rLP2086-specific GMTs did not appear to differ among the different vaccine dose groups after each immunization. While these serum IgG responses indicate the magnitude of the antibody response to rLP2086 vaccination, serum bactericidal assays (SBA) provide an assessment of functional antibody responses and are recognized as a correlate of protection against invasive meningococcal disease.Citation25 Immunogenicity analysis in this study was limited to IgG GMTs. SBA titers using human serum as the source of complement (hSBA) have been determined in previous clinical trials of the bivalent rLP2086 vaccine.Citation26-Citation28 These studies have shown robust responses (hSBA titers ≥ 1:4 in up to 100% of vaccinated participants) to multiple MnB test strains expressing LP2086 variants that are homologous and heterologous to vaccine antigens.Citation26-Citation28 Additional studies of the final vaccine formulation that include hSBA analyses will be important to determining the optimal dose required to elicit protective immune responses.
A meningococcal B vaccine consisting of several outer membrane protein antigens, including a fusion protein of non-lipidated subfamily B LP2086, is also in clinical development. Vaccinated individuals have shown immune responses to MnB strains selected based on homology to three individual vaccine components including fHBP.Citation29,Citation30 The ability of this vaccine to induce broad protection will depend on the geographical variations of protein antigens in endemic and epidemic MnB strains.
Overall, these results indicate that the bivalent rLP2086 vaccine is generally well tolerated and is not associated with clinically relevant laboratory abnormalities or serious AEs in healthy adults. These data support further development of the bivalent rLP2086 vaccine in larger clinical trials and in additional age groups at risk of IMD caused by MnB.
Methods
This phase 1, randomized, open-label, active- and placebo-controlled, parallel-group study was performed between April 2009 and March 2010 at a single center in Miami, Florida. Male and female subjects (≥ 18 and ≤ 40 y of age) were enrolled if they were in good health as determined by medical history and physical examination. Laboratory blood and urinalysis test values were required to be within normal ranges as defined by the study protocol. Laboratory evaluations included hematology values (white and red blood cell counts, red blood cell indices, platelet count, hematocrit and hemoglobin levels), chemistry values (alanine and aspartate aminotransferase, alkaline phosphatase, total bilirubin, total protein, albumin, creatinine, blood urea nitrogen, lactate dehydrogenase, creatine phosphokinase and gamma glutamyl transferase), coagulation profile (prothrombin time, partial thromboplastin time, international normalized ratio, fibrinogen and D-dimer) and urinalysis (protein, glucose and blood). Female subjects were excluded from the study if they were pregnant or breast-feeding, and any subject considered biologically capable of having children agreed to use a reliable method of birth control for the duration of the study and for 30 d after study completion. Ineligible subjects included those with a history of any invasive disease caused by N. meningitidis or Neisseria gonorrhoeae and those who had previously received a MnB vaccine. Subjects were also ineligible if they had a history of anaphylactic or severe vaccine-associated adverse reaction, known hypersensitivity to any study vaccine component, or known or suspected diseases of the immune system. Subjects were excluded if they were receiving immunosuppressive therapy, including systemic corticosteroids (excluding topical, inhaled and intra-articular corticosteroids). Subjects receiving blood products (including gamma globulin) 6 mo before the first study dose or an investigational drug or device 6 weeks before the first study dose were considered ineligible. Subjects were excluded if they received a vaccine containing any Tdap components within the past 5 y or have a contraindication to a Tdap vaccine.
As a phase 1 trial, the study sample size was not based on statistical considerations. Eligible subjects were randomly assigned to receive 60, 120 or 200 μg of the bivalent rLP2086 or control regimen in a 1:1:1:1 ratio. Subjects were initially scheduled to receive single injections at 0, 2 and 6 mo. The vaccination window for dose 3 was broadened to 6–9 mo to accommodate a study pause due to a vaccine-related SAE in a separate study. In the related study, the safety profile of the rLP2086 vaccine was determined by the project independent safety review team to be unchanged and all studies were restarted.
The active control regimen consisted of a Tdap vaccine (tetanus toxoid 5Lf, diphtheria toxoid 2Lf and acellular pertussis [detoxified pertussis toxin 2.5 μg; filamentous hemagglutinin 5 μg; pertactin 3 μg; and fimbriae types 2 and 3, 5 μg]; Adacel, Sanofi Pasteur) given at the first injection and saline placebo for the second and third injections. The rLP2086 vaccine contained equal amounts (30, 60 or 100 μg) of purified lipidated recombinant LP2086 proteins cloned from MnB strains PMB1745 (LP2086 variant A05) and PMB1135 (B01) and aluminum phosphate in a pre-formulated, 0.5 mL, liquid preparation. All vaccines were administered intramuscularly in the upper deltoid muscle.
Blood and urine samples were taken before the first dose and 2–3 d after each vaccination for clinical laboratory assessments. Subjects recorded local and systemic reactions for 7 d after each vaccination using an electronic diary. Unsolicited adverse events (AEs) were reported throughout the study. All laboratory abnormalities, regardless of their clinical significance, were recorded as AEs.
Additional blood samples were obtained before the first injection, 1 mo after injection 2, immediately before injection 3 and 1 mo after injection 3 to evaluate immune responses. rLP2086-specific IgG titers were assessed using a Luminex-based assay (Luminex) that quantified antibody titers specific for homologous subfamily A and B rLP2086 antigens.Citation31 The IgG antibody titers were expressed in arbitrary units per milliliter (U/mL). Meningococcal IgG Luminex assays were performed at Pfizer (Vaccine Research and Early Development). Laboratory personnel were blinded to subject randomization.
The immunogenicity population included all randomized participants who presented for a scheduled blood draw and had assay results available (calculated per immune sample time-point). IgG titers were summarized as the GMT and 2-sided 95% confidence interval (CI) at each sampling time point by vaccine group and for each LP2086 subfamily. The CIs were calculated using the Student t-distribution. The safety population included all randomized subjects who received at least 1 vaccination. The safety endpoints described above were descriptively summarized.
Ethical Statements
The trial was registered with clinicaltrials.gov (identifier NCT00879814) and was conducted in accordance with the International Conference on Harmonization Guideline for Good Clinical Practice which has its origins in the Declaration of Helsinki. All participants provided written and informed consent.
| Abbreviations: | ||
| IgG | = | Immunoglobulin G |
| fHBP | = | factor H binding protein, LP2086 |
| GMT | = | geometric mean titer |
| hSBA | = | serum bactericidal assay using human complement |
| IMD | = | invasive meningococcal disease |
| MLST | = | multi locus sequence typing |
| MnB | = | Neisseria meningitidis Serogroup B |
| MnC | = | Neisseria meningitidis Serogroup C |
| rLP2086 | = | Bivalent recombinant LP2086 vaccine |
Acknowledgments
Editorial/medical writing support was provided by Robert Glover and John Clinton Earnheart at Scientific Strategy Partners and was funded by Pfizer Inc. We thank Annaliesa Anderson, Pfizer, for review of the manuscript. This work was presented in part at the 14th Annual Conference on Vaccine Research in Baltimore, MD, on May 16–18, 2011.
Financial Support
This study was sponsored by Wyeth, which was acquired by Pfizer Inc in October 2009. The sponsor was involved in all study stages, from the study design to the data analysis and preparation of the manuscript. The corresponding author had the final responsibility for the decision to submit the manuscript for publication.
Conflict of Interest
P.C.G., Q.J. and J.L.P. are employees of Pfizer Inc. E.S. and H.S. have no conflicts to report but their institution has received clinical research and grant funding from Wyeth, which was acquired by Pfizer Inc in October 2009. Financial compensation was not provided to E.S. or H.S. for manuscript preparation or for participation in the study reported herein.
References
- Christensen H, May M, Bowen L, Hickman M, Trotter CL. Meningococcal carriage by age: a systematic review and meta-analysis. Lancet Infect Dis 2010; 10:853 - 61; http://dx.doi.org/10.1016/S1473-3099(10)70251-6; PMID: 21075057
- Thompson MJ, Ninis N, Perera R, Mayon-White R, Phillips C, Bailey L, et al. Clinical recognition of meningococcal disease in children and adolescents. Lancet 2006; 367:397 - 403; http://dx.doi.org/10.1016/S0140-6736(06)67932-4; PMID: 16458763
- Bilukha OO, Rosenstein N, National Center for Infectious Diseases, Centers for Disease Control and Prevention (CDC). Prevention and control of meningococcal disease. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2005; 54:RR-7 1 - 21; PMID: 15917737
- Edmond K, Clark A, Korczak VS, Sanderson C, Griffiths UK, Rudan I. Global and regional risk of disabling sequelae from bacterial meningitis: a systematic review and meta-analysis. Lancet Infect Dis 2010; 10:317 - 28; http://dx.doi.org/10.1016/S1473-3099(10)70048-7; PMID: 20417414
- Girard MP, Preziosi MP, Aguado MT, Kieny MP. A review of vaccine research and development: meningococcal disease. Vaccine 2006; 24:4692 - 700; http://dx.doi.org/10.1016/j.vaccine.2006.03.034; PMID: 16621189
- Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine 2009; 27:Suppl 2 B51 - 63; http://dx.doi.org/10.1016/j.vaccine.2009.04.063; PMID: 19477562
- Zollinger WD, Boslego J. Immunologic Methods for Diagnosis of Infections by Gram-Negative Cocci. In: Rose NR, ed. Manual of clinical laboratory immunology. Washington, DC: ASM Press, 1997:473-83.
- Pace D, Pollard AJ, Messonier NE. Quadrivalent meningococcal conjugate vaccines. Vaccine 2009; 27:Suppl 2 B30 - 41; http://dx.doi.org/10.1016/j.vaccine.2009.05.003; PMID: 19477560
- Trotter CL, Ramsay ME, Kaczmarski EB. Meningococcal serogroup C conjugate vaccination in England and Wales: coverage and initial impact of the campaign. Commun Dis Public Health 2002; 5:220 - 5; PMID: 12434692
- United Kingdom Health Protection Agency. Meningococcal Reference Unit: Isolates of Neisseria menengitidis; England and Wales, by serogroup & calendar year, 1998-2010*. 2010: http://www.hpa.org.uk/web/HPAweb&HPAwebStandard/HPAweb_C/1234859712887. Accessed February 2012.
- de Greeff SC, de Melker HE, Spanjaard L, Schouls LM, van Derende A. Protection from routine vaccination at the age of 14 months with meningococcal serogroup C conjugate vaccine in the Netherlands. Pediatr Infect Dis J 2006; 25:79 - 80; http://dx.doi.org/10.1097/01.inf.0000195594.41449.c6; PMID: 16395110
- Slinko VG, Sweeny A. Reduction in invasive meningococcal disease in Queensland: a success for immunisation. Commun Dis Intell 2007; 31:227 - 32; PMID: 17725000
- Australian Meningococcal Surveillance Programme. Annual report of the Australian Meningococcal Surveillance Programme, 2009. Commun Dis Intell 2010; 34:291 - 302; PMID: 21090184
- van Driel JJ, Bekker V, Spanjaard L, van der Ende A, Kuijpers TW. Epidemiologic and microbiologic characteristics of recurrent bacterial and fungal meningitis in the Netherlands, 1988-2005. Clin Infect Dis 2008; 47:e42 - 51; http://dx.doi.org/10.1086/590251; PMID: 18643757
- Bruge J, Bouveret-Le Cam N, Danve B, Rougon G, Schulz D. Clinical evaluation of a group B meningococcal N-propionylated polysaccharide conjugate vaccine in adult, male volunteers. Vaccine 2004; 22:1087 - 96; http://dx.doi.org/10.1016/j.vaccine.2003.10.005; PMID: 15003635
- Wyle FA, Artenstein MS, Brandt BL, Tramont EC, Kasper DL, Altieri PL, et al. Immunologic response of man to group B meningococcal polysaccharide vaccines. J Infect Dis 1972; 126:514 - 21; http://dx.doi.org/10.1093/infdis/126.5.514; PMID: 4197754
- Jelfs J, Munro R, Wedege E, Caugant DA. Sequence variation in the porA gene of a clone of Neisseria meningitidis during epidemic spread. Clin Diagn Lab Immunol 2000; 7:390 - 5; PMID: 10799451
- Martin DR, Ruijne N, McCallum L, O’Hallahan J, Oster P. The VR2 epitope on the PorA P1.7-2,4 protein is the major target for the immune response elicited by the strain-specific group B meningococcal vaccine MeNZB. Clin Vaccine Immunol 2006; 13:486 - 91; http://dx.doi.org/10.1128/CVI.13.4.486-491.2006; PMID: 16603616
- Tondella ML, Popovic T, Rosenstein NE, Lake DB, Carlone GM, Mayer LW, et al, The Active Bacterial Core Surveillance Team. Distribution of Neisseria meningitidis serogroup B serosubtypes and serotypes circulating in the United States. J Clin Microbiol 2000; 38:3323 - 8; PMID: 10970378
- Madico G, Welsch JA, Lewis LA, McNaughton A, Perlman DH, Costello CE, et al. The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J Immunol 2006; 177:501 - 10; PMID: 16785547
- Schneider MC, Exley RM, Chan H, Feavers I, Kang YH, Sim RB, et al. Functional significance of factor H binding to Neisseria meningitidis. J Immunol 2006; 176:7566 - 75; PMID: 16751403
- Murphy E, Andrew L, Lee KL, Dilts DA, Nunez L, Fink PS, et al. Sequence diversity of the factor H binding protein vaccine candidate in epidemiologically relevant strains of serogroup B Neisseria meningitidis. J Infect Dis 2009; 200:379 - 89; http://dx.doi.org/10.1086/600141; PMID: 19534597
- Jiang HQ, Hoiseth SK, Harris SL, McNeil LK, Zhu D, Tan C, et al. Broad vaccine coverage predicted for a bivalent recombinant factor H binding protein based vaccine to prevent serogroup B meningococcal disease. Vaccine 2010; 28:6086 - 93; http://dx.doi.org/10.1016/j.vaccine.2010.06.083; PMID: 20619376
- Fletcher LD, Bernfield L, Barniak V, Farley JE, Howell A, Knauf M, et al. Vaccine potential of the Neisseria meningitidis 2086 lipoprotein. Infect Immun 2004; 72:2088 - 100; http://dx.doi.org/10.1128/IAI.72.4.2088-2100.2004; PMID: 15039331
- Borrow R, Carlone GM, Rosenstein N, Blake M, Feavers I, Martin D, et al. Neisseria meningitidis group B correlates of protection and assay standardization--international meeting report Emory University, Atlanta, Georgia, United States, 16-17 March 2005. Vaccine 2006; 24:5093 - 107; http://dx.doi.org/10.1016/j.vaccine.2006.03.091; PMID: 16838413
- Marshall H, Nissen MD, Richmond P, Lambert SB, Roberton D, Gruber W, et al. A Randomized, Plaebo-Controlled, Double-Blind, Phase 1 Trial of Ascending Doses of Meningococcal Group B rLP2086 Vaccine in Healthy Adults. Rotterdam, The Netherlands: Presented at the Sixteenth International Pathogenic Conference, 2008.
- Marshall H, Richmond P, Nissen MD, Jiang Q, Anderson A, Jansen KU, et al. Phase 1 Randomized Controlled Clinical Trial of Safety and Immunogenicity of a Meningococcal B Bivalent Vaccine in Healthy Toddlers. The Hague, The Neatherlands: Presented at the Twenty-ninth Annual Meeting of the European Society for Paediatric Infectious Diseases, 2011.
- Richmond P, Marshall H, Nissen M, Jiang Q, Jansen KU, Garces-Sanchez M, et al. Phase 2 Randomised Controlled Trial of Safety and Immunogenicity of a Meningococcal B Bivalent Vaccine (rLP2086) in Healthy Adolescents. The Hague, The Netherlands: Presented at the Twenty-ninth Annual Meeting of the European Society for Paediatric Infectious Diseases, 2011.
- Gossger N, Snape MD, Yu LM, Finn A, Bona G, Esposito S, et al, European MenB Vaccine Study Group. Immunogenicity and tolerability of recombinant serogroup B meningococcal vaccine administered with or without routine infant vaccinations according to different immunization schedules: a randomized controlled trial. JAMA 2012; 307:573 - 82; http://dx.doi.org/10.1001/jama.2012.85; PMID: 22318278
- Santolaya ME, O’Ryan ML, Valenzuela MT, Prado V, Vergara R, Muñoz A, et al, V72P10 Meningococcal B Adolescent Vaccine Study group. Immunogenicity and tolerability of a multicomponent meningococcal serogroup B (4CMenB) vaccine in healthy adolescents in Chile: a phase 2b/3 randomised, observer-blind, placebo-controlled study. Lancet 2012; 379:617 - 24; http://dx.doi.org/10.1016/S0140-6736(11)61713-3; PMID: 22260988
- Ala’aldeen DA, Flint M, Oldfield NJ, Omer SA, McNeil LK, Jiang Q, et al. Human antibody responses to the meningococcal factor H binding protein (LP2086) during invasive disease, colonization and carriage. Vaccine 2010; 28:7667 - 75; http://dx.doi.org/10.1016/j.vaccine.2010.09.038; PMID: 20875489