Abstract
Co-administration of meningococcal serogroups A, C, W-135 and Y conjugate vaccine (ACWY-TT) with seasonal influenza vaccine was investigated in a subset of adults enrolled in a larger study evaluating lot-to-lot consistency of ACWY-TT and non-inferiority to licensed tetravalent meningococcal polysaccharide vaccine (MenPS). Subjects in this sub-study were randomized (3:1:1) to receive ACWY-TT alone (ACWY-TT group) or with seasonal influenza vaccine (Coad), or licensed MenPS alone. Serum bactericidal antibodies (rSBA) and serum haemagglutination-inhibition (HI) antibody titers were measured pre- and 1 mo post-vaccination. Non-inferiority of the Coad group compared with ACWY-TT group was demonstrated in terms of rSBA geometric mean antibody titers (GMTs) to serogroups A, W-135 and Y. For serogroup C the pre-defined non-inferiority limit was marginally exceeded. Post-vaccination rSBA GMTs were significantly higher (exploratory analysis) in the Coad group compared with the MenPS group for serogroups A, W-135, and Y and were similar to the MenPS group for serogroup C. Overall, > 97% of subjects achieved rSBA titers ≥ 1:128 for all serogroups. The Coad group met all criteria defined by the Committee on Human Medicinal Products (CHMP) for seroprotection, seroconversion and seroconversion factor for HI antibodies for all three influenza strains. Grade 3 solicited local/general symptoms were reported by ≤ 1.9% of subjects in any group. These data support the co-administration of ACWY-TT with seasonal influenza vaccine when protection is needed against both diseases.
This study is registered at clinicaltrials.gov NCT00453986
Introduction
Infection with Neisseria meningitidis causes serious, potentially life-threatening disease. Approximately 10% of invasive meningococcal infections are fatal, despite appropriate antibiotic treatment and supportive care.Citation1 The majority of invasive meningococcal disease (IMD) is caused by 6 serogroups: A, B, C, W-135,Y and X, whose distribution varies globally.Citation1,Citation2 The incidence of IMD is highest in infants, but disease occurs in all age groups, with a substantial proportion of cases that occur in adults.Citation3 In older age groups case fatality increases with increasing age.Citation3 Adult populations particularly at risk of IMD include travelers to meningococcal endemic regions. As global travel activity continues to rise, regional differences in IMD incidence and serogroup distribution pose increasing risk for international travelers to acquire and facilitate the intercontinental spread of meningococcal disease. In particular, travelers to the Hajj face an increased risk of meningococcal disease, and meningococcal vaccination against serogroups A, C, W-135 and Y is now required prior to Hajj attendance for all pilgrims over 2 y of age.Citation4,Citation5
Travel also has an important role in disseminating influenza outbreaks, as evident during the recent influenza pandemic.Citation6 Prior to travel it is often necessary to administer multiple vaccines simultaneously. Given the global endemicity of both N. meningitidis and influenza virus, immunogenicity and safety data of co-administered meningococcal conjugate and inactivated influenza vaccines are desirable.
The investigational tetravalent polysaccharide conjugate vaccine against N. meningitidis serogroups A, C, W-135 and Y, using tetanus toxoid as the carrier protein [ACWY-TT, GlaxoSmithKline Biologicals (GSK) Belgium] is immunogenic in toddlers, children and adolescents.Citation7-Citation13 Immunogenicity and safety of co-administration of ACWY-TT and seasonal influenza vaccine (Fluarix™, GSK Biologicals) was assessed in adults 18–55 y of age as a sub-study nested in a partially double-blinded, controlled, non-inferiority study. Within the sub-study (the Influenza cohort), subjects were randomized 1:1:1:1:1 to receive a single dose of ACWY-TT (one of three manufacturing lots); or Mencevax™ ACWY (MenPS group), or ACWY-TT co-administered with the seasonal influenza vaccine (Coad group). Results of the ‘Influenza' cohort are reported here. In the same study, manufacturing consistency using three different manufacturing lots was established and pooled serological results were compared against the tetravalent polysaccharide vaccine control (MenPS: Mencevax™ ACWY, GSK Biologicals). These results are reported separately.
Results
A total of 520 adults were included in the total vaccinated Influenza cohort (ACWY-TT, Lot A, n = 311; Coad, n = 105; MenPS, n = 104). Three subjects (all in the ACWY-TT group) were eliminated from the according to protocol (ATP) Influenza cohort for immunogenicity: two subjects received a vaccine forbidden by the protocol (one subject received an inactivated influenza vaccine and one subject received a tetanus toxoid vaccine within the 30 d period post-vaccination) and one subject had no post-vaccination blood sample, leaving 517 subjects in the ATP Influenza cohort for immunogenicity. More male subjects than female subjects were enrolled (56.9% vs. 43.1%). The predominance of males was more pronounced in the Coad group (61.9% males vs. 55.9% and 54.8% in the ACWY-TT and MenPS groups, respectively). The mean age of subjects was 35.6 y (standard deviation 10.57 y, range 18–55). All but one subject was of South East Asian descent. No subject withdrew during the study.
The pre-defined statistical criterion of non-inferiority of serum bactericidal activity geometric mean titers (using rabbit complement as the exogenous complement source: rSBA GMTs) (ACWY-TT group over Coad group) was met for serogroups A, W-135 and Y [, the upper limit of the 2-sided 95% confidence interval (CI) on the GMT ratio (ACWY-TT/Coad group) was ≤ 2.0). For serogroup C the upper limit of 2.0 for the ratio of rSBA GMTs was marginally exceeded (by 0.03) (). When compared with the MenPS control group, an exploratory analysis showed that the upper limit of the 2-sided 95% CI on the GMT ratio (ACWY-TT/Men-PS group) was ≤ 2.0 for all serogroups ().
Table 1. Evaluation of primary non-inferiority hypotheses and exploratory analyses: Coad group vs. ACWY-TT and MenPS groups (ATP Influenza cohort for immunogenicity)
A vaccine response (defined as an rSBA titer ≥ 1:32 in initially seronegative subjects [pre-vaccination titer < 1:8], or a ≥ 4-fold increase in titer for initially seropositive subjects [pre-vaccination titer ≥ 1:8] against individual serogroups was observed in 76.5–88.7% of subjects in the Coad group, 80.6–92.0% in the ACWY-TT group and 63.6–87.3% in the MenPS group. Exploratory analyses did not detect any statistically significant differences between groups in terms of vaccine response rates (). All initially seronegative subjects mounted a booster response to vaccination for each serogroup with the following exception: in the ACWY-TT group, one subject for serogroup A (2.2%) and one subject for serogroup Y (4.2%), in the MenPS group, one subject for serogroup W-135 (5.6%) and one subject for serogroup C (5.9%), and in the Coad group one subject for serogroup W-135 (6.2%).
Overall, at least 99.0% and 97.1% of subjects in all vaccine groups achieved rSBA titers ≥ 1:8 and ≥ 1:128 respectively, for each of the 4 serogroups (data not shown). No differences between groups were observed in terms of the percentage of subjects reaching the 1:8 and 1:128 cut-offs (data not shown).
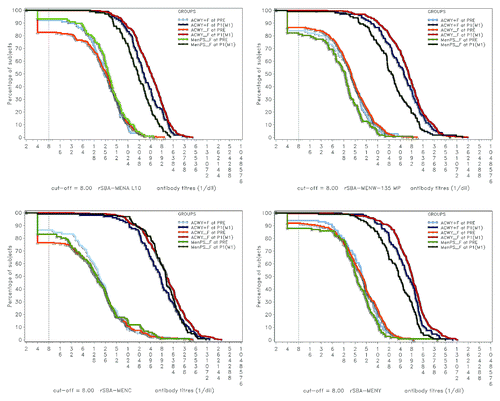
Exploratory analysis showed that rSBA GMTs adjusted for age strata and pre-vaccination titer were statistically significantly lower in the Coad group than in the ACWY-TT group for serogroups A and C, and statistically significantly higher in the Coad group than in the MenPS group for serogroups A, W-135 and Y (). Reverse cumulative curves (RCCs) showing pre and post-vaccination rSBA titers indicate robust increases in GMTs in all treatment groups for all 4 serogroups after vaccination ().
Figure 1. rSBA GMTs in each group one month after vaccination (ATP Influenza cohort for immunogenicity). *statistically significant difference between the indicated group and the Coad group: differences between groups were done on GMT values adjusted for pre-vaccination measurements and age strata, exploratory analysis, whereas the GMTs displayed are unadjusted.
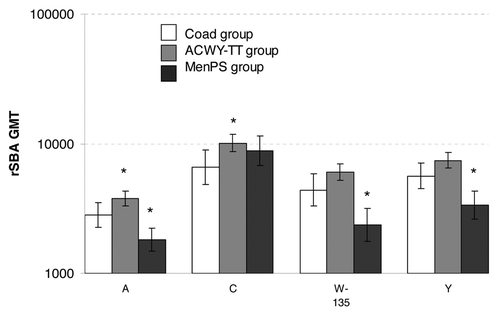
Figure 2. Reverse cumulative curves showing rSBA titers for N. meningitidis serogroups A, C, W-135 and Y. ACWY+F, Coad group; ACWY_F, ACWY-TT group; MenPS_F, MenPS group
The Coad group met all pre-defined statistical criteria set out by the European Medicines Agency Committee for Proprietary Medicinal Products (CHMP) for antibody responses against influenza antigens A/H1N1, A/H3N2, and B ().
Table 2. Influenza humoral immune responses one month after vaccination (ATP Influenza cohort for immunogenicity)
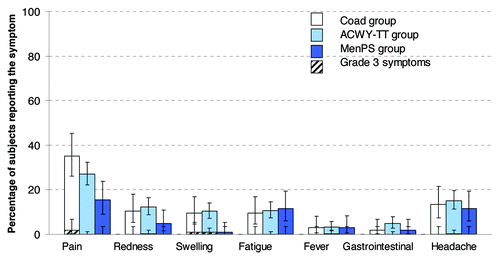
The most frequently reported solicited local and general Adverse Events (AEs) in the Coad, ACWY-TT and MenPS groups were injection site pain and headache, respectively (). Grade 3 solicited local/general AEs were reported by no more than 1.9% of subjects in any vaccination group.
Figure 3. Percentage of subjects reporting solicited local and general symptoms during the 4-day post-vaccination period (total vaccinated Influenza cohort). Note: For the Co-ad group, local symptoms refer to the percentage of subjects with at least one local symptom at either injection site. Gastrointestinal symptoms included nausea, vomiting, diarrhea and/or abdominal pain. Grade 3: Redness and swelling > 50mm, fever > 39.5°C, Preventing normal everyday activity for all other symptoms.
Four subjects in the Influenza cohort reported seven serious AEs (SAEs) (all in the ACWY-TT group) during the study period. Of these, one subject reported abdominal pain and gastritis with onset 5 d after vaccination that was considered by the investigator to be related to vaccination. At the conclusion of the 6-mo safety follow-up, no subject in the Influenza cohort reported rash, an emergency room visit or new onset of chronic disease.
Discussion
ACWY-TT co-administered with seasonal influenza vaccine induced robust immune responses, with at least 76.5% of subjects having a vaccine response to each of the meningococcal serogroups, and a seroconversion rate of at least 61.9% to all three influenza strains. The statistical criterion of non-inferiority of Coad compared with ACWY-TT was met for serogroups A, W-135, and Y, but was not achieved for serogroup C by a small margin (0.03). Nevertheless, a high percentage of subjects achieved post-vaccination titers ≥ 1:128 (98.1%), a level considered a conservative threshold of protection.Citation15-Citation18 Exploratory analyses did not detect any statistically significant differences between the Coad group and the ACWY-TT group in the percentages of subjects with titers ≥ 1:8 and ≥ 1:128, or in the vaccine response rate for any meningococcal serogroup, indicating that both groups exhibited robust immune responses to meningococcal vaccination. Furthermore, no statistically significant difference in rSBA-MenC GMTs between the Coad group and the MenPS control group were observed (exploratory analysis), demonstrating a comparable immune response after co-administration of ACWY-TT and seasonal influenza vaccine to the standard of care in the Philippines at the time of the study. Finally, the post-vaccination rSBA-MenC GMT in the Coad group was within the range of GMTs previously reported following administration of ACWY conjugate vaccines to adolescent and adult populations, in studies employing the same rSBA.Citation7,Citation19
The magnitude of the post-vaccination rSBA GMT was lower in the Coad group than the ACWY-TT group for all meningococcal vaccine serogroups (significant only for serogroups A and C), suggesting the possibility of immune interference when ACWY-TT and influenza vaccine are co-administered. However, all but one subject in the Coad group achieved rSBA titers ≥ 1:8 and no more than 2.9% of subjects failed to achieve rSBA titers ≥ 1:128, for any one vaccine serogroup. Additionally, rSBA GMTs were significantly higher in the Coad group than the MenPS group for 3 out of 4 vaccine serogroups. Although the data from this study suggest some reduction in meningococcal rSBA titers when ACWY-TT is co-administered with trivalent inactivated influenza vaccine, the available data also suggest that any potential interference is unlikely to have clinical consequences, given the robust immune responses as illustrated by the RCC curves, and the fact that the post-vaccination titers are within previously-reported ranges. This, taken with the observation that all 3 CHMP criteria defining an acceptable immune response to influenza vaccine were met for all 3 tested influenza strains suggests that ACWY-TT and seasonal influenza vaccine can be co-administered, while maintaining acceptable immune responses to both vaccines.
We identified only one other study where a meningococcal vaccine (MenC-CRM197) was co-administered with an influenza vaccine.Citation20 The study was designed to assess the impact of an immuno-modulating agent on responses to co-administered MenC-CRM197 and influenza vaccines. The response to both vaccines in these individuals appeared adequate. However, comparison with the current study is precluded by use of different assay methods, and interpretation of the results in terms of the immunogenicity of co-administration cannot be made in the absence of a group that received MenC-CRM197 alone.
The present study is potentially limited by the absence of control groups to evaluate MenPS co-administered with influenza vaccine, or influenza vaccine alone. However, the criteria for clinical acceptability of the immune response were based on CHMP objective criteria for the yearly registration of inactivated influenza vaccine, and not on a non-inferiority comparison. Thus, the decision not to include a group that received influenza vaccine alone is justified based on the manner in which regulatory agencies determine whether the yearly change in influenza vaccine formulation can be marketed in a given year. Additionally, because ACWY-TT was administered intramuscularly while MenPS was given subcutaneously, the study was conducted open-blind with respect to ACWY-TT and Men-PS administration. Potential bias in the analysis of immunogenicity was reduced by blinding laboratory personnel to age and group. The open design may have influenced attribution of the relationship of adverse events to vaccination, but reporting bias would more likely be against the investigational product.
ACWY-TT had an acceptable safety profile in healthy adults, which was not affected by co-administration with influenza vaccine. Co-administration of vaccines reduces the number of visits required to complete vaccination and improves coverage of the co-administered antigens, which may be particularly important in travelers who need to be vaccinated against multiple antigens in a short duration of time. The administration of seasonal influenza vaccine may coincide with that of ACWY-TT in adults 18–55 y of age annually for an approximately four-month season. This is the first reported study describing the co-administration of an ACWY conjugate vaccine with seasonal influenza vaccine. The data support co-administration for combined vaccination against invasive meningococcal disease and seasonal influenza in healthy adult travelers.
Materials and Methods
Study design
The study was a Phase III, randomized, partially double-blinded, controlled, non-inferiority study conducted at one study center in Lebanon and in three centers in the Philippines (109067/NCT00453986) between April 2007 and May 2008. The study was conducted according to good clinical practice and in accordance with the Declaration of Helsinki. The protocol and associated documents were reviewed and approved by ethics committees at each study center. Written informed consent was obtained from subjects before study entry.
Enrolled healthy adults 18–55 y of age received a single dose of one of three manufacturing lots of ACWY-TT (ACWY-TT group, lots A, B and C), or Mencevax™ ACWY (MenPS group), or ACWY-TT Lot A co-administered with the seasonal influenza vaccine, (Fluarix™: GSK Biologicals, Coad group), respectively. The full study consisted of three cohorts with separate objectives: (1) lot-to-lot consistency of three clinical lots of ACWY-TT, which will be reported separately, (2) immunogenicity and safety of ACWY-TT vs. MenPS vaccine, which is reported separately and (3) safety and immunogenicity when ACWY-TT and seasonal influenza vaccine were co-administered as compared with ACWY-TT alone as assessed in the ‘Influenza' cohort, and reported here. Subjects in the Influenza cohort were healthy adults enrolled at two sites in the Philippines randomized 1:1:1:1:1 to the ACWY-TT group (pooled lots A, B and C), MenPS or Coad groups.
Study objectives
The co-primary study objectives were: (1) demonstration of non-inferiority of ACWY-TT co-administered with seasonal influenza vaccine compared with ACWY-TT alone, in terms of serum bactericidal antibodies geometric mean titers (rSBA GMTs) for serogroups A, C, W-135, and Y 1 mo post-vaccination, and (2) demonstration of acceptable immunogenicity of seasonal influenza vaccine co-administered with ACWY-TT in terms of haemagglutination inhibition (HI) antibody titers, based on criteria defined by the CHMP.Citation14
Non-inferiority was prospectively defined as the upper limit of the 2-sided 95% CI on the GMT ratio (ACWY-TT/Coad group) adjusted for pre-vaccination rSBA titers being ≤ 2.0. Immunogenicity 1 mo post-vaccination with respect to influenza antigens (A/H1N1, A/H3N2, B) was pre-defined as acceptable if one of the following CHMP standard criteria for the evaluation of inactivated influenza vaccines was satisfied: the lower limit of the 2-sided exact 95% CI in the seroconversion rate for anti-HI was > 40%; the lower limit of the 2-sided standardized asymptotic 95% CI in the fold increase in anti-HI GMT was > 2.5; the lower limit of the 2-sided exact 95% CI in the proportion of subjects with anti-HI ≥ 1:40 was > 70%. The power of the study to meet both objectives was > 96.7%.
Immunogenicity assessment
Blood samples were collected from all subjects prior to and one month after vaccination. Pre- and post-vaccination sera were tested for rSBA for each meningococcal serogroup to measure functional antibody levels following ACWY-TT vaccination as previously described.Citation21 The assay works by killing the target meningococcal strain in the presence of complement and serial dilutions of vaccinated subject sera. Both rabbit and human complement have been used as the exogenous complement source; there is currently no international consensus on the standard source of human complement to be used.Citation22 The serum bactericidal titer is expressed as the reciprocal serum dilution yielding ≥ 50% killing as compared with the number of viable cells prior to incubation. The cut-off of the rSBA assay was a 1:8 dilution and was considered indicative of seroprotection.Citation15,Citation16 A conservative cut-off of 1:128 was also considered.
Pre and post-vaccination samples in the Coad group were also tested for HI antibody titers against influenza virus vaccine strains. HI is dependent on the ability of serial dilutions of vaccinated subject sera to inhibit the interaction between the virus and erythrocyte sialic acid. The HI titer is defined as the dilution factor of the highest dilution that completely inhibits hemagglutination. Seroconversion was defined as antibody titer ≥ 1:40 after vaccination in initially seronegative subjects (anti-HI < 1:10), and as a ≥ 4-fold increase in initially seropositive subjects. The seroconversion factor was defined as the geometric mean ratio {mean [log10(post-vaccination GMT/pre-vaccination GMT)]}.
Safety assessment
Local and general AEs were solicited for 4 d after vaccination. Other (unsolicited) non-serious AEs were recorded for 31 d after vaccination, and specific AEs (rash, new onset of chronic disease and AEs leading to emergency room visits) and SAEs were collected through 6 mo after vaccination via scripted phone call.
Statistical analysis
The analysis of immunogenicity was conducted on the ATP immunogenicity cohort that included all vaccinated subjects who complied with protocol-defined procedures. For the purposes of the analysis, immunogenicity and safety results from the 3 ACWY-TT lots were pooled.
The 95% CIs of the rSBA GMT ratios between vaccine groups were calculated using an ANCOVA model on the log10 transformation of the titers, using the pre-vaccination log10 transformation of the titers and age strata as covariates. Antibody titers below the cut-off of the assay were given an arbitrary value of half the cut-off for the purpose of GMT calculation. Vaccine groups were considered significantly different if the 95% CI for the GMT ratio between groups did not contain the value 1, or, if the asymptotic standardized 95% CI for the difference in threshold rates or vaccine response rates between groups did not contain the value 0. Due to the multiplicity of endpoints, statistically significant findings from the exploratory analyses should be interpreted with caution.
The analysis of safety was performed on the total vaccinated cohort that included all vaccinated subjects. The incidence and intensity of symptoms were calculated with exact 95% CI for each group.
Analyses were performed using SAS® software version 9.1 (SAS Institute Inc.) and Proc StatXact 7.0.
FLUARIX and MENCEVAX are trademarks of the GlaxoSmithKline group of companies.
| Abbreviations: | ||
| ACWY–TT | = | Tetravalent serogroups A, C, W-135 and Y vaccine with all serogroups conjugated to the tetanus toxoid carrier protein |
| (S)AE | = | (serious) adverse event |
| ATP cohort | = | according-to-protocol cohort |
| CI | = | confidence interval |
| CHMP | = | Committee on Human Medicinal Products |
| GMT | = | geometric mean antibody titer |
| GSK | = | GlaxoSmithKline Biologicals |
| HI | = | hemagglutinin inhibition |
| MenPS | = | meningococcal tetravalent polysaccharide vaccine |
| rSBA | = | meningococcal bactericidal titers using rabbit complement as exogenous complement source |
Acknowledgments
The authors thank the individuals who participated in the study, and all investigators involved in conducting the study. The authors also thank Sally Gatchalian, Sameh Anis, Aline Stukkens and Jane Nappa for their assistance in coordination of the study; Dr. Fotios Vikas (GSK) for assistance in preparation of study reports; Laurence Fissette (GSK) for performing the statistical analysis and Dr. Brigitte Cheuvart (GSK) for statistical input in the protocol and study set-up; Dr. Dominique Boutriau and Dr. Peter Vink for input into protocol development; Dr. Pascal Lestrate and Koen Maleux for conducting the laboratory assays; Dr. Joanne Wolter (on behalf of GSK) for preparation of the first draft of the manuscript and Virginie Durbecq and Juliette Gray (Xpe Pharma and Science) for coordination and editorial assistance.
Sources of Support
GlaxoSmithKline Biologicals was the funding source and was involved in all stages of the study conduct and analysis. GSK Biologicals also funded all costs associated with the development and the publishing of the present manuscript. The corresponding author had full access to the data.
Disclosure of Potential Conflicts of Interest
MRADLR, GD and ED have received consulting fees and honoraria from GSK within the past three years. NM declares no conflict of interest. VB, YB and JM are employees of GSK Biologicals. YB and JM report ownership of GSK Biologicals stocks and stock options.
References
- Pollard AJ. Global epidemiology of meningococcal disease and vaccine efficacy. Pediatr Infect Dis J 2004; 23:Suppl S274 - 9; PMID: 15597069
- Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine 2009; 27:Suppl 2 B51 - 63; http://dx.doi.org/10.1016/j.vaccine.2009.04.063; PMID: 19477562
- Cohn AC, MacNeil JR, Harrison LH, Hatcher C, Theodore J, Schmidt M, et al. Changes in Neisseria meningitidis disease epidemiology in the United States, 1998-2007: implications for prevention of meningococcal disease. Clin Infect Dis 2010; 50:184 - 91; http://dx.doi.org/10.1086/649209; PMID: 20001736
- Wilder-Smith A, Goh KT, Barkham T, Paton NI. Hajj-associated outbreak strain of Neisseria meningitidis serogroup W135: estimates of the attack rate in a defined population and the risk of invasive disease developing in carriers. Clin Infect Dis 2003; 36:679 - 83; http://dx.doi.org/10.1086/367858; PMID: 12627350
- Saudi Ministry of Health Requirements—Hajj. Cited 2012 Jan 9. Available from: http://www.hajinformation.com/main/p3001.htm
- Kenah E, Chao DL, Matrajt L, Halloran ME, Longini IM Jr. The global transmission and control of influenza. PLoS One 2011; 6:e19515; http://dx.doi.org/10.1371/journal.pone.0019515; PMID: 21573121
- Østergaard L, Lebacq E, Poolman J, Maechler G, Boutriau D. Immunogenicity, reactogenicity and persistence of meningococcal A, C, W-135 and Y-tetanus toxoid candidate conjugate (MenACWY-TT) vaccine formulations in adolescents aged 15-25 years. Vaccine 2009; 27:161 - 8; http://dx.doi.org/10.1016/j.vaccine.2008.08.075; PMID: 18834910
- Knuf M, Kieninger-Baum D, Habermehl P, Muttonen P, Maurer H, Vink P, et al. A dose-range study assessing immunogenicity and safety of one dose of a new candidate meningococcal serogroups A, C, W-135, Y tetanus toxoid conjugate (MenACWY-TT) vaccine administered in the second year of life and in young children. Vaccine 2010; 28:744 - 53; http://dx.doi.org/10.1016/j.vaccine.2009.10.064; PMID: 19887137
- Baxter R, Baine Y, Ensor K, Bianco V, Friedland LR, Miller JM. Immunogenicity and safety of an investigational quadrivalent meningococcal ACWY tetanus toxoid conjugate vaccine in healthy adolescents and young adults 10 to 25 years of age. Pediatr Infect Dis J 2011; 30:e41 - 8; http://dx.doi.org/10.1097/INF.0b013e3182054ab9; PMID: 21200360
- Vesikari T, Karvonen A, Bianco V, Van der Wielen M, Miller J. Tetravalent meningococcal serogroups A, C, W-135 and Y conjugate vaccine is well tolerated and immunogenic when co-administered with measles-mumps-rubella-varicella vaccine during the second year of life: An open, randomized controlled trial. Vaccine 2011; 29:4274 - 84; http://dx.doi.org/10.1016/j.vaccine.2011.03.043; PMID: 21443965
- Memish ZA, Dbaibo G, Montellano M, Verghese VP, Jain H, Dubey AP, et al. Immunogenicity of a single dose of tetravalent meningococcal serogroups A, C, W-135, and Y conjugate vaccine administered to 2- to 10-year-olds is noninferior to a licensed-ACWY polysaccharide vaccine with an acceptable safety profile. Pediatr Infect Dis J 2011; 30:e56 - 62; http://dx.doi.org/10.1097/INF.0b013e31820e6e02; PMID: 21278617
- Knuf M, Pantazi-Chatzikonstantinou A, Pfletschinger U, Tichmann-Schumann I, Maurer H, Maurer L, et al. An investigational tetravalent meningococcal serogroups A, C, W-135 and Y-tetanus toxoid conjugate vaccine co-administered with Infanrix™ hexa is immunogenic, with an acceptable safety profile in 12-23-month-old children. Vaccine 2011; 29:4264 - 73; http://dx.doi.org/10.1016/j.vaccine.2011.03.009; PMID: 21420417
- Bermal N, Huang L-M, Dubey AP, Jain H, Bavdekar A, Lin T-Y, et al. Safety and immunogenicity of a tetravalent meningococcal serogroups A, C, W-135 and Y conjugate vaccine in adolescents and adults. Hum Vaccin 2011; 7:239 - 47; http://dx.doi.org/10.4161/hv.7.2.14068; PMID: 21343698
- Nauta JJP, de Bruijn IA. The European CHMP criteria for influenza vaccine immunogenicity cannot be improved by the use of confidence intervals. Vaccine 2006; 24:6643 - 4; http://dx.doi.org/10.1016/j.vaccine.2006.05.053; PMID: 16797109
- Andrews N, Borrow R, Miller E. Validation of serological correlate of protection for meningococcal C conjugate vaccine by using efficacy estimates from postlicensure surveillance in England. Clin Diagn Lab Immunol 2003; 10:780 - 6; PMID: 12965904
- Borrow R, Balmer P, Miller E. Meningococcal surrogates of protection--serum bactericidal antibody activity. Vaccine 2005; 23:2222 - 7; http://dx.doi.org/10.1016/j.vaccine.2005.01.051; PMID: 15755600
- Campbell H, Andrews N, Borrow R, Trotter C, Miller E. Updated postlicensure surveillance of the meningococcal C conjugate vaccine in England and Wales: effectiveness, validation of serological correlates of protection, and modeling predictions of the duration of herd immunity. Clin Vaccine Immunol 2010; 17:840 - 7; http://dx.doi.org/10.1128/CVI.00529-09; PMID: 20219881
- Borrow R, Andrews N, Goldblatt D, Miller E. Serological basis for use of meningococcal serogroup C conjugate vaccines in the United Kingdom: reevaluation of correlates of protection. Infect Immun 2001; 69:1568 - 73; http://dx.doi.org/10.1128/IAI.69.3.1568-1573.2001; PMID: 11179328
- Weston WM, Friedland LR, Wu X, Howe B. Immunogenicity and reactogenicity of co-administered tetanus-diphtheria-acellular pertussis (Tdap) and tetravalent meningococcal conjugate (MCV4) vaccines compared to their separate administration. Vaccine 2011; 29:1017 - 22; http://dx.doi.org/10.1016/j.vaccine.2010.11.057; PMID: 21134450
- Chioato A, Noseda E, Felix SD, Stevens M, Del Giudice G, Fitoussi S, et al. Influenza and meningococcal vaccinations are effective in healthy subjects treated with the interleukin-1 beta-blocking antibody canakinumab: results of an open-label, parallel group, randomized, single-center study. Clin Vaccine Immunol 2010; 17:1952 - 7; http://dx.doi.org/10.1128/CVI.00175-10; PMID: 20962212
- Maslanka SE, Gheesling LL, Libutti DE, Donaldson KB, Harakeh HS, Dykes JK, et al, The Multilaboratory Study Group. Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. Clin Diagn Lab Immunol 1997; 4:156 - 67; PMID: 9067649
- Gill CJ, Ram S, Welsch JA, Detora L, Anemona A. Correlation between serum bactericidal activity against Neisseria meningitidis serogroups A, C, W-135 and Y measured using human versus rabbit serum as the complement source. Vaccine 2011; 30:29 - 34; http://dx.doi.org/10.1016/j.vaccine.2011.10.068; PMID: 22075087