Abstract
Rotavirus vaccines were licensed in Spain between late 2006 and early 2007. Rotavirus vaccination was recommended but not reimbursed by the Spanish National Health System. Significant coverage rates have been reached in Galicia, with an average of 47% since the period July 2007–June 2008. We aim to explore eventual variations in the incidence of hospitalizations for acute gastroenteritis (AGE) among children < 5 y of age before and after vaccine introduction. The annual and monthly hospitalization rates for rotavirus-related AGE and all cause AGE, before and after rotavirus vaccine introduction, were calculated by using the official surveillance system for hospital data. The annual hospitalization rates for rotavirus-related AGE in children < 5 y of age decreased by 14.8% for the period July 2008 to June 2009 and by 44.5% for the period July 2009 to June 2010 as compared with the median rate of the pre-vaccination period (July 2003 to June 2007). The corresponding decreases for all cause AGE were 29.9% and 49.0%, respectively. In children < 12 mo of age a more marked decrease was observed. Compared with pre-vaccination years, a decrease in rotavirus-related and all cause AGE hospitalization rates was observed, with a greater decline in the July 2009 to June 2010 period.
Introduction
Rotavirus is one of the most common causes of diarrhea-associated hospitalizations in infants and young children worldwide.Citation1 The global impact of this infection in terms of diarrheal deaths and disease burden has been extensively documented.Citation2-Citation4 Two effective rotavirus vaccines are available and recommended by the World Health Organization (WHO) for routine immunization of all infants.Citation5 Different studies have shown that rotavirus vaccines implemented within national childhood immunization programs have had a substantial impact on reduction in hospitalizations for diarrhea and rotavirus infections.Citation6-Citation9
The monovalent attenuated human rotavirus vaccine Rotarix® (GlaxoSmithkline) was marketed on August 2006 and the pentavalent human-bovine reassortant vaccine Rotateq® (sanofi pasteur MSD) was licensed on January 2007. In 2008, rotavirus vaccination was recommended for universal use in children from 6 weeks to 24 or 26 weeks of age, depending on the vaccine, by the Vaccination Advisory Committee but not reimbursed by the Spanish National Health System. However, some areas have shown significant coverage rates. In the Autonomous Community of Galicia (North-western Spain), the estimated coverage of vaccination, assuming that all children receive the complete vaccination schedule, increased from 12% in the period July 2006–June 2007 to 43% in July 2007–June 2008, and 51% in July 2008–June 2009 and then stabilizes in 46% between July 2009 and June 2010 (sales data provided by IMS Health). This circumstance might allow observing changes in the incidence of diarrhea-associated hospitalizations in the targeted-age groups before and after non-routine immunization program in our community.
The aim of this study was to estimate the incidence of hospitalizations due to acute gastroenteritis caused by rotavirus infection in children under 5 y of age in Galicia and to assess the eventual variations in these rates between the periods pre and post vaccine introduction.
Results
A total of 3,564 hospitalizations in children < 5 y of age with AGE episodes during the study period (July 2003–June 2010) were retrieved. There were 1,989 (55.8%) records for RV-AGE and 1,575 for AGE not coded as RV-AGE. More than 70% of admissions occurred in infants ≤ 23 mo of age (). Overall, the mean (standard deviation, SD) number of RV-AGE was 284.1 (78.3) per year and 509.1 (118.5) for all cause AGE hospitalizations.
Table 1. Number of hospitalizations (%) related to rotavirus gastroenteritis and gastroenteritis of all causes in children aged < 5 y
During the study period (July 2003–June 2010), the mean annual hospitalization rates (SD) for RV-AGE and AGE of all causes was 284.2 (85.0) per 100,000 and 510.6 (138.6) per 100,000, respectively. shows the annual hospitalizations rates among the period. In the post-vaccination years, rates of RV-AGE hospitalizations decreased, globally, by 14.8% and 4.5% in the periods July 2008-June 2009 and July 2009–June 2010 respectively, as compared with the median rate of the pre-vaccination period (July 2003–June 2007) (). The corresponding decreases in the annual hospitalization rates due to AGE of all causes in the post vaccination periods as compared with the median rate of the pre vaccination years were 29.9% (July 2008–June 2009) and 49.0% (July 2009–June 2010) (). In children < 12 mo of age a more marked decrease was observed: Compared with the pre-vaccination period, the hospitalization rates decreased by 32.0% and 35.2% respectively for RV-AGE and all cause AGE in the first post vaccination period (July 2008–June 2009), and by 57.3% and 58.7%, respectively, for the period July 2009–June 2010 ().
Table 2. Annual rates of hospitalizations due to acute infectious gastroenteritis and rotavirus gastroenteritis in children aged < 5 y
Table 3. Changes in hospitalization rates due to acute infectious gastroenteritis and rotavirus gastroenteritis in children aged < 5 y
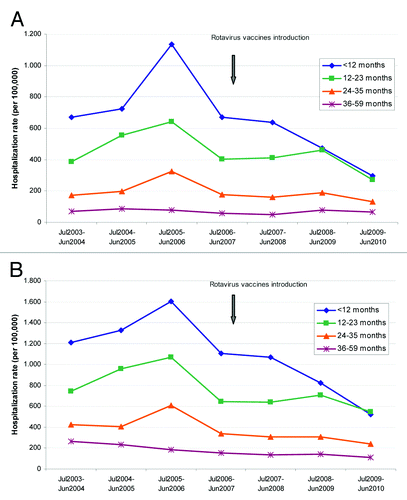
As shown in , before vaccines introduction, around 47% of RV-AGE hospitalizations occurred in children < 12 mo of age while in the post-vaccination periods (July 2008- June 2009 and July 2009-June 2010) hospital admissions in children < 12 mo represented only 35.5% of the total.
The annual hospitalization rates by age groups during the study period for RV-AGE and AGE of all causes are shown in . In the first period after vaccines introduction in which children < 12 mo of age were vaccinated with an estimated coverage above 40% (July 2008–June 2009), a decrease of the hospitalizations rates related to RV-AGE () was observed in this group of age. In the period July 2009–June 2010, rates in children < 12 mo of age continued decreasing and a decline was also observed among children of 12–23 mo of age (vaccinated with an estimated 43% coverage in the 2007–2008 period) ().
Figure 1. Annual hospitalization rates for rotavirus-related AGE and acute gastroenteritis (AGE) of all causes in Galicia from June 2003 to July 2010 by group of age. (A) Hospitalization rates RV-AGE. (B) Hospitalization rates all cause-AGE.
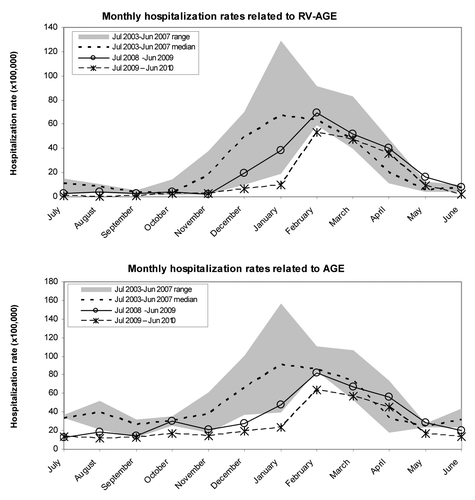
A seasonal pattern was observed for either RV-AGE or AGE of all causes with higher number of admissions during the winter-spring months. In the pre vaccination years 73.9% of admissions related to RV-AGE and 55.6% of the ones related to all cause AGE occurred between December and March (). In the periods between July 2008 and June 2009 and July 2009 and June 2010 a delay in season onset was observed and, especially for the latest period analyzed (July 2009–June 2010), the RV-AGE hospitalizations rates peak was shorter in time and reduced in comparison to the median rates of the year previous to the vaccines introduction ().
Table 4. Monthly number of hospitalizations related to rotavirus gastroenteritis and acute gastroenteritis (AGE) of all causes in children aged < 5 y
Discussion
Our study shows a decrease in the annual hospitalization rates due to RV-AGE and AGE of all causes in children under 5 y of age admitted to hospitals of the SERGAS network in the periods after the introduction of rotavirus vaccine. Before vaccines introduction, rotavirus disease generated a considerable burden of severe disease requiring hospital treatment in Spain, as it was previously reported.Citation10-Citation14 In the present study, the mean hospitalization rate for RV-AGE per year was 284.2 admissions per 100,000 children < 5 y. The annual hospitalization rates for RV-AGE were higher in the younger age groups and during the winter months, a well known tendency also described in other studies.Citation10,Citation15-Citation17 The decrease in the annual hospitalization rates due to RV-AGE was especially marked among the age groups targeted for vaccination. In children ≤ 12 mo of age, the reduction of RV related hospitalization rate was of 32.0% for the period of July 2008-June 2009, and 57.3% for the period of July 2009–June 2010 in comparison with the pre-vaccination years. Rates of hospitalization of the group of children of 12–23 mo of age, who were vaccinated with an estimated coverage of ≈40% in the 2007–2008 period also experienced a decrease in the last post vaccination period assessed.
A similar decrease in the rate of hospitalizations was also observed among all cause-AGE admissions. These findings are in line with previous studies conducted in different countries and geographical areas with different vaccination coverage, in which reductions of RV-AGE and all-cause AGE cases have been described not only within the hospital setting but also among Emergency Department and outpatient offices.Citation8,Citation9,Citation18-Citation26 Reduction in AGE hospitalizations non coded specifically as rotavirus suggest that even in settings where rotavirus testing is routine, testing and coding practices may underestimate the true rate of rotavirus hospitalizations.Citation9,Citation27 The observed sustained decrease in the second post- vaccination season, especially in children < 2 y of age, supports this hypothesis. Besides, other factors such as the natural secular fluctuations in rotavirus disease epidemiology, the year-to-year variability in gastroenteritis caused by other pathogens with winter seasonality (e.g., norovirus), the role of the vaccine in mixed infections or even changes in the coding or clinical practices, may have also influenced our findings.Citation28
Post-marketing studies have shown high effectiveness of both RV vaccines regarding RV-associated hospital-admission rates and RV-morbidity.Citation29 RV vaccine effectiveness was found similarly high in our setting.Citation30 Although the proportion of the hospitalization rate reduction directly attributable to vaccination could not be determined, the decrease observed in our study apparently exceeded what could have been expected, considering the vaccination coverage -around 40–50%- and vaccine effectiveness in preventing severe disease cases. Other authors have observed a larger than expected effect in RV disease after the introduction of rotavirus vaccination suggesting an indirect reduction of infections in unvaccinated children as a consequence of the vaccination-induced herd effect derived from the disrupted transmission of rotavirus among household and community contacts.Citation8,Citation31,Citation32
Continued surveillance and further population-based studies will provide more accurate data on the direct and indirect effects of vaccination in the reduction of rotavirus-related disease.
A delay in rotavirus season onset was observed in our results, together with a reduction of the epidemic peak. This finding was consistent within post-licensure studies performed in different regions and it was especially remarkable in studies from the United States and Belgium.Citation20,Citation21,Citation33 During the 2010 rotavirus season in the United-States, rotavirus activity was below the epidemic threshold for the first time in 19 y of rotavirus surveillance.Citation34,Citation35 Curns et al. showed remarkable alterations in the spatiotemporal spread of rotavirus disease in the US after vaccination.Citation36 These findings also emphasize the need of surveillance programs over time and extended to the months of the year when rotavirus is not typically expected to fully understand the public health importance of shifts in rotavirus epidemiology.
Some considerations related to the limitations of the study should be commented on. The national administrative database for hospital admissions was used as data source, since in Spain rotavirus infections are not subject to special surveillance, which means that information may be incomplete. Furthermore, considering that data from the CMBD-HA is anonymous, it is impossible to identify if the same child is hospitalized more than once in the same year. However, since primary rotavirus infection generally protects against subsequent severe illness, it could be considered that any contribution of re-admitted cases to the results would likely be small.Citation37,Citation38 Infra-notification is usually another of the limitations for studies performed by using databases as source of information, but in the present study this risk should be low as the CMBD is a mandatory register with an estimated coded valid discharge rate of almost 100%. Diagnosis codes may not correspond to laboratory-confirmed cases, although, other studies have shown the ICD-9-MC code for rotavirus to be highly specific.Citation10 Anyway, as it has been previously discussed, the number of rotavirus related admissions may be underestimated, also because pediatricians in many hospitals may have been discouraged from ordering diagnostics tests for rotavirus that increase the cost of medical care but not significantly influence treatment decisions and rotavirus tests protocol for use in children admitted for AGE may have changed after vaccine implementation;Citation27 Hsu et al. study on the validation of rotavirus hospitalization estimates by using the ICD-9-CM code concluded that the sensitivity of the rotavirus specific code was limited and only 25% of those with rotavirus positive test results were actually assigned a rotavirus code.Citation27 Other authors have previously demonstrated the limitations of using the rotavirus-specific code for the estimation of the rate of hospitalizations for rotavirus-related AGE.Citation8,Citation9,Citation18,Citation19,Citation23,Citation26,Citation39 For these reasons we also relied on all cause AGE admissions assess RV vaccines impact in our study. Finally, although estimation of vaccination coverage using sales is a usual practice, the percentage obtained may be overestimated as the distributed doses may not necessarily reflect the actual number of doses administered.
Despite these limitations, the present study provides reliable information on the burden of rotavirus disease in Galicia. The epidemiologic pattern described in this study is likely to reflect the situation throughout Spain. This pattern and the severe disease reduction observed, despite limited vaccination coverage, is similar to that described in other industrialized countries. Continued surveillance and further population-based studies are needed to monitor the direct and indirect effects of vaccination on reduction of rotavirus-related disease, particularly in the long-term.
Materials and Methods
Setting and study design
A retrospective, observational and hospital-based surveillance study of rotavirus gastroenteritis in children was conducted in the Autonomous Community of Galicia, Spain. The Autonomous Community of Galicia is situated in the northwest of Spain with a total area of 29,574 km2. Total population for the region as a whole (January 2006 census) is 2,767,524 people (approximately 100,000 under 5 y of age).Citation40
Health care is provided through the Galician Health Service (Servicio Gallego de Salud, SERGAS) which provides public health services in the region of Galicia. The hospital network of SERGAS is constituted by 8 hospitals complexes and 7 hospitals (constituting a total of 32 hospitals). About 242,000 hospital discharges are generated in the SERGAS hospital network each year.Citation41
The study period, between July 2003 and June 2010, included pre and post-vaccination seasons. The primary objective of the study was to estimate the number and rate of hospitalizations due to rotavirus-related acute gastroenteritis (RV-AGE) in children < 5 y of age in the SERGAS hospital network during the study period. Secondary objectives of the study also include: (1) to estimate the number and rate of hospitalizations due to AGE episodes in children < 5 y of age; and (2) to assess the annual and monthly changes in the incidence of AGE-related hospitalizations.
The study was conducted using data from the hospitals of the SERGAS network. Inclusion criteria were admission for an AGE episode in one of the study hospitals, children aged less than 5 y and residents in the area of the hospitals included in the study. No exclusion criteria were established.
Data collection and study procedures
The SERGAS official surveillance system for hospital data, known as Conjunto Mínimo Básico de Datos de Hospitalización de Agudos (CMBD-HA, Minimum Set of Acute Hospitalizations database) was used as the information source. This database includes personal, administrative and medical data of all patients admitted to the hospital in Galicia, with diagnoses codified according to the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM). The CMBD-HA has maintained a coded valid discharge rate and since 2003, 100% of the hospitalizations are coded.Citation42 A search strategy was applied based on disease category codes corresponding to diagnosis at discharge of intestinal infectious gastroenteritis (ICD-9-CM codes 001 to 009) and non-infectious gastroenteritis (ICD-9-CM code 558.9), in any diagnostic position. Among these hospitalizations with ICD-9-MC codes 001–009, the code 008.6, which specifically relates to rotavirus infection, was then selected. The diagnosis, age, and date of admission was collected for each case identified. The study protocol was reviewed and approved by the institutional review board of Galicia (Comité Ético de Investigación Clínica de Galicia).
Statistical analysis
This was a hospital-based surveillance study and therefore no sample size was calculated. The annual and monthly hospitalization rates for RV-AGE and AGE of all causes during the study period were calculated either for the whole study population (children < 5 y of age) or by age groups. The population used as denominator for the calculation of rates was the population of children < 5 y of age living in the Autonomous Community of Galicia during the corresponding years of the study period. These figures were obtained from the Galician Institute of Statistics. The percentage of decrease in the hospitalizations rates for RV-AGE and AGE of all causes during post-vaccination period (July 2008–June 2009 and July 2009–June 2010) as compared with the median rate of the pre-vaccination period (July 2003–June 2007) was also calculated. The transition period between July 2007 and June 2008 was excluded for this analysis because rotavirus vaccines were introduced between August 2006 and January 2007 and recommendation was first published in January 2008. The statistical analyses were performed using SPSS for Windows version 17.0 (SPSS Inc.).
| Abbreviations: | ||
| AGE | = | acute gastroenteritis |
| CMBD-HA | = | Minimum Set of Acute Hospitalizations database |
| ICD-9-CM | = | International Classification of Disease, 9th Revision, Clinical Modification |
| SERGAS | = | Galician Health Service |
Acknowledgments
The authors thank Marta Pulido, MD, for editing the manuscript and editorial assistance. The fees of medical editing were supported by Sanofi Pasteur MSD.
Funding
This study and the group research activities were supported by grants from Consellería de Economía e Industria / Xunta de Galicia (Promoción Xeral de Investigación 10PXIB918184PR), Consellería de Sanidade / Xunta de Galicia (RHI07/2-intensificación actividad investigadora and PS09749), Fundación de Investigación Médica Mutua Madrileña (2010), Sanofi Pasteur MSD (unrestricted grant RTQ02E), Instituto Carlos III (Intensificación de la actividad investigadora) and Fondo de Investigación Sanitaria (FIS; PI1000540)) del plan nacional de I+D+I and ‘Fondos FEDER’ given to Federico Martinón-Torres.
Disclosure of Potential Conflicts of Interest
Federico Martinón-Torres has received research grants and/or honoraria as consultant/advisor and/or speaker from GlaxoSmithKline, Sanofi Pasteur MSD, Pfizer Inc., Wyeth, Novartis, and Medimmune Inc. María San-Martín is employee of Sanofi Pasteur MSD, company that commercialize the rotavirus vaccine Rotateq®
No conflicts of interest to be declared for the remaining authors.
References
- Bernstein DI. The changing epidemiology of rotavirus gastroenteritis. Introduction. Pediatr Infect Dis J 2009; 28:Suppl S49; http://dx.doi.org/10.1097/INF.0b013e3181967bda; PMID: 19252422
- Patel MM, Steele D, Gentsch JR, Wecker J, Glass RI, Parashar UD. Real-world impact of rotavirus vaccination. Pediatr Infect Dis J 2011; 30:Suppl S1 - 5; http://dx.doi.org/10.1097/INF.0b013e3181fefa1f; PMID: 21183833
- Parashar UD, Burton A, Lanata C, Boschi-Pinto C, Shibuya K, Steele D, et al. Global mortality associated with rotavirus disease among children in 2004. J Infect Dis 2009; 200:Suppl 1 S9 - 15; http://dx.doi.org/10.1086/605025; PMID: 19817620
- Diez-Domingo J, Suriñach NL, Alcalde NM, Betegón L, Largeron N, Trichard M. Burden of paediatric Rotavirus Gastroenteritis (RVGE) and potential benefits of a universal Rotavirus vaccination programme with a pentavalent vaccine in Spain. BMC Public Health 2010; 10:469; http://dx.doi.org/10.1186/1471-2458-10-469; PMID: 20698958
- WHO. Meeting of the Strategic Advisory Group on Immunization, October 2009 conclusions and recommendations. Wkly Epidemiol Rec 2009; 84:518
- Molto Y, Cortes JE, De Oliveira LH, Mike A, Solis I, Suman O, et al. Reduction of diarrhea-associated hospitalizations among children aged < 5 Years in Panama following the introduction of rotavirus vaccine. Pediatr Infect Dis J 2011; 30:Suppl S16 - 20; http://dx.doi.org/10.1097/INF.0b013e3181fefc68; PMID: 21183835
- Chang HG, Smith PF, Tserenpuntsag B, Markey K, Parashar U, Morse DL. Reduction in hospitalizations for diarrhea and rotavirus infections in New York state following introduction of rotavirus vaccine. Vaccine 2010; 28:754 - 8; http://dx.doi.org/10.1016/j.vaccine.2009.10.075; PMID: 19896451
- Yen C, Tate JE, Wenk JD, Harris JM 2nd, Parashar UD. Diarrhea-associated hospitalizations among US children over 2 rotavirus seasons after vaccine introduction. Pediatrics 2011; 127:e9 - 15; http://dx.doi.org/10.1542/peds.2010-1393; PMID: 21172995
- Buttery JP, Lambert SB, Grimwood K, Nissen MD, Field EJ, Macartney KK, et al. Reduction in rotavirus-associated acute gastroenteritis following introduction of rotavirus vaccine into Australia’s National Childhood vaccine schedule. Pediatr Infect Dis J 2011; 30:Suppl S25 - 9; http://dx.doi.org/10.1097/INF.0b013e3181fefdee; PMID: 21183837
- López-de-Andrés A, Jiménez-García R, Carrasco-Garrido P, Alvaro-Meca A, Galarza PG, de Miguel AG. Hospitalizations associated with rotavirus gastroenteritis in Spain, 2001-2005. BMC Public Health 2008; 8:109; http://dx.doi.org/10.1186/1471-2458-8-109; PMID: 18397512
- Luquero Alcalde FJ, Eiros Bouza JM, Rubio AP, Bachiller Luque MR, Castrodeza Sanz JJ, Ortiz de Lejarazu Leonardo R. Gastroenteritis by rotavirus in Spanish children. Analysis of the disease burden. Eur J Pediatr 2008; 167:549 - 55; http://dx.doi.org/10.1007/s00431-007-0550-8; PMID: 17653572
- Soriano-Gabarró M, Mrukowicz J, Vesikari T, Verstraeten T. Burden of rotavirus disease in European Union countries. Pediatr Infect Dis J 2006; 25:Suppl S7 - 11; http://dx.doi.org/10.1097/01.inf.0000197622.98559.01; PMID: 16397431
- Martinón-Torres F, Bouzón-Alejandro M, López-Sousa M, Redondo-Collazo L, Almeida-Agudín S, Astorgano-Fernández C, et al. An estimation of indirect costs caused by acute rotavirus gastroenteritis in a Galician area, Spain. Eur J Pediatr 2008; 167:337 - 9; http://dx.doi.org/10.1007/s00431-007-0466-3; PMID: 17364172
- Gimenez-Sanchez F, Delgado-Rubio A, Martinon-Torres F, Bernaola-Iturbe E, Rotascore Research Group. Multicenter prospective study analysing the role of rotavirus on acute gastroenteritis in Spain. Acta Paediatr 2010; 99:738 - 42; PMID: 20096025
- Díez-Domingo J, Martín IO, Sanz AB, López AG, Martínez CC, Boronat CP, et al. Rotavirus gastroenteritis among children under five years of age in Valencia, Spain. Pediatr Infect Dis J 2006; 25:455 - 7; http://dx.doi.org/10.1097/01.inf.0000217378.30444.21; PMID: 16645514
- Téllez Castillo CJ, Tirado Balaguer MD, Colomer Revuelta J, Moreno Muñoz R, Beltrán Garrido JM. [Ten-year retrospective study of rotavirus infection in the province of Castellón (Spain)]. An Pediatr (Barc) 2008; 68:39 - 44; PMID: 18194626
- Van Damme P, Giaquinto C, Huet F, Gothefors L, Maxwell M, Van der Wielen M, REVEAL Study Group. Multicenter prospective study of the burden of rotavirus acute gastroenteritis in Europe, 2004-2005: the REVEAL study. J Infect Dis 2007; 195:Suppl 1 S4 - 16; http://dx.doi.org/10.1086/516714; PMID: 17387650
- Bégué RE, Perrin K. Reduction in gastroenteritis with the use of pentavalent rotavirus vaccine in a primary practice. Pediatrics 2010; 126:e40 - 5; http://dx.doi.org/10.1542/peds.2009-2069; PMID: 20587671
- Curns AT, Steiner CA, Barrett M, Hunter K, Wilson E, Parashar UD. Reduction in acute gastroenteritis hospitalizations among US children after introduction of rotavirus vaccine: analysis of hospital discharge data from 18 US states. J Infect Dis 2010; 201:1617 - 24; http://dx.doi.org/10.1086/652403; PMID: 20402596
- Raes M, Strens D, Vergison A, Verghote M, Standaert B. Reduction in pediatric rotavirus-related hospitalizations after universal rotavirus vaccination in Belgium. Pediatr Infect Dis J 2011; 30:e120 - 5; http://dx.doi.org/10.1097/INF.0b013e318214b811; PMID: 21436757
- Tate JE, Cortese MM, Payne DC, Curns AT, Yen C, Esposito DH, et al. Uptake, impact, and effectiveness of rotavirus vaccination in the United States: review of the first 3 years of postlicensure data. Pediatr Infect Dis J 2011; 30:Suppl S56 - 60; http://dx.doi.org/10.1097/INF.0b013e3181fefdc0; PMID: 21183842
- Gagneur A, Nowak E, Lemaitre T, Segura JF, Delaperrière N, Abalea L, et al, IVANHOE investigators. Impact of rotavirus vaccination on hospitalizations for rotavirus diarrhea: the IVANHOE study. Vaccine 2011; 29:3753 - 9; http://dx.doi.org/10.1016/j.vaccine.2011.03.035; PMID: 21443962
- Uhlig U, Kostev K, Schuster V, Uhlig HH. Rotavirus vaccination in Germany: analysis of nationwide surveillance data 2006 to 2010. Pediatr Infect Dis J 2011; 30:e244 - 7; http://dx.doi.org/10.1097/INF.0b013e31822d1408; PMID: 21817956
- Trimis G, Koutsoumbari I, Kottaridi C, Palaiologou N, Assimakopoulou E, Spathis A, et al. Hospital-based surveillance of rotavirus gastroenteritis in the era of limited vaccine uptake through the private sector. Vaccine 2011; 29:7292 - 5; http://dx.doi.org/10.1016/j.vaccine.2011.07.092; PMID: 21816195
- Payne DC, Staat MA, Edwards KM, Szilagyi PG, Weinberg GA, Hall CB, et al, New Vaccine Surveillance Network (NVSN). Direct and indirect effects of rotavirus vaccination upon childhood hospitalizations in 3 US Counties, 2006-2009. Clin Infect Dis 2011; 53:245 - 53; http://dx.doi.org/10.1093/cid/cir307; PMID: 21705316
- Clarke MF, Davidson GP, Gold MS, Marshall HS. Direct and indirect impact on rotavirus positive and all-cause gastroenteritis hospitalisations in South Australian children following the introduction of rotavirus vaccination. Vaccine 2011; 29:4663 - 7; http://dx.doi.org/10.1016/j.vaccine.2011.04.109; PMID: 21575665
- Hsu VP, Staat MA, Roberts N, Thieman C, Bernstein DI, Bresee J, et al. Use of active surveillance to validate international classification of diseases code estimates of rotavirus hospitalizations in children. Pediatrics 2005; 115:78 - 82; PMID: 15629984
- Sánchez-Fauquier A, Montero V, Colomina J, González-Galán V, Aznar J, Aisa ML, et al. Global study of viral diarrhea in hospitalized children in Spain: results of structural surveillance of viral gastroenteritis net work (VIGESS-net) 2006-2008. J Clin Virol 2011; 52:353 - 8; http://dx.doi.org/10.1016/j.jcv.2011.08.025; PMID: 21963269
- Soares-Weiser K, Maclehpse H, Ben-Aharon I, et al. Vaccines for preventing rotavirus diarrhoea: vaccines in use. Cochrane Database Sust Rev. 2012: CD008521.
- Martinón-Torres F, Bouzón Alejandro M, Redondo Collazo L, Sánchez Lastres JM, Pértega Díaz S, Seoane Pillado MT, et al, ROTACOST research team. Effectiveness of rotavirus vaccination in Spain. Hum Vaccin 2011; 7:757 - 61; http://dx.doi.org/10.4161/hv.7.7.15576; PMID: 21521947
- Glass RI. Unexpected benefits of rotavirus vaccination in the United States. J Infect Dis 2011; 204:975 - 7; http://dx.doi.org/10.1093/infdis/jir477; PMID: 21878426
- Paulke-Korinek M, Kundi M, Rendi-Wagner P, de Martin A, Eder G, Schmidle-Loss B, et al. Herd immunity after two years of the universal mass vaccination program against rotavirus gastroenteritis in Austria. Vaccine 2011; 29:2791 - 6; http://dx.doi.org/10.1016/j.vaccine.2011.01.104; PMID: 21320539
- Centers for Disease Control and Prevention (CDC). Reduction in rotavirus after vaccine introduction--United States, 2000-2009. MMWR Morb Mortal Wkly Rep 2009; 58:1146 - 9; PMID: 19847149
- Torok TJ, Kilgore PE, Clarke MJ, et al. Visualizing geographich and temporal trends in rotavirus activity in the United States, 1991, 1996. National respiratory and enteric virus surveillance system collaborating laboratories. Pediatr Infect Dis J 1997; 16:941 - 6; PMID: 9380468
- Turcios RM, Curns AT, Holman RC, Pandya-Smith I, LaMonte A, Bresee JS, et al, National Respiratory and Enteric Virus Surveillance System Collaborating Laboratories. Temporal and geographic trends of rotavirus activity in the United States, 1997-2004. Pediatr Infect Dis J 2006; 25:451 - 4; http://dx.doi.org/10.1097/01.inf.0000214987.67522.78; PMID: 16645512
- Curns AT, Panozzo CA, Tate JE, Payne DC, Patel MM, Cortese MM, et al. Remarkable postvaccination spatiotemporal changes in United States rotavirus activity. Pediatr Infect Dis J 2011; 30:Suppl S54 - 5; http://dx.doi.org/10.1097/INF.0b013e3181fefda9; PMID: 21183841
- García-Basteiro AL, Bosch A, Sicuri E, Bayas JM, Trilla A, Hayes EB. Hospitalizations due to rotavirus gastroenteritis in Catalonia, Spain, 2003-2008. BMC Res Notes 2011; 4:429; http://dx.doi.org/10.1186/1756-0500-4-429; PMID: 22013948
- Velázquez FR, Matson DO, Calva JJ, Guerrero L, Morrow AL, Carter-Campbell S, et al. Rotavirus infections in infants as protection against subsequent infections. N Engl J Med 1996; 335:1022 - 8; http://dx.doi.org/10.1056/NEJM199610033351404; PMID: 8793926
- Wang FT, Mast TC, Glass RJ, Loughlin J, Seeger JD. Effectiveness of the pentavalent rotavirus vaccine in preventing gastroenteritis in the United States. Pediatrics 2010; 125:e208 - 13; http://dx.doi.org/10.1542/peds.2009-1246; PMID: 20100757
- Instituto Gallego de Estadística (Galician Institute of Statistics). . (http://www.ige.eu/web/mostrar_actividade_estatistica.jsp?idioma=es&codigo=0201001002).
- Informe anual del Sistema Nacional de Salud 2007. Ministerio de Sanidad y Política Social y Consellería de Sanidade de la Xunta de Galicia. (http://www.msc.es/organizacion/sns/planCalidadSNS/pdf/equidad/informeAnual2007/GaliciaSNS2007.pdf).
- Boletines de codificación diagnóstica. Consellería de Sanidade de la Xunta de Galicia. (http://www.sergas.es/MostrarContidos_N2_T01.aspx?IdPaxina=61674).