Abstract
The present extension study, conducted in children originally vaccinated at 12–14 mo or 3–5 y of age, assessed antibody persistence and immune memory induced by an investigational tetravalent meningococcal serogroups A, C, W-135 and Y tetanus toxoid conjugate vaccine (MenACWY-TT). In the original study, participants were randomized to receive one dose of MenACWY-TT or licensed age-appropriate meningococcal control vaccines. Fifteen months post-vaccination, all participants underwent serum sampling to evaluate antibody persistence and participants previously vaccinated as toddlers received a polysaccharide challenge to assess immune memory development.
Exploratory comparisons showed that (1) All children and ≥ 92.3% of the toddlers maintained serum bactericidal (rSBA) titers ≥ 1:8 at 15 mo post MenACWY-TT vaccination; statistically significantly higher rSBA geometric mean titers (GMTs) were observed compared with control vaccines. (2) At one month after polysaccharide challenge, all toddlers primed with MenACWY-TT or with the monovalent serogroup C conjugate vaccine had rSBA titers ≥ 1:8 and ≥ 1:128 for serogroup C and similar rSBA-GMTs; rSBA-GMTs for serogroups A, W-135 and Y were statistically significantly higher in toddlers primed with MenACWY-TT compared with the control vaccine. Thus, a single dose of MenACWY-TT induced persisting antibodies in toddlers and children and immune memory in toddlers.
This study has been registered at www.clinicaltrials.gov NCT00126984.
Infections caused by Neisseria meningitidis can be devastating, with case fatality rates of 10–15% and up to 20% of the survivors developing long-term sequelae.Citation1,Citation2 Meningococci are classified into 13 serogroups on the basis of the capsular polysaccharides; of these, six cause the majority of disease: MenA, MenB, MenC, MenW-135, MenY, and more recently, MenX.Citation1 Vaccination is the best strategy to prevent meningococcal diseases and meningococcal plain polysaccharide vaccines have been available for this purpose for many years. However, these vaccines may induce hyporesponsiveness, at least for some serogroups, do not elicit long-term protection or immune memory, and are poorly immunogenic in young children, who are at highest risk.Citation2-Citation4 Immunogenicity of the meningococcal vaccines can be increased or enabled by conjugation of the polysaccharides to carrier proteins, as first demonstrated by monovalent MenC conjugate vaccines.Citation5 Currently, two tetravalent meningococcal conjugate vaccines offering protection against serogroups A, C, W-135, and Y, using diphtheria toxoid or a non-toxic cross-reacting mutant of diphtheria toxoid (CRM197) as carrier proteins, have been licensed in various countries.
In addition, an investigational tetravalent meningococcal serogroups A, C, W-135 and Y conjugate vaccine, using tetanus toxoid (TT) as carrier protein (MenACWY-TT) has been shown to be immunogenic and to have a clinically acceptable safety profile in toddlers, children, adolescents, and young adults.Citation6-Citation12 The present study evaluated the persistence of the immune response in toddlers and children 15 mo after priming with a single dose of MenACWY-TT. In addition, participants who were vaccinated as toddlers received a reduced dose of meningococcal polysaccharide vaccine to mimic exposure to meningococcal bacteria and to assess whether immune memory had been induced.
This phase II, open, controlled study conducted in 30 centers in Germany and five centers in Austria between November 2006 and February 2008 was an extension of the previously reported study evaluating four different formulations of MenACWY-TT.Citation6 The extension study compared the antibody persistence and the immune memory induced by the MenACWY-TT formulation containing 5 µg of each capsular polysaccharide conjugated to TT (~44 µg) to that of licensed age-appropriate control vaccines. The randomization ratio was 1:1 for these two groups in the primary study.Citation6 The control vaccine was a monovalent MenC conjugate vaccine using mutant diphtheria toxoid (CRM197) as carrier protein (Meningitec™, Pfizer, hereafter referred as MenC-CRM197) for the toddlers aged 12–14 mo at the time of vaccination or a tetravalent meningococcal serogroups A, C, W-135 and Y plain polysaccharide vaccine (Mencevax™ ACWY, GlaxoSmithKline Biologicals, hereafter referred as MenPS) for the children aged 3–5 y at the time of vaccination.
Participants from the primary study were not included in the extension study if they had received a meningococcal vaccine not planned in the protocol, immunoglobulin, blood products, any investigational product, or immune-modifying drug during the study period. Written informed consent was obtained from each parent/guardian prior to study entry. The study was conducted in accordance with Good Clinical Practice and the Declaration of Helsinki and the protocol and informed consent were approved by national or regional ethics committees. This study has been registered at www.clinicaltrials.gov NCT00126984.
Blood samples were collected from all the participants at 15 mo post-primary vaccination. Participants who were vaccinated as toddlers in the primary study received a polysaccharide challenge (1/5 dose of MenPS, or a 10 µg dose of the capsular polysaccharides for meningococcal serogroups A, C, W-135 and Y) and an additional blood sample was collected from these participants one month later. The choice of 1/5th dose of MenPS was selected based on the design of a previous study, in which 1/5 dose of a bivalent polysaccharide vaccine against meningococcal serogroups A and C (Mengivac A + C®; Sanofi Pasteur Mérieux) containing 10µg of each meningococcal serogroup was administered in children to demonstrate that monovalent meningococcal serogroup C conjugate vaccines induced immune memory.Citation13 Immune memory was not assessed in the cohort vaccinated at 3–5 y of age because of concerns of inducing hyporesponsiveness after a second vaccination with meningococcal polysaccharide vaccines in the control group.Citation4
Immunogenicity analyses were conducted on the according to protocol (ATP) cohort for persistence at 15 mo post-primary vaccination and on the ATP cohort for immune memory at one month after polysaccharide challenge administration. Functional antibody responses against the four serogroups were assessed by serum bactericidal antibody assays using baby rabbit serum as complement source (rSBA).Citation14 The cut-off of the assay was a rSBA titer ≥ 1:8, which has been shown to correlate with protection for serogroup C, and evaluation of this threshold was extended to the other serogroups.Citation15,Citation16 The percentages of participants with rSBA titers ≥ 1:128, which is the more conservative threshold to define seroprotection, were also evaluated.Citation17,Citation18 An exploratory statistical analysis was performed to evaluate the potential differences between the MenACWY-TT and the control groups. Exploratory analyses were supplementary analyses that were not prospectively stated as primary objectives of the study, but were nonetheless pre-defined in the study protocol and statistical analysis plan. Two groups were considered statistically significantly different if the asymptotic standardised 95% confidence interval (CI) for (1) the difference in percentage of participants with titers above the proposed cut-offs between the two groups did not contain the value ‘0’ or (2) the GMT ratio adjusted for pre-vaccination titers between the two groups did not contain the value ‘1’. Statistically significant findings must be interpreted with caution given their exploratory nature. As the study was not powered to evaluate the exploratory objectives and there was no adjustment for multiplicity of endpoints, the relevance of potential statistically significant differences is not clear.
Local and general solicited adverse events (AEs) were recorded for seven days and unsolicited AEs for 30 d following administration of the polysaccharide challenge by the parent/ guardian on a standardised diary card. Occurrence of any serious adverse event (SAE) was recorded throughout the entire study. The statistical analyses were performed using the SAS® software version 9.1 (SAS Institute Inc.) and StatXact 7.0 (Cytel).
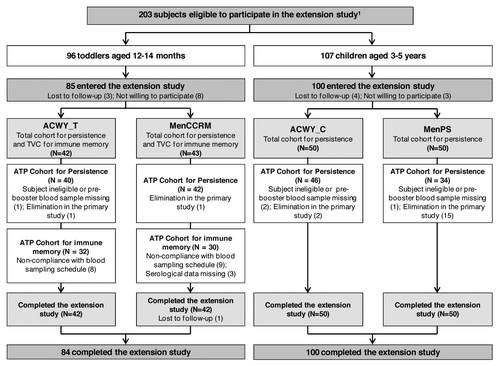
At 15 mo post-primary vaccination, 185 of the 203 eligible participants were enrolled in the extension study (). Demographic characteristics of both groups were comparable within each age stratum and consistent with the primary study (data not shown).Citation6
Figure 1. Participant flow. ACWY_T, toddlers vaccinated with MenACWY-TT at 12–14 mo of age; MenCCRM, toddlers vaccinated with MenC-CRM197 at 12–14 mo of age; ACWY_C, children vaccinated with MenACWY-TT at 3–5 y of age; MenPS, children vaccinated with MenPS at 3–5 y of age; ATP, according to protocol; TVC, total vaccinated cohort; N, number of participants. 1Several formulations were evaluated in the primary study, but participants who received the selected formulation for further development or control vaccine were eligible to participate in the persistence study. Participants who withdrew from the primary phase were described in the previous publication.Citation6
All the children vaccinated with MenACWY-TT and 59.4% to 93.8% of those who received MenPS at 3–5 y of age in the primary study had persisting rSBA titers ≥ 1:8 for the individual serogroups at 15 mo post-primary vaccination (). Between one month and 15 mo post-vaccination, the rSBA GMTs for MenA, MenC, MenW-135 and MenY decreased 4.7-, 7.8-, 6.1-, and 5.2-fold, respectively, in the children vaccinated with MenACWY-TT and 7.0-, 15.9-, 7.5- and 10.0-fold, respectively, in the children vaccinated with MenPS (). Exploratory analyses showed statistically significantly higher rSBA GMTs for all serogroups and percentages of children with rSBA titers ≥ 1:8 and ≥ 1:128 for MenA, MenC, and MenY in the MenACWY-TT recipients compared with the MenPS recipients (Table S2). These results are in line with those of the primary study, which showed that rSBA GMTs against each serogroup were statistically higher at one month post-MenACWY-TT vaccination compared with MenPS.Citation6 These findings were expected as it has previously been observed that conjugate vaccines induce a more robust immune response than polysaccharide vaccines with a slower rate of decay of GMTs.Citation2,Citation3,Citation19
Table 1. Percentage of participants with rSBA titers above defined thresholds at pre-vaccination, at 1 mo and 15 mo after primary vaccination (ATP cohort for persistence) and at 1 mo after the polysaccharide challenge (ATP cohort for immune memory)
Figure 2. Geometric mean titers for rSBA at pre-vaccination, at 1 moCitation1 and 15 moCitation1 after primary vaccination (ATP cohort for persistence) and at 1 mo after the polysaccharide challenge (ATP cohort for immune memory). Notes: ATP, according to protocol; ACWY_T, toddlers primed with MenACWY-TT at 12–14 mo of age; MenCCRM, toddlers primed with MenC-CRM197 at 12–14 mo of age; ACWY_C, children primed with MenACWY-TT at 3–5 y of age; MenPS, children primed with MenPS at 3–5 y of age; Errors bars, exact 95% confidence interval; Month 0, pre-primary vaccination; Month 1, 1 mo post-primary vaccination; Month 15, 15 mo post-primary vaccination; Month 16, one month post-polysaccharide challenge. 1These numbers are not the same as those presented in the previous publication at the same timepoints because the analyses for the present study were conducted on the ATP cohorts for persistence and immune memory (Month 15) and in the previous publication the analyses were performed on the ATP cohort for immunogenicity (Month 1). *Statistically significantly higher values compared with the control group (exploratory analysis)
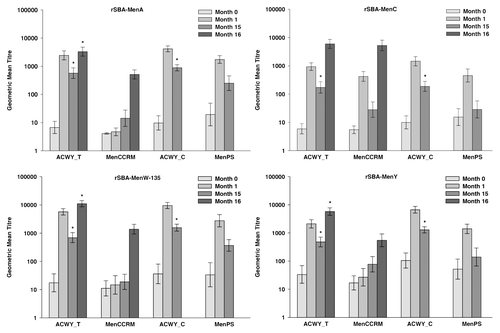
The percentages of toddlers vaccinated at 12–14 mo of age who had persisting rSBA-MenC titers ≥ 1:8 at 15 mo post-primary vaccination were 92.3% and 60.0% in the MenACWY-TT and MenC-CRM197 recipients, respectively (). The decrease in rSBA-MenC GMTs between 1 and 15 mo post-vaccination was less for in the toddlers who received the MenACWY-TT vaccine than with the MenC-CRM197 vaccine (5.4-fold vs. 15-fold) (). Exploratory analyses for the common antigen between the two groups showed statistically significantly higher proportions of toddlers with rSBA-MenC titers ≥ 1:8 or ≥ 1:128 and rSBA-MenC GMTs at 15 mo after vaccination with MenACWY-TT compared with MenC-CRM197 (Table S2). This observation is consistent with results of a previous study in which a monovalent vaccine using TT as carrier (MenC-TT) was compared with two monovalent vaccines using CRM197 as carrier protein.Citation13-Citation20 In the toddlers vaccinated with MenACWY-TT, the rSBA GMTs for MenA, MenW-135 and MenY decreased 4.3-, 8.2- and 4.4-fold, respectively, between one month and 15 mo post-vaccination (). At 15 mo post-primary vaccination, rSBA GMTs for MenA, MenW-135 and MenY induced by MenACWY-TT were statistically significantly higher than antibodies derived from natural immunity, as measured in toddlers primed with MenC-CRM197 (Table S2).
All toddlers who were primed with MenACWY-TT or MenC-CRM197 in the primary study, had rSBA-MenC titers ≥ 1:8 and ≥ 1:128 at one month post-challenge in the extension study (). Similar rSBA-MenC GMTs were observed in both groups after the administration of the polysaccharide challenge, which induced a 34.3- and 153.1-fold increase in rSBA-MenC GMTs in the MenACWY-TT vaccine and the MenC-CRM197 control vaccine, respectively (; Table S2). This difference in fold-increases for rSBA-MenC GMTs was driven by the lower persistence in the group originally vaccinated with MenC-CRM197. Thus, both the MenACWY-TT vaccine and the MenC-CRM197 control vaccine were observed to prime for immunological memory. These results confirm those of a previous study, in which three MenC conjugate vaccines induced immunologic memory after a single dose in toddlers.Citation13
All the toddlers vaccinated with MenACWY-TT in the primary study had rSBA titers ≥ 1:128 for MenA, MenW-135 and MenY at one month after administration of the polysaccharide challenge and this percentage ranged between 90.0% and 96.7% in the toddlers who received MenC-CRM197 in the primary study (). In the toddlers who were primed with MenACWY-TT, the polysaccharide challenge induced a 6.1-, 17.2- and 13.0-fold increase in rSBA GMTs for MenA, MenW-135, and MenY, respectively (). Exploratory analyses showed that post-polysaccharide challenge GMTs for MenA, MenW-135 and MenY were statistically significantly higher in the toddlers primed with MenACWY-TT compared with those who received MenC-CRM197 in the primary study and were unprimed for these serogroups (Table S2), suggesting the induction of immune memory in the recipients of MenACWY-TT for these serogroups.
The incidence of local reactions after administration of the polysaccharide challenge was similar in toddlers primed with MenACWY-TT or MenC-CRM197 (data not shown). One grade 3 solicited local symptom and three grade 3 solicited general symptoms were reported in toddlers primed with MenC-CRM197. Four participants experienced one or more SAEs throughout the study, but none were considered related to vaccination.
A potential limitation of this study was its open design, which would not have influenced the immunogenicity results because the laboratory personnel was blinded during immunological assays but may have induced a bias in the safety profile of the polysaccharide challenge. Theoretically, functional antibody levels could have been biased if participants were exposed to N. meningitidis during the 15-mo post-vaccination follow-up. Although there is limited epidemiological information on the seroprevalence of antibodies against meningococcal serogroups A, C, W-135, and Y in Europe, some results are available for the United Kingdom (UK). Before the introduction of monovalent meningococcal serogroup C conjugate vaccines, it was observed that the majority of individuals lacked functional antibody titers against this serogroup and the lowest titers were found in young children.Citation21 After the introduction of the meningococcal serogroup C conjugate vaccines, the prevalence of protective antibodies against meningococcal serogroup C increased from 10–15% between 1996 and 1999 to 32% between 2000 and 2004 among children between one and five years of age.Citation21,Citation22 A more recent study conducted in 2009 showed that natural immunity against meningococcal serogroups W-135 and Y was detected in 7% and 13% of children younger than five years of age, respectively.Citation23
Some results are also available for Turkey, where a previous study showed that, regardless of age, 60.5%, 27.2%, 12.3% and 19.2% of inhabitants had serogroup-specific antibody concentrations ≥ 2 µg/mL for serogroups A, C, W-135 and Y, respectively,Citation24 but these data may not correlate with functional bactericidal activity. Christensen, et al., performed a meta-analysis of meningococcal carriage, and concluded that rates of meningococcal carriage are lowest in infants (4.5%) and increase to 7.7% in children 10 y of age.Citation25 Given the limited evidence for extensive carriage and seroprevalence of functional anti-meningococcal antibodies in young children, it is likely that boosting from circulating meningococci had a limited impact on the antibody persistence observed in this study.
In conclusion, this extension study showed the persistence of the antibody responses (rSBA ≥ 1:8) induced by MenACWY-TT for 15 mo after vaccination in > 92% of the toddlers aged 12–14 mo and in all the children aged 3–5 y. Moreover, this study showed that a single dose of MenACWY-TT was able to induce immune memory for the four serogroups included in the vaccine. This observation confirms and extends the results of a previous study showing that a single dose of three different monovalent meningococcal serogroup C vaccines in toddlers induced the development of immune memory.Citation13
Meningitec is a trademark of Pfizer, formerly Wyeth; Mencevax is a trademark of the GlaxoSmithKline Biologicals group of companies.
| Abbreviations: | ||
| ATP | = | according to protocol |
| CI | = | confidence interval |
| GMT | = | geometric mean antibody titres |
| MenACWY-TT | = | meningococcal tetravalent serogroups A,C,W-135 and Y vaccine with all serogroups conjugated to the tetanus toxoid (TT) carrier protein |
| MenC-CRM197 | = | meningococcal monovalent serogroup C vaccine conjugated to the mutant diphtheria toxoid (CRM197) carrier protein |
| MenC-TT | = | meningococcal monovalent serogroup C vaccine conjugated to TT as carrier protein |
| MenPS | = | meningococcal tetravalent polysaccharide vaccine |
| rSBA | = | serum bactericidal antibody assays using baby rabbit serum as exogenous complement source |
| (S)AE | = | (serious) adverse event |
| SD | = | standard deviation |
Additional material
Download Zip (113.4 KB)Acknowledgments
The authors are indebted to the study participants and their parents, clinicians, nurses, and laboratory technicians at the study site as well as to the sponsor’s project staff and to the study managers for their support and contributions throughout the study. We are grateful to Doctors Angermayr, Busse, Doering, Grunert, Hoernlein, Kieninger-Baum, Kimmig, Knecht, Maurer, Taube, Van Stiphout, Wagner, Wittermann and all teams for their contribution to this study, as well as Werner Kroeniger, Margit Spacek and Sophie Ledant (GSK Biologicals) for their supports. The authors would also like to thank Koen Maleux and Pascal Lestrate, who coordinated the laboratory testing for this study, Laurence Fissette (GSK Biologicals) for performing the statistical analysis and Emmanuel Aris (GSK Biologicals) for statistical advice. Finally we thank Claire Verbelen (XPE Pharma and Science) who provided medical writing services and Stephanie Harbers (GSK Biologicals), Virginie Durbecq and Juliette Gray (XPE Pharma and Science) for editorial assistance and manuscript coordination.
Disclosure of Potential Conflicts of Interest
MK received honoraria or consulting fees as well as support for meetings, travel or accommodation expenses from GSK in the past 3 y. YB, VB, DB and JM are employees of GSK Biologicals. YB, DB and JM declare stock ownership in GSK. DB is also inventor of certain GSK Biologicals patents.
Funding
GSK Biologicals was the funding source and was involved in all stages of the study conduct and analysis. GSK Biologicals also took responsibility for all costs associated with the development and publishing of the present manuscript.
Supplementary Material
Supplementary materials can be found at: www.landesbioscience.com/journals/vaccines/article/20229
References
- Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine 2009; 27:Suppl 2 B51 - 63; http://dx.doi.org/10.1016/j.vaccine.2009.04.063; PMID: 19477562
- Girard MP, Preziosi MP, Aguado MT, Kieny MP. A review of vaccine research and development: meningococcal disease. Vaccine 2006; 24:4692 - 700; http://dx.doi.org/10.1016/j.vaccine.2006.03.034; PMID: 16621189
- Harrison LH. Prospects for vaccine prevention of meningococcal infection. Clin Microbiol Rev 2006; 19:142 - 64; PMID: 16418528
- Bröker M, Veitch K. Quadrivalent meningococcal vaccines: hyporesponsiveness as an important consideration when choosing between the use of conjugate vaccine or polysaccharide vaccine. Travel Med Infect Dis 2010; 8:47 - 50; http://dx.doi.org/10.1016/j.tmaid.2009.12.001; PMID: 20188306
- Borrow R, Miller E. Long-term protection in children with meningococcal C conjugate vaccination: lessons learned. Expert Rev Vaccines 2006; 5:851 - 7; http://dx.doi.org/10.1586/14760584.5.6.851; PMID: 17184222
- Knuf M, Kieninger-Baum D, Habermehl P, Muttonen P, Maurer H, Vink P, et al. A dose-range study assessing immunogenicity and safety of one dose of a new candidate meningococcal serogroups A, C, W-135, Y tetanus toxoid conjugate (MenACWY-TT) vaccine administered in the second year of life and in young children. Vaccine 2010; 28:744 - 53; http://dx.doi.org/10.1016/j.vaccine.2009.10.064; PMID: 19887137
- Östergaard L, Lebacq E, Poolman J, Maechler G, Boutriau D. Immunogenicity, reactogenicity and persistence of meningococcal A, C, W-135 and Y-tetanus toxoid candidate conjugate (MenACWY-TT) vaccine formulations in adolescents aged 15-25 years. Vaccine 2009; 27:161 - 8; http://dx.doi.org/10.1016/j.vaccine.2008.08.075; PMID: 18834910
- Baxter R, Baine Y, Ensor K, Bianco V, Friedland LR, Miller JM. Immunogenicity and safety of an investigational quadrivalent meningococcal ACWY tetanus toxoid conjugate vaccine in healthy adolescents and young adults 10 to 25 years of age. Pediatr Infect Dis J 2011; 30:e41 - 8; http://dx.doi.org/10.1097/INF.0b013e3182054ab9; PMID: 21200360
- Bermal N, Huang L-M, Dubey AP, Jain H, Bavdekar A, Lin T-Y, et al. Safety and immunogenicity of a tetravalent meningococcal serogroups A, C, W-135 and Y conjugate vaccine in adolescents and adults. Hum Vaccin 2011; 7:239 - 47; http://dx.doi.org/10.4161/hv.7.2.14068; PMID: 21343698
- Knuf M, Pantazi-Chatzikonstantinou A, Pfletschinger U, Tichmann-Schumann I, Maurer H, Maurer L, et al. An investigational tetravalent meningococcal serogroups A, C, W-135 and Y-tetanus toxoid conjugate vaccine co-administered with Infanrix™ hexa is immunogenic, with an acceptable safety profile in 12-23-month-old children. Vaccine 2011; 29:4264 - 73; http://dx.doi.org/10.1016/j.vaccine.2011.03.009; PMID: 21420417
- Memish ZA, Dbaibo G, Montellano M, Verghese VP, Jain H, Dubey AP, et al. Immunogenicity of a single dose of tetravalent meningococcal serogroups A, C, W-135, and Y conjugate vaccine administered to 2- to 10-year-olds is noninferior to a licensed-ACWY polysaccharide vaccine with an acceptable safety profile. Pediatr Infect Dis J 2011; 30:e56 - 62; http://dx.doi.org/10.1097/INF.0b013e31820e6e02; PMID: 21278617
- Vesikari T, Karvonen A, Bianco V, Van der Wielen M, Miller J. Tetravalent meningococcal serogroups A, C, W-135 and Y conjugate vaccine is well tolerated and immunogenic when co-administered with measles-mumps-rubella-varicella vaccine during the second year of life: An open, randomized controlled trial. Vaccine 2011; 29:4274 - 84; http://dx.doi.org/10.1016/j.vaccine.2011.03.043; PMID: 21443965
- Richmond P, Borrow R, Goldblatt D, Findlow J, Martin S, Morris R, et al. Ability of 3 different meningococcal C conjugate vaccines to induce immunologic memory after a single dose in UK toddlers. J Infect Dis 2001; 183:160 - 3; http://dx.doi.org/10.1086/317646; PMID: 11078484
- Maslanka SE, Gheesling LL, Libutti DE, Donaldson KB, Harakeh HS, Dykes JK, et al, The Multilaboratory Study Group. Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. Clin Diagn Lab Immunol 1997; 4:156 - 67; PMID: 9067649
- Andrews N, Borrow R, Miller E. Validation of serological correlate of protection for meningococcal C conjugate vaccine by using efficacy estimates from postlicensure surveillance in England. Clin Diagn Lab Immunol 2003; 10:780 - 6; PMID: 12965904
- Borrow R, Andrews N, Goldblatt D, Miller E. Serological basis for use of meningococcal serogroup C conjugate vaccines in the United Kingdom: reevaluation of correlates of protection. Infect Immun 2001; 69:1568 - 73; http://dx.doi.org/10.1128/IAI.69.3.1568-1573.2001; PMID: 11179328
- Borrow R, Balmer P, Miller E. Meningococcal surrogates of protection--serum bactericidal antibody activity. Vaccine 2005; 23:2222 - 7; http://dx.doi.org/10.1016/j.vaccine.2005.01.051; PMID: 15755600
- Campbell H, Andrews N, Borrow R, Trotter C, Miller E. Updated postlicensure surveillance of the meningococcal C conjugate vaccine in England and Wales: effectiveness, validation of serological correlates of protection, and modeling predictions of the duration of herd immunity. Clin Vaccine Immunol 2010; 17:840 - 7; http://dx.doi.org/10.1128/CVI.00529-09; PMID: 20219881
- Gill CJ, Baxter R, Anemona A, Ciavarro G, Dull P. Persistence of immune responses after a single dose of Novartis meningococcal serogroup A, C, W-135 and Y CRM-197 conjugate vaccine (Menveo®) or Menactra® among healthy adolescents. Hum Vaccin 2010; 6:881 - 7; http://dx.doi.org/10.4161/hv.6.11.12849; PMID: 21339701
- Borrow R, Andrews N, Findlow H, Waight P, Southern J, Crowley-Luke A, et al. Kinetics of antibody persistence following administration of a combination meningococcal serogroup C and haemophilus influenzae type b conjugate vaccine in healthy infants in the United Kingdom primed with a monovalent meningococcal serogroup C vaccine. Clin Vaccine Immunol 2010; 17:154 - 9; http://dx.doi.org/10.1128/CVI.00384-09; PMID: 19906895
- Trotter C, Borrow R, Andrews N, Miller E. Seroprevalence of meningococcal serogroup C bactericidal antibody in England and Wales in the pre-vaccination era. Vaccine 2003; 21:1094 - 8; http://dx.doi.org/10.1016/S0264-410X(02)00630-8; PMID: 12559785
- Trotter CL, Borrow R, Findlow J, Holland A, Frankland S, Andrews NJ, et al. Seroprevalence of antibodies against serogroup C meningococci in England in the postvaccination era. Clin Vaccine Immunol 2008; 15:1694 - 8; http://dx.doi.org/10.1128/CVI.00279-08; PMID: 18827191
- Trotter CL, Findlow H, Borrow R. Seroprevalence of serum bactericidal antibodies against group W135 and Y meningococci in England in 2009. Clin Vaccine Immunol 2012; 19:219 - 22; http://dx.doi.org/10.1128/CVI.05515-11; PMID: 22190393
- Ceyhan M, Yildirim I, Balmer P, Riley C, Laher G, Andrews N, et al. Age-specific seroprevalence of serogroup C meningococcal serum bactericidal antibody activity and serogroup A, C, W135 and Y-specific IgG concentrations in the Turkish population during 2005. Vaccine 2007; 25:7233 - 7; http://dx.doi.org/10.1016/j.vaccine.2007.07.019; PMID: 17707957
- Christensen H, May M, Bowen L, Hickman M, Trotter CL. Meningococcal carriage by age: a systematic review and meta-analysis. Lancet Infect Dis 2010; 10:853 - 61; http://dx.doi.org/10.1016/S1473-3099(10)70251-6; PMID: 21075057