Abstract
The first prophylactic vaccine, Hecolin®, against hepatitis E virus (HEV) infection and the HEV associated disease was approved by China's State Food and Drug Administration (SFDA) in December 2011. Key milestones during the 14-year HEV vaccine development are summarized in this commentary. After years of innovative research the recombinant virus-like particle (VLP) based antigen with virion-like epitopes was successfully produced in E. coli production platform on a commercial scale. Safety and efficacy of this vaccine was demonstrated in a large scale phase III clinical trial.
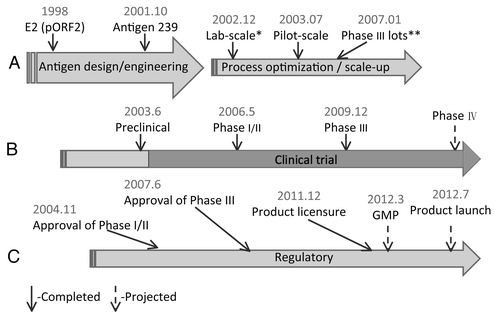
Ever since the publication of the 2010 Lancet paperCitation7 with 112,604 volunteers during a large Phase III clinical trial, a lot of attention and enthusiasm were put on the highly expected first human vaccine against Hepatitis E. Here we recount the main milestones during the development of this vaccine for Hepatitis E disease. Vaccine development since 1998 has been a long journey in our laboratories side by side with the development of diagnostic kits. Here we focus on key progress, particularly the milestones () of bioprocessing, clinical trials and regulatory interface with Chinese State of Food and Drug Administration (SFDA) during the 14-y development of this HE vaccine.
Optimization of Antigen For Prophylactic Vaccination
The HEV genome contains three open reading frames (ORFs), ORF1, ORF2 and ORF3, among which ORF2 encodes the only capsid protein, i.e., pORF2. Therefore, pORF2 is the virion capsid protein, with recombinant pORF2 alone being capable of particle assembly into virus-like or subviral particles. This protein was also shown to be crucial for binding to host cells and eliciting of neutralizing and protective antibodies during preclinical studies.Citation8,Citation9 Along with human serum samples from Hepatitis E patients during acute phase or post recovery, a large panel of hundreds of mouse monoclonal antibodies were developed for epitope characterization of HEV capsid. These reagents and clinical samples were used to understand and define the key epitopes on HEV virions and on different lengths of wild-type and mutated recombinant pORF2. In-depth characterization of the immune-reactivity of mono- and polyclonal antibodies (Abs) against HEV showed that the immunodominant epitopes reside on pORF2. Further immunochemical and structural analyses revealed that the immunodominant epitopes are at the unique groove regions near the dimeric interface in the E2 homodimers – the building blocks of recombinant virus-like-particles (VLPs) or native viral capsids.Citation10,Citation11
The dimer form of pORF2 showed high affinity to polyclonal Abs in patient sera of HEV-infected individuals.Citation12 To further enhance immunogenicity, a particulate form or VLPs of the pORF2 (referred to as “239” in reflection of the number of aa residues) is used as the immunogen. Much higher (~240-fold) immunogenicity and efficacy of the particulate form of this HEV 239 vaccine were demonstrated in non-human primates, as compared with a shortened (by 26 amino acids) dimer form or E2.Citation8 Rhesus monkeys twice vaccinated with a 10μg or a 20μg formulation of this vaccine showed essentially the same antibody response, whereas the response to a 5μg formulation was delayed but reached similar antibody levels. Proof of concept of this vaccine was demonstrated in that all three vaccine formulations afford complete protection against infection with 104 genome-equivalent doses of the homologous genotype 1 virus and heterogeneous genotype 4 virus. At higher virus dose of 107, the same vaccine formulation partially protected against infection and completely protected against hepatitis.
Clinical Milestones
All clinical studies were conducted in China during 2005–2009. A Phase I clinical trial was initiated in early 2005 in Guangxi province.Citation13 We recruited 44 volunteers of ages 21–55. A two-dose vaccination regimen was given at 0–1 mo with 20 μg HE vaccine per injection. After safety of the vaccine was preliminarily demonstrated in Phase I, Phase II trials were performed with the purpose of testing dose schedule and dose escalation. A total of 457 subjects were enrolled in a Phase IIa trial. Two dose schedules of 0,-6 and 0–1-6 mo were tested in this trial. Data showed that in patients of the ‘3-dose group’, the anti-HEV IgG seroconversion rate is higher than those in the ‘2-dose group’ (100% vs. 98%) but the difference was not statistically significant. In addition, the geometric mean concentration (GMC) of anti-HEV IgG was significantly higher in the ‘3-dose group’ than ‘2-dose group’ (15.9 vs. 8.6 WHO units/ml, p < 0.05).Citation13
In a Phase IIb clinical trial, 155 subjects were recruited and randomly assigned to four groups, with each group receiving 3 doses of one of four dosage levels - 10, 20, 30, or 40 μg HE vaccine per injection. All four groups showed 100% seroconversion. Detailed analysis showed that the high-dosage groups (20, 30, 40 μg) showed higher anti-HEV IgG GMV levels than the 10-μg dosage group (p < 0.05). The adverse event rates were similar among the four dosage groups, indicating comparable reactogenicity or tolerability of the vaccine at all levels.Citation13
From August 2007 to May 2009, a large-scale randomized double-blind placebo-controlled Phase III trial was conducted in Dongtai, Jiangsu Province.Citation7 We recruited 112,604 volunteers with ages ranging 16–65 y. These volunteers were randomly assigned into two groups, receiving 3 doses of 30 μg HE vaccine or 5 μg recombinant Hepatitis B virus (HBV) vaccine (as “placebo”) at 0–1-6 mo. HEV vaccine, similar to placebo, was well tolerated and no vaccine-related serious adverse events (SAE) were reported.Citation7 As part of this large-scale Phase III trial, there were cases of pregnancy occurred post-screening stage with the volunteers having received at least one dose of HE vaccine. Safety of the vaccine for pregnant women was demonstrated by preliminary analysis in 37 pregnant women who inadvertently received 1, 2 or 3 doses of HE vaccine during pregnancy. The vaccine was well tolerated, and the babies born in the vaccine group were as healthy as those born in the control group.Citation14
An active acute hepatitis surveillance system comprising 205 sentinels was established for the trial. An independent data and safety monitoring board reviewed the clinical and laboratory results of hepatitis-like patients and confirmed the diagnoses of hepatitis E before unblinding. The Hepatitis E case definition included three conditions that should all be fulfilled: (1) clinical symptoms: acute hepatitis-like illness lasting for at least three days; (2) diagnosis of acute hepatitis: alanine transaminase (ALT) level exceeding 2.5-times the upper limit of normal range (ULN); (3) two positive HEV markers: anti-HEV IgM, HEV RNA, ≥ 4-fold increase in anti-HEV IgG. During the 12 mo after 30 d from receipt of the third dose, 15 per-protocol participants in the placebo group developed hepatitis E compared with none in the vaccine group. Vaccine efficacy after three doses was 100% (95% CI, 72.1–100).Citation7 Due to lack of international gold-standard for hepatitis E case-definition where a specific ALT criterion is used, efficacy calculations based on different ALT criterion were described in . Since most viral liver disease in China is due to chronic hepatitis and not acute hepatitis, the conventional standard of care is to look for lower levels of ALT that are consistent with chronic liver injury. Acute hepatitis E has a different pathophysiology. In India, clinicians suggest that hepatitis E patients should be diagnosed only if the ALT level is ≥ 10-times ULN.Citation15 Lower levels of ALT were considered to offer an excessive risk of falsely confirming acute liver injury. The abnormal ALT criterion in our study is 2.5 ULN, which is similar to that for the Phase II clinical trial in Nepal of another HE vaccine candidate by GlaxoSmithKline,Citation16 based on the consideration that all clinically consequential cases associated with substantial liver injury during the acute phase were counted, while mild syndromes having little or no clinical consequences were excluded.
Table 2. Different scoring of efficacy of Hecolin® vaccine against hepatitis E based on four different criteria for the disease or its protection against overall HEV infection in participants who received all three doses
By comparing the antibody levels of paired samples with a 12-mo interval, HEV infection episodes after vaccination were diagnosed based on positive seroconversion or ≥ 4 fold increase of antibody level. During the two successive years after vaccination, 101 episodes of HEV infection occurred among 3,548 participants in the placebo group and 22 episodes occurred among 3,567 participants in the vaccine group. All were asymptomatic, except for 3 symptomatic hepatitis E cases in placebo group. The overall vaccine efficacy against HEV infection for the two-year period after vaccination is 78.3% (95% CI, 65.6 - 86.3) ( and ).
Table 1. Clinical trials of the recombinant HE vaccine during late-stage development of Hecolin®
Regulatory Milestones
A clinical trial application for innovation drug (IND) was submitted to SFDA in June 2003. Phase I and II trials were approved in December 2004. After submitting results of the Phase I and II trials in July 2006, it took another year for SFDA to approve the initiation of the Phase III trial. Data from the Phase III trial were submitted at the end of 2009. Upon the completion of Phase III data analysis, the vaccine was named Hecolin® (Hepatitis E vaccine made in E. coli). In December 2011, China approved the HEV Hecolin® for use in subjects > 16 y old. The GMP inspection by SFDA for commercial vaccine production is ongoing, and product launch is expected in the second half of 2012.
Future prospects
Initial introduction of Hecolin® will focus on high-risk populations, such as child-bearing-age women, food industry workers, aged persons, blood donors, organ transplantation receptors and travelers. The manufacturer is expected to work with non-government organizations to make the vaccine available to people in need worldwide. For high-risk populations such as pregnant women,Citation3 chronic liver disease patients,Citation4 and children o < 2 y old,Citation5 more clinical trials are needed to test the benefit and safety of this HEV vaccine.
References
- Kamar N, Bendall R, Legrand-Abravanel F, Xia NS, Ijaz S, Izopet J, et al. Hepatitis E. Lancet 2012; 379 In press
- Purcell RH, Emerson SU. Hepatitis E: an emerging awareness of an old disease. J Hepatol 2008; 48:494 - 503; http://dx.doi.org/10.1016/j.jhep.2007.12.008; PMID: 18192058
- Navaneethan U, Al Mohajer M, Shata MT. Hepatitis E and pregnancy: understanding the pathogenesis. Liver Int 2008; 28:1190 - 9; http://dx.doi.org/10.1111/j.1478-3231.2008.01840.x; PMID: 18662274
- Dalton HR, Hazeldine S, Banks M, Ijaz S, Bendall R. Locally acquired hepatitis E in chronic liver disease. Lancet 2007; 369:1260; http://dx.doi.org/10.1016/S0140-6736(07)60595-9; PMID: 17434400
- Teshale EH, Howard CM, Grytdal SP, Handzel TR, Barry V, Kamili S, et al. Hepatitis E epidemic, Uganda. Emerg Infect Dis 2010; 16:126 - 9; http://dx.doi.org/10.3201/eid1601.090764; PMID: 20031058
- Zhuang H, Cao XY, Liu CB, Wang GM. Epidemiology of hepatitis E in China. Gastroenterol Jpn 1991; 26:Suppl 3 135 - 8; PMID: 1909252
- Zhu FC, Zhang J, Zhang XF, Zhou C, Wang ZZ, Huang SJ, et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet 2010; 376:895 - 902; http://dx.doi.org/10.1016/S0140-6736(10)61030-6; PMID: 20728932
- Li SW, Zhang J, Li YM, Ou SH, Huang GY, He ZQ, et al. A bacterially expressed particulate hepatitis E vaccine: antigenicity, immunogenicity and protectivity on primates. Vaccine 2005; 23:2893 - 901; http://dx.doi.org/10.1016/j.vaccine.2004.11.064; PMID: 15780738
- Zhang J, Gu Y, Ge SX, Li SW, He ZQ, Huang GY, et al. Analysis of hepatitis E virus neutralization sites using monoclonal antibodies directed against a virus capsid protein. Vaccine 2005; 23:2881 - 92; http://dx.doi.org/10.1016/j.vaccine.2004.11.065; PMID: 15780737
- Tang X, Yang C, Gu Y, Song C, Zhang X, Wang Y, et al. Structural basis for the neutralization and genotype specificity of hepatitis E virus. Proc Natl Acad Sci U S A 2011; 108:10266 - 71; http://dx.doi.org/10.1073/pnas.1101309108; PMID: 21642534
- Li S, Tang X, Seetharaman J, Yang C, Gu Y, Zhang J, et al. Dimerization of hepatitis E virus capsid protein E2s domain is essential for virus-host interaction. PLoS Pathog 2009; 5:e1000537; http://dx.doi.org/10.1371/journal.ppat.1000537; PMID: 19662165
- Zhang JZ, Ng MH, Xia NS, Lau SH, Che XY, Chau TN, et al. Conformational antigenic determinants generated by interactions between a bacterially expressed recombinant peptide of the hepatitis E virus structural protein. J Med Virol 2001; 64:125 - 32; http://dx.doi.org/10.1002/jmv.1027; PMID: 11360244
- Zhang J, Liu CB, Li RC, Li YM, Zheng YJ, Li YP, et al. Randomized-controlled phase II clinical trial of a bacterially expressed recombinant hepatitis E vaccine. Vaccine 2009; 27:1869 - 74; http://dx.doi.org/10.1016/j.vaccine.2008.12.061; PMID: 19168109
- Wu T, Zhu FC, Huang SJ, Zhang XF, Wang ZZ, Zhang J, et al. Safety of the hepatitis E vaccine for pregnant women: A preliminary analysis. Hepatology 2011; In press http://dx.doi.org/10.1002/hep.25522; PMID: 22161542
- Sarin SK, Kumar M. A vaccine for hepatitis e: has it finally arrived?. Gastroenterology 2011; 140:1349 - 52, discussion 1352-3; http://dx.doi.org/10.1053/j.gastro.2011.02.040; PMID: 21354166
- Shrestha MP, Scott RM, Joshi DM, Mammen MP Jr., Thapa GB, Thapa N, et al. Safety and efficacy of a recombinant hepatitis E vaccine. N Engl J Med 2007; 356:895 - 903; http://dx.doi.org/10.1056/NEJMoa061847; PMID: 17329696