Abstract
Neisseria meningitidis infection results in life-threatening illnesses, including bacteremia, sepsis and meningitis. Early diagnosis and treatment are a challenge due to rapid disease progression, resulting in high mortality and morbidity in survivors. Disease can occur in healthy individuals, however, risk of infection is higher in patients with certain risk factors. N meningitidis carriage and case-fatality rates are high in adolescents and young adults. The absolute incidence of meningococcal disease has decreased partially due to increasing meningococcal vaccination rates. Maintaining protective levels of circulating antibodies by vaccination is necessary for clinical protection against disease. The Centers for Disease Control and Prevention Advisory Committee on Immunization Practices guidelines recommend vaccination for all individuals aged 11 through 12 years, followed by a booster dose at age 16 years for maintenance of protective antibody levels throughout the high-risk years. Despite these guidelines, many adolescents remain unvaccinated and susceptible to infection and disease.
Introduction
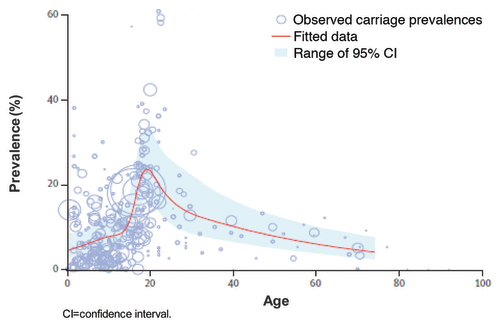
Meningococcal infection, caused by the gram-negative aerobic bacteria N meningitidis, can result in severe disease, including meningitis and sepsis, and death. The organism is spread through respiratory droplets and secretions. Transmission often results in nasopharyngeal colonization, but fortunately only a small percentage of colonized individuals develop invasive disease.Citation1 Colonized individuals serve as a reservoir for transmission, and eradication or prevention of the carrier state may be useful in reducing disease burden in a community. Prevalence of asymptomatic carriage in the general population varies depending on the region and/or group studied.Citation2 Results from a meta-analysis found that asymptomatic meningococcal carriage rates were highest in adolescents and young adults, occurring in approximately 24% of people in this group. In infants, this carriage rate was much lower, at an estimated 4.5%, and in adults aged 50 y, carriage rates were estimated to be around 8% ().Citation3 Carriage prevalence does not reliably predict disease risk. Virulence of meningococcal strain, host health, and immune response, as well as environmental factors, all influence susceptibility to disease.Citation1
Figure 1.Neisseria meningitidis carriage rates by age group.Citation3 Reprinted from Lancet Infect Dis. 10(12), Christensen H, May M, Bowen L, Hickman M, Trotter C. Meningococcal carriage by age: a systemic review and meta-analysis. 853-861, Copyright (2010), with permission from Elsevier.
Invasive meningococcal disease occurs when bacteria cross the mucosal surfaces (possibly due to irritated or damaged mucosal epithelium) and enter the bloodstream. Exposure to tobacco smoke or other irritants as well as concomitant viral respiratory infections or other similar processes may damage the mucosal epithelium and increase risk for invasive meningococcal disease.Citation4
Adolescents appear to be at high risk for N meningitidis colonization as well as invasive infection and death due to infection. This article discusses the importance of circulating antibodies to protect against invasive meningococcal disease.
Disease burden and clinical features
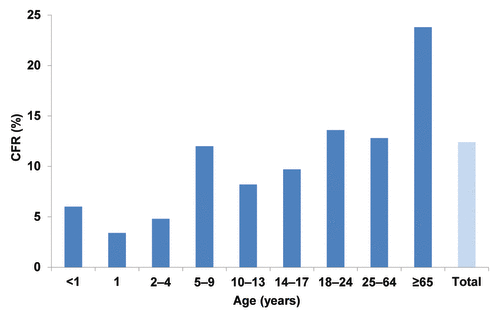
Meningococcal disease is characterized by severe and rapidly progressing symptoms, often resulting in death or serious permanent sequelae, including limb amputations.Citation5 Case-fatality rates for meningococcal disease can be as high as 14%, even with prompt and appropriate medical treatment.Citation6,Citation7 Because case-fatality rates increase with age, adolescents and young adults have higher rates than infants and toddlers, and the highest case-fatality rates occur in adults aged 65 y ().Citation7 In the United States between 1998 and 2007, 337 of 1,525 total meningococcal cases and 41 of 189 meningococcal deaths were among adolescents and young adults (aged 14-24 y), resulting in a case fatality rate of 12%.
Figure 2. US case-fatality rates associated with meningococcal disease by age group, 1998-2007.Citation7
Infection due to N meningitidis can cause a range of clinical presentations, most often as meningococcal meningitis and sepsis, but it can also present as pneumonia, conjunctivitis, arthritis, or pericarditis.Citation8-Citation10 Meningitis is an infection of the meninges surrounding the brain and spinal cord.Citation11 Meningococcal septicemia (meningococcemia) occurs when pathogenic organisms or their toxins accumulate in the bloodstream, and may be accompanied by disseminated intravascular coagulation, causing ischemic tissue damage and bleeding.Citation9,Citation11,Citation12 These conditions can be life-threatening and require immediate medical care, however, early diagnosis and treatment can be challenging, due to the nonspecific nature of the initial symptoms, which may appear similar to more common self-limiting viral infections.Citation13 Symptoms specific for meningococcal meningitis or septicemia, such as leg pain, abnormal skin color, photophobia, and stiff neck, may not appear until 5 to 18 h after the onset of early-stage symptoms.5Nonspecific symptoms during the early stage of infection (4-8 h) include irritability, headache, fever, and loss of appetite. Symptoms typically progress rapidly over the next several hours to include hemorrhagic rash, altered mental state, loss of consciousness, and meningismus. Meningismus appears to be more common and to occur earlier in older adolescents (aged 15-16 y) as compared with younger patients.Citation5 Death can result within 24 to 48 h after the initial onset of symptoms.Citation5 Timely recognition of early symptoms of meningococcal disease is critical to insure early diagnosis and treatment of this life-threatening infection.
Survivors of meningococcal disease are often left with substantial morbidity and permanent sequelae, including neurologic damage, hearing loss, renal failure, or limb amputation.Citation6,Citation9,Citation14 Long-term management of the disease and these sequelae result in substantial health care costs.Citation15
Risk factors
Adolescents and young adults often engage in many of the behaviors associated with increased risk for acquiring and transmitting meningococcal disease - including active or passive smoking, patronizing bars and nightclubs, drinking alcohol, intimate personal contact (eg, kissing), and residing in crowded living conditions such as dormitories and barracks.Citation2,Citation14,Citation16
The rate of meningococcal disease among US college freshmen living in dormitories during the 1998 to 1999 school year was 5.1 cases per 100,000 population, higher than that seen for any age group other than children aged < 2 y.Citation14,Citation17 As noted above, meningococcal carriage is highest in adolescents and young adults (24%).Citation3 Military recruits, many of whom are in the adolescent/young adult age group, are also at increased risk for meningococcal disease due to crowded living conditions and exposure to new meningococcal strains.Citation2 Carriage rates in military recruits have been reported from 36% to 71%.Citation16
Other risk factors for meningococcal disease that could apply to any age group include traveling to or residing in countries where N meningitidis is hyperendemic or epidemic, having a terminal complement deficiency, and having anatomic or functional asplenia.Citation2,Citation18-Citation21
Epidemiology
Considerable differences in disease incidence and serogroup distribution are observed across geographic regions, over time, and by individual age groups.Citation22 The reported incidence of meningococcal disease in the United States from 1999 through 2009 ranged from 0.32 to 0.92 per 100,000, with the highest rate of disease in 1999.Citation23 Approximately 1,000 to 2,700 cases of meningococcal disease per year were reported in the United States during that time.Citation23 In the European Union, 4,637 cases were reported in 2009: the reported incidence of meningococcal disease ranged from 0.3 cases per 100,000 population in Italy to 3.37 cases per 100,000 population in Ireland.Citation24
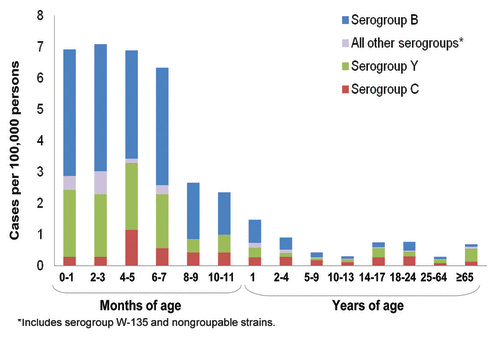
The burden of meningococcal disease is highest in infants, adolescents, and elderly adults. In the United States, the incidence of meningococcal disease was highest in children aged < 5 y, with additional peaks occurring in adolescents and young adults aged 14 through 24 y and those aged 65 y and older ().Citation7 Data from the European Union show that from 1999 to 2006 there were more than 14,000 cases of meningococcal disease in infants and children aged < 5 y and more than 5,000 cases in adolescents aged 15 to 19 y.Citation25
Figure 3. Incidence of meningococcal disease by age group in the United States, 1998-2007.Citation7
Although 13 serogroups of N meningitidis have been identified on the basis of the bacterial polysaccharide capsule, 5 serogroups, A, B, C, W-135, and Y, cause the vast majority of meningococcal disease globally.Citation22 In the United States, serogroups B, C, and Y currently cause most meningococcal disease, and serogroup A is uncommon ().Citation26 Serogroups B and C are also responsible for the majority of cases in Central and South America, as well as in Europe.Citation9 In Africa and Asia, serogroups A and C are predominant,Citation9 whereas serogroup X, which is uncommon in the rest of the world, has exhibited epidemic potential in sub-Saharan Africa.Citation22
Figure 4. Changing serogroup distribution in the United States, 1989-2009.Citation26,Citation27
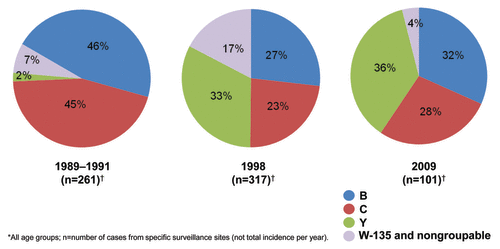
Changes in distribution of disease-causing serogroups within regions is unpredictable and can occur over relatively short periods of time.Citation7 Globally, serogroup W-135 was a rare cause of invasive disease until outbreaks during the Hajj pilgrimage in 2000. At that time, pilgrims returning to their home countries spread disease caused by serogroup W-135 to Africa, Europe, Asia, Saudi Arabia, and North America.Citation22,Citation28 Serogroup B was the most common serogroup in the United States from 2000 through 2005,Citation26 however, by 2009, the most common serogroup was serogroup Y ().Citation26,Citation27 Brazil has seen a dramatic increase in the proportion of disease caused by serogroup C; in 1993, serogroup C caused 31% of meningococcal cases, but by 2005 it was responsible for 71% of cases.Citation29,Citation30 The opposite occurred in the United Kingdom between 1999 and 2001, when the proportion of disease caused by serogroup C declined 87%- from 38% to 16%.Citation31
Serogroup distribution also varies among age groups. In the United States, serogroup B currently causes the majority of cases in infants aged < 1 y, while serogroups C, Y, and W-135 are responsible for 75% of cases in persons aged 11 y.Citation7,Citation14 Similarly, in South Africa in 2001 and 2002, serogroup B caused the majority of meningococcal cases in infants aged < 1 y, and serogroup A was predominant in adolescents and young adults aged 15 through 24 y.Citation32 Serogroup B causes the majority of meningococcal disease in Europe across all age groups, however, the proportion of disease due to serogroup B is lower in adolescents than in young children and infants.Citation24
Dynamic serogroup changes may occur as a result of multiple factors, including exposure to other serogroup strains during international travel (subsequently introducing that strain to other areas), capsular switching, and other genetic modifications.Citation33 The use of multivalent vaccines such as conjugate vaccines against serogroups A, C, W-135, and Y has the potential to reduce the incidence of infection across a broad range of serogroups.
Prevention of meningococcal disease
People who are in close contact with individuals who have meningococcal disease are at risk of exposure and colonization by the organism, and are at higher than average risk of developing invasive meningococcal disease. Identifying close contacts of patients diagnosed with meningococcal disease and offering prompt and effective antimicrobial chemoprophylaxis has been a successful strategy in reducing secondary cases of disease, although this approach is labor intensive and costly. In the United States, the agents recommended as prophylaxis for exposed individuals include rifampin, ceftriaxone, and ciprofloxacin.Citation14 Antibiotic resistance has been observed, raising the possibility of antibiotic failure. Three cases of ciprofloxacin-resistant serogroup B N meningitidis were identified in the United States between 2007 and 2008.Citation34 Resistance was due to both point mutations and horizontal gene transfer from a resistant strain of Neisseria lactamica (a commensal of the human upper respiratory tract that is rarely pathogenic).Citation34 It is unknown whether ciprofloxacin resistance will increase over time.
Immunization of at-risk populations provides another line of defense, and is encouraged and supported by the CDC. In a recent CDC report, 63% of adolescents aged 13 through 17 y received quadrivalent meningococcal conjugate or meningococcal-unknown type vaccine in 2010, up from 54% in 2009, 42% in 2008, and 32% in 2007.Citation35-Citation37 Increased vaccination rates in adolescents and young adults not only may protect the vaccine recipient against meningococcal disease, but may also reduce the carrier-state reservoir from which infection is transmitted to others.
Importance of circulating antibodies
After initial vaccination, circulating functional antibody levels naturally begin to decline; if further booster vaccination does not occur, there may be a waning of clinical protection over time.Citation33 Long-term protection after immunization depends on the maintenance of 3 mechanisms: memory response, the persistence of functional antibodies, and herd protection.Citation38
If immune memory is functioning properly, it will mount a defense against a recognized antigen with antibodies and T cells in 2 to 7 d.Citation39 In the interim, the host relies upon the innate immune response to combat the infection.
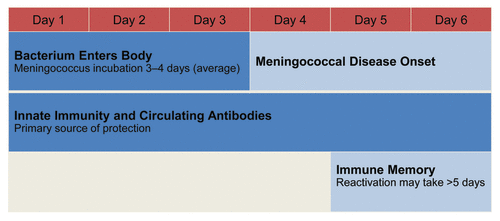
While some diseases, such as hepatitis B, do not require high circulating antibodies because of their slow pathogenesis (average incubation of 90 d),Citation40 innate immunity and high levels of protective circulating bactericidal antibodies are the primary immune defenses against rapidly progressing diseases such as those caused by N meningitidis.Citation39 When the immune system encounters invasive meningococcal infection, the stimulated response from memory B cells may not occur quickly enough because protection resulting from antibodies raised by B-cell immune memory takes up to 5 d, and the incubation period of meningococcal disease is 3 to 4 d ().Citation18,Citation39 Thus, maintaining the presence of circulating functional antibodies against N meningitidis may be necessary for clinical protection against disease.Citation41
Figure 5. Meningococcal incubation and immune memory reactivation.Citation18,Citation39,Citation42,Citation43
A correlate of protection against meningococcal disease was established through seminal work conducted by Goldschneider and colleagues in the 1960s.Citation44 The ability of a meningococcal vaccine to induce antimeningococcal bactericidal antibodies is measured by a SBA using human (h) or rabbit (r) serum as the complement source in the assay. In general, an hSBA titer of 1:4 or 1:8 and an rSBA titer of 1:128 are accepted as protective thresholds.Citation45-Citation47The standard measure of meningococcal vaccine immunogenicity for regulatory authorities in the United States is now hSBA; and both hSBA and rSBA have been used by regulatory bodies globally.
As host defenses driven by immunologic memory can lag behind the pace of infection, the best defense against rapidly progressing encapsulated bacteria, such as Hib and N meningitidis, is to maintain clinically protective levels of circulating antibodies either directly by vaccination or through a booster dose after initial priming doses.Citation42,Citation48 For example, despite the presence of demonstrated immune memory, MenC and Hib conjugate vaccine failures have occurred in infants in the United Kingdom and Canada.Citation49,Citation50 Between January 2000 and December 2003, there were 465 confirmed cases of serogroup C meningococcal disease in the United Kingdom. Of these, 53 cases occurred in subjects who had received MCC vaccine primary immunization (2, 3, and 4 mo) without a booster. Vaccine failure was defined as a laboratory confirmed case of meningococcal serogroup C disease occurring 10 d after the last vaccine dose in the primary series.Citation49 An anamnestic response was shown in subjects with vaccine failure, suggesting that serogroup C meningococcal disease occurred despite the MCC vaccine priming for immune memory.Citation49 The presence of circulating antibodies at the time of exposure may therefore be a more appropriate correlate of long-term protection for MCC vaccines than the ability to generate a memory response.Citation49
Similarly, in the United Kingdom and Ireland from 1992 to 2001, 93 children were diagnosed with invasive Hib disease despite having received 3 doses of Hib conjugate vaccine.Citation50 During this time an accelerated vaccination schedule was in place, but it lacked requirement of a booster in the second year of life.Citation50 Higher antibody responses to invasive Hib disease were also observed in the vaccinated children with meningitis, reflecting priming for immunologic memory by the Hib vaccine. Although a majority of children were protected from Hib disease by immunization, the relative roles of immunologic memory and other immune mechanisms in conferring disease protection remains unclear.Citation50
The researchers of these studies concluded that the vaccine failures may have resulted from rapid waning of immunity in early childhood after infant vaccinations and a lack or insufficiency in circulating functional antibodies that would have provided long-term protection against the disease. In light of these experiences, the United Kingdom began requiring booster doses of Hib and meningococcal vaccines to maintain circulating antibodies at protective levels. Similarly, Canada,Citation51 Greece, Ireland, Italy, Spain, and PortugalCitation24,Citation52 now recommend booster doses for toddlers in their meningococcal vaccine schedules.
In addition to the natural decay of antibody levels postvaccination, other factors may contribute to immunogenic variation among individuals, potentially leaving a vaccinated person susceptible to disease.Citation53 These include functional or anatomic asplenia (the spleen traps and disposes of bacteria; without it bacteremia can result)Citation21,Citation54 and deficiencies in the terminal common complement pathway.Citation18,Citation54,Citation55 Immune defects in T-cell responsiveness and immunoglobulin deficiencies may also lead to impaired production of bactericidal antibodies postvaccination.Citation53,Citation56-Citation58
Vaccine strategies
A decrease in the absolute incidence of meningococcal serogroup C disease has occurred due to vaccination and prevention programs in the United Kingdom and Canada.Citation59,Citation60 The MenC conjugate vaccination program introduced in the United Kingdom and Wales in 1999 initially provided routine vaccination to the infant population and a catch-up vaccination to everyone aged 1 through 18 y.Citation61 A year after the introduction of the MenC vaccine campaign in 1999, a survey conducted by the UK Meningococcal Carriage Group found an average 66% decrease in serogroup C carriage in students aged 15 through 17 y.Citation62 The catch-up MenC vaccine age group was extended in 2002 to include everyone aged 25 y.Citation61 By 2009, this approach led to a 99% reduction in the incidence of serogroup C meningococcal disease in individuals aged 20 y and to a 98.7% reduction for all age groups.Citation61 This finding shows that catch-up campaigns with conjugate vaccines in groups with high carriage rates, such as adolescents and young adults, can have a dramatic impact on the carriage of meningococci, the benefits of which can lead to herd immunity.Citation8,Citation63
Polysaccharide vaccines have shown effectiveness in adolescents and adults. In young children and infants, antibody levels decline swiftly, but detectible antibody levels in young adults have been reported 10 y after initial vaccination.Citation1,Citation33,Citation64 Unlike conjugate vaccines such as those used in the UK immunization campaign, polysaccharide vaccines do not induce immune memory or booster response (particularly in young children). Additionally, they produce only a short-term, transient effect on nasopharyngeal carriage rates and thus are not expected to reduce transmission or lead to herd immunity.Citation33,Citation65,Citation66 Repeated immunization with polysaccharide vaccines may lead to hyporesponsiveness, such that antibody levels following subsequent doses are lower than antibody levels following the initial dose.Citation33,Citation65,Citation67,Citation68 Therefore, meningococcal conjugate vaccines are now being implemented in schedules globally, including in the United States.Citation48
ACIP and AAP recommendations in adolescents
In the United States, significant numbers of cases and sporadic outbreaks due to all serogroups continue to cause substantial morbidity and mortality. In 2005, the ACIP recommended routine quadrivalent (serogroups A, C, W-135, and Y) conjugate meningococcal vaccination for youths aged 11 through 12 y.Citation14 Despite the routine implementation of a meningococcal conjugate vaccine in the adolescent group and an overall decline in the estimated number of meningococcal cases in the United States since 2000, the meningococcal disease peak (due to serogroup C and Y infections) in older adolescents and young adults remains.Citation48
The CDC's ACIP and the AAP now recommend meningococcal vaccination with a quadrivalent conjugate vaccine at age 11 through 12 y followed by a second dose at age 16.Citation48,Citation69 This 2-dose vaccine series results in protective antibody levels throughout the period of time that adolescents remain at high risk for meningococcal disease. These recommendations were made based on data which suggested that many adolescents who receive a single dose of vaccine may lose protection after 5 y. A case-controlled study evaluating vaccine effectiveness of MCV4-DT showed that vaccine effectiveness dropped significantly 2 to 5 y after vaccination; the overall vaccine effectiveness in persons vaccinated 0 to 5 y earlier was 78% (95% CI: 29, 93). As length of time from primary vaccination increased, vaccine effectiveness decreased. The vaccine effectiveness for persons vaccinated < 1 y earlier, 1 y earlier, and 2 through 5 y earlier was 95% (CI: 10, 100), 91% (CI: 10, 101), and 58% (CI: -72, 89), respectively.Citation48 In addition, clinical studies have shown waning of antibodies over time. Five years after vaccination with a quadrivalent conjugate vaccine, approximately 50% of adolescents had bactericidal antibody levels protective against meningococcal disease.Citation48,Citation69 Therefore, persons immunized with a single vaccine dose at age 11 through 12 y become susceptible to infection and disease by age 16, when their risk for meningococcal disease is highest.Citation48
The ACIP considered other vaccination strategies, such as delaying the initial single vaccination from age 11 or 12 to age 14 or 15, but concerns surrounding lack of coverage due to decreased likelihood of physician visits by this older age group led to the current recommendation of a booster dose with a quadrivalent meningococcal conjugate vaccine at age 16.Citation48 The impact of this strategy on the incidence of disease remains to be seen. However, in the same report, the ACIP presented results from an economic analysis that concluded that administering a booster dose is estimated to prevent twice the number of cases and deaths as administering a single dose at age 11 y or age 15 y, with equivalent cost per quality-adjusted life-year.Citation48
The characteristics of conjugate vaccines which are believed to be critical in establishing long-term protection against a bacterial pathogen include production of protective circulating antibody, memory T-cell response, and herd immunity.Citation38,Citation48 Although vaccination primes the immune system, the T-cell memory response which may occur after loss of protective antibody may not progress quickly enough to protect against the rapidly replicating bacteria. Thus, circulating antibodies are critical for protection against meningococcal disease.
Conclusion
In summary, adolescents and young adults are a population at increased risk for meningococcal disease. Waning immunity following a single dose of meningococcal vaccine may not offer sufficient protection and leaves adolescents and young adults at risk for meningococcal disease. Without the presence of circulating antibodies, the immune system's memory response may be too slow to offer protection against the rapid progression of meningococcal disease following infection. The CDC's updated recommendations for meningococcal conjugate vaccination, recommends initial vaccination at age 11 through 12 y and a booster at age 16 y. This strategy aims to provide protective circulating antibody throughout the period of high risk.
| Abbreviations: | ||
| AAP | = | American Academy of Pediatrics |
| ACIP | = | Advisory Committee on Immunization Practices |
| CDC | = | Centers for Disease Control and Prevention |
| CI | = | confidence interval |
| Hib | = | Haemophilus influenzae type b |
| hSBA | = | serum bactericidal assay using human complement |
| MCC | = | meningococcal serogroup C conjugate |
| MCV4-DT | = | meningococcal diphtheria toxoid conjugate vaccine |
| MenC | = | meningococcal C conjugate |
| N. meningitidis | = | Neisseria meningitides |
| rSBA | = | serum bactericidal assay using rabbit complement |
Acknowledgments
Writing and editorial assistance was provided by International Meetings and Science Inc., which was supported by Novartis Vaccines and Diagnostics. The authors retained content and editorial control throughout and received no other compensation.
Disclosure of Potential Conflicts of Interest
K.SE has received honoraria from Novartis Vaccines and Diagnostics for lectures and service on an advisory board. B.L.C has received honoraria and consulting fees from Novartis Vaccines and Diagnostics.
References
- Stephens DS. Conquering the meningococcus. FEMS Microbiol Rev 2007; 31:3 - 14; http://dx.doi.org/10.1111/j.1574-6976.2006.00051.x; PMID: 17233633
- Brigham KS, Sandora TJ. Neisseria meningitidis: epidemiology, treatment and prevention in adolescents. Curr Opin Pediatr 2009; 21:437 - 43; http://dx.doi.org/10.1097/MOP.0b013e32832c9668; PMID: 19421058
- Christensen H, May M, Bowen L, Hickman M, Trotter CL. Meningococcal carriage by age: a systematic review and meta-analysis. Lancet Infect Dis 2010; 10:853 - 61; http://dx.doi.org/10.1016/S1473-3099(10)70251-6; PMID: 21075057
- van Deuren M, Brandtzaeg P, van der Meer JW. Update on meningococcal disease with emphasis on pathogenesis and clinical management. Clin Microbiol Rev 2000; 13:144 - 66; http://dx.doi.org/10.1128/CMR.13.1.144-166.2000; PMID: 10627495
- Thompson MJ, Ninis N, Perera R, Mayon-White R, Phillips C, Bailey L, et al. Clinical recognition of meningococcal disease in children and adolescents. Lancet 2006; 367:397 - 403; http://dx.doi.org/10.1016/S0140-6736(06)67932-4; PMID: 16458763
- Centers for Disease Control and Prevention (CDC). Vaccines and Immunizations. Factsheet: Meningococcal diseases and meningococcal vaccines. http://www.cdc.gov/vaccines/vpd-vac/mening/vac-mening-fs.htm. Updated November 16, 2011. Accessed February 20, 2012.
- Cohn AC, MacNeil JR, Harrison LH, Hatcher C, Theodore J, Schmidt M, et al. Changes in Neisseria meningitidis disease epidemiology in the United States, 1998-2007: implications for prevention of meningococcal disease. Clin Infect Dis 2010; 50:184 - 91; http://dx.doi.org/10.1086/649209; PMID: 20001736
- World Health Organization (WHO). Meningococcal disease. Geneva, Switzerland: WHO Document Production Services; 2010. The immunological basis for immunization series. Module 15.
- Rosenstein NE, Perkins BA, Stephens DS, Popovic T, Hughes JM. Meningococcal disease. N Engl J Med 2001; 344:1378 - 88; http://dx.doi.org/10.1056/NEJM200105033441807; PMID: 11333996
- Winstead JM, McKinsey DS, Tasker S, De Groote MA, Baddour LM. Meningococcal pneumonia: characterization and review of cases seen over the past 25 years. Clin Infect Dis 2000; 30:87 - 94; http://dx.doi.org/10.1086/313617; PMID: 10619738
- Herf C, Nichols J, Fruh S, Holloway B, Anderson CU. Meningococcal disease: recognition, treatment, and prevention. Nurse Pract 1998; 23:30 - , 33-6, 39-40 passim; PMID: 9718600
- Mertens R, Peschgens T, Granzen B, Heimann G. Diagnosis and stage-related treatment of disseminated intravascular coagulation in meningococcal infections. Klin Padiatr 1999; 211:65 - 9; http://dx.doi.org/10.1055/s-2008-1043767; PMID: 10407813
- Granoff D, Harrison LH, Borrow R. Meningococcal vaccines. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. Philadelphia, PA: WB Saunders, 2008:399-434.
- Bilukha OO, Rosenstein N, National Center for Infectious Diseases, Centers for Disease Control and Prevention (CDC). Prevention and control of meningococcal disease. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2005; 54:RR-7 1 - 21; PMID: 15917737
- Ortega-Sanchez IR, Meltzer MI, Shepard C, Zell E, Messonnier ML, Bilukha O, et al, Active Bacterial Core Surveillance Team. Economics of an adolescent meningococcal conjugate vaccination catch-up campaign in the United States. Clin Infect Dis 2008; 46:1 - 13; http://dx.doi.org/10.1086/524041; PMID: 18171206
- Stephens DS, Greenwood B, Brandtzaeg P. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis.. Lancet 2007; 369:2196 - 210; http://dx.doi.org/10.1016/S0140-6736(07)61016-2; PMID: 17604802
- Bruce MG, Rosenstein NE, Capparella JM, Shutt KA, Perkins BA, Collins M. Risk factors for meningococcal disease in college students. JAMA 2001; 286:688 - 93; http://dx.doi.org/10.1001/jama.286.6.688; PMID: 11495618
- Centers for Disease Control and Prevention (CDC). Meningococcal disease. In: Atkinson W, Wolfe S, Hamborsky J, eds. The Pink Book: Epidemiology and Prevention of Vaccine-Preventable Diseases. 12th ed. Washington DC: Public Health Foundation, 2011:193-203.
- Biggar WD, Bogart D, Holmes B, Good RA. Impaired phagocytosis of pneumococcus type 3 in splenectomized rats. Proc Soc Exp Biol Med 1972; 139:903 - 8; PMID: 4401921
- Francke EL, Neu HC. Postsplenectomy infection. Surg Clin North Am 1981; 61:135 - 55; PMID: 7010637
- Krivit W. Overwhelming postsplenectomy infection. Am J Hematol 1977; 2:193 - 201; http://dx.doi.org/10.1002/ajh.2830020210; PMID: 329670
- Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine 2009; 27:Suppl 2 B51 - 63; http://dx.doi.org/10.1016/j.vaccine.2009.04.063; PMID: 19477562
- Centers for Disease Control and Prevention (CDC). Summary of notifiable diseases: United States, 2009. MMWR Morb Mortal Wkly Rep 2011; 58:1 - 100; PMID: 21566560
- European Centre for Disease Prevention and Control (ECDC). Surveillance of invasive bacterial diseases in Europe 2008/09. Stockholm, Sweden: ECDC; 2011. Surveillance report.
- European Union Invasive Bacterial Infections Surveillance Network (EU-IBIS). Age distribution of culture confirmed Neisseria meningitidis cases in all reporting countries, by year, 1999-2006. http://www.hpa-bioinformatics.org.uk/euibis/php/meningo_age_chart.php?item=culture&country=All+countries. Updated June 2008. Accessed March 2012.
- Centers for Disease Control and Prevention (CDC). Active bacterial core surveillance (ABCs) report: emerging infections program network, Neisseria meningitidis, 1998-2009. http://www.cdc.gov/abcs/reports-findings/surv-reports.html. Accessed February 20, 2012.
- Jackson LA, Wenger JD. Laboratory-based surveillance for meningococcal disease in selected areas, United States, 1989-1991. MMWR CDC Surveill Summ 1993; 42:21 - 30; PMID: 8510639
- Lingappa JR, Al-Rabeah AM, Hajjeh R, Mustafa T, Fatani A, Al-Bassam T, et al. Serogroup W-135 meningococcal disease during the Hajj, 2000. Emerg Infect Dis 2003; 9:665 - 71; http://dx.doi.org/10.3201/eid0906.020565; PMID: 12781005
- Ciccone HF, Suzuki E, Pellini ACG, Freitas AC, Vila??a PJ, Carvalhanas TR, et al. Meningococcal disease: communitarian outbreak investigation in Grajau, in the city of Sao Paulo, July, 2006. Bol Epidemiol Paulista 2006; 3:7 - 12
- Lemos AP, Brando AP, Gorla MC, Paiva MV, Simonsen V, Melles CE. Phenotypic characterization of Neisseria meningitidis strains isolated from invasive disease in Brazil from 1990 to 2001. J Med Microbiol 2006; 55:751 - 7; http://dx.doi.org/10.1099/jmm.0.46451-0; PMID: 16687595
- Balmer P, Borrow R, Miller E. Impact of meningococcal C conjugate vaccine in the UK. J Med Microbiol 2002; 51:717 - 22; PMID: 12358061
- Coulson GB, von Gottberg A, du Plessis M, Smith AM, de Gouveia L, Klugman KP, Group for Enteric, Respiratory and Meningeal Disease Surveillance in South Africa. Meningococcal disease in South Africa, 1999-2002. Emerg Infect Dis 2007; 13:273 - 81; http://dx.doi.org/10.3201/eid1302.051553; PMID: 17479891
- Harrison LH. Prospects for vaccine prevention of meningococcal infection. Clin Microbiol Rev 2006; 19:142 - 64; http://dx.doi.org/10.1128/CMR.19.1.142-164.2006; PMID: 16418528
- Wu HM, Harcourt BH, Hatcher CP, Wei SC, Novak RT, Wang X, et al. Emergence of ciprofloxacin-resistant Neisseria meningitidis in North America. N Engl J Med 2009; 360:886 - 92; http://dx.doi.org/10.1056/NEJMoa0806414; PMID: 19246360
- Centers for Disease Control and Prevention (CDC). National, state, and local area vaccination coverage among adolescents aged 13-17 years --- United States, 2009. MMWR Morb Mortal Wkly Rep 2010; 59:1018 - 23; PMID: 20724968
- Centers for Disease Control and Prevention (CDC). National and state vaccination coverage among adolescents aged 13 through 17 years--United States, 2010. MMWR Morb Mortal Wkly Rep 2011; 60:1117 - 23; PMID: 21866084
- Centers for Disease Control and Prevention (CDC). National, state, and local area vaccination coverage among adolescents aged 13-17 years--United States, 2008. MMWR Morb Mortal Wkly Rep 2009; 58:997 - 1001; PMID: 19763075
- Pollard AJ, Perrett KP, Beverley PC. Maintaining protection against invasive bacteria with protein-polysaccharide conjugate vaccines. Nat Rev Immunol 2009; 9:213 - 20; http://dx.doi.org/10.1038/nri2494; PMID: 19214194
- Pichichero ME. Booster vaccinations: can immunologic memory outpace disease pathogenesis?. Pediatrics 2009; 124:1633 - 41; http://dx.doi.org/10.1542/peds.2008-3645; PMID: 19933727
- Mast EE, Ward JW. Hepatitis B vaccine. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. Philadelphia, PA: WB Saunders, 2008:205-41.
- Borrow R, Miller E. Long-term protection in children with meningococcal C conjugate vaccination: lessons learned. Expert Rev Vaccines 2006; 5:851 - 7; http://dx.doi.org/10.1586/14760584.5.6.851; PMID: 17184222
- Blanchard Rohner G, Snape MD, Kelly DF, John T, Morant A, Yu LM, et al. The magnitude of the antibody and memory B cell responses during priming with a protein-polysaccharide conjugate vaccine in human infants is associated with the persistence of antibody and the intensity of booster response. J Immunol 2008; 180:2165 - 73; http://dx.doi.org/10.1182/blood-2009-03-211052; PMID: 18250423
- Snape MD, Kelly DF, Salt P, Green S, Snowden C, Diggle L, et al. Serogroup C meningococcal glycoconjugate vaccine in adolescents: persistence of bactericidal antibodies and kinetics of the immune response to a booster vaccine more than 3 years after immunization. Clin Infect Dis 2006; 43:1387 - 94; http://dx.doi.org/10.1086/508776; PMID: 17083009
- Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med 1969; 129:1307 - 26; http://dx.doi.org/10.1084/jem.129.6.1307; PMID: 4977280
- Andrews N, Borrow R, Miller E. Validation of serological correlate of protection for meningococcal C conjugate vaccine by using efficacy estimates from postlicensure surveillance in England. Clin Diagn Lab Immunol 2003; 10:780 - 6; PMID: 12965904
- Jodar L, Stephens D, Feavers IM. Assay parameters and methods of data analysis for the comparison of complement sources in the Neisseria meningitidis serogroup C serum bactericidal assay. Biologicals 2002; 30:323 - 9; http://dx.doi.org/10.1006/biol.2002.0341; PMID: 12474864
- Santos GF, Deck RR, Donnelly J, Blackwelder W, Granoff DM. Importance of complement source in measuring meningococcal bactericidal titers. Clin Diagn Lab Immunol 2001; 8:616 - 23; http://dx.doi.org/10.1128/CDLI.8.3.616-623.2001; PMID: 11329468
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of meningococcal conjugate vaccines --- Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep 2011; 60:72 - 6; PMID: 21270745
- Auckland C, Gray S, Borrow R, Andrews N, Goldblatt D, Ramsay M, et al. Clinical and immunologic risk factors for meningococcal C conjugate vaccine failure in the United Kingdom. J Infect Dis 2006; 194:1745 - 52; http://dx.doi.org/10.1086/509619; PMID: 17109348
- McVernon J, Johnson PD, Pollard AJ, Slack MP, Moxon ER. Immunologic memory in Haemophilus influenzae type b conjugate vaccine failure. Arch Dis Child 2003; 88:379 - 83; http://dx.doi.org/10.1136/adc.88.5.379; PMID: 12716702
- National Advisory Committee on Immunization (NACI). An update on the invasive meningococcal disease and meningococcal vaccine conjugate recommendations. An Advisory Committee Statement (ACS). Can Commun Dis Rep 2009; 35:ACS-3 1 - 40; PMID: 19400026
- World Health Organization. Immunization surveillance, assessment and monitoring. National immunization schedules. http://www.who.int/immunization_monitoring/data/data_subject/en/index.html. Updated December 1, 2011. Accessed February 20, 2012.
- Foster RA, Carlring J, Lees A, Borrow R, Ramsay M, Kacsmarski E, et al. Functional T-cell deficiency in adolescents who experience serogroup C meningococcal disease despite receiving the meningococcal serogroup C conjugate vaccine. Clin Vaccine Immunol 2010; 17:1104 - 10; http://dx.doi.org/10.1128/CVI.00481-09; PMID: 20463106
- Ram S, Lewis LA, Rice PA. Infections of people with complement deficiencies and patients who have undergone splenectomy. Clin Microbiol Rev 2010; 23:740 - 80; http://dx.doi.org/10.1128/CMR.00048-09; PMID: 20930072
- Figueroa JE, Densen P. Infectious diseases associated with complement deficiencies. Clin Microbiol Rev 1991; 4:359 - 95; http://dx.doi.org/10.1128/CMR.4.3.359; PMID: 1889047
- Foster RA, Carlring J, McKendrick MW, Lees A, Borrow R, Read RC, et al. Evidence of a functional B-cell immunodeficiency in adults who experience serogroup C meningococcal disease. Clin Vaccine Immunol 2009; 16:692 - 8; http://dx.doi.org/10.1128/CVI.00485-08; PMID: 19279166
- Ladhani S, Oeser C, Sheldon J, Ramsay M, Booy R, Heath PT. Immunoglobulin deficiency in children with Hib vaccine failure. Vaccine 2011; 29:9137 - 40; http://dx.doi.org/10.1016/j.vaccine.2011.09.107; PMID: 21983357
- Skattum L, Gullstrand B, Holmstr??m E, Oxelius VA, Truedsson L. Serum bactericidal activity against Neisseria meningitidis in patients with C3 nephritic factors is dependent on IgG allotypes. Clin Immunol 2008; 129:123 - 31; http://dx.doi.org/10.1016/j.clim.2008.06.010; PMID: 18667363
- Bettinger JA, Scheifele DW, Le Saux N, Halperin SA, Vaudry W, Tsang R, Canadian Immunization Monitoring Program, Active (IMPACT). The impact of childhood meningococcal serogroup C conjugate vaccine programs in Canada. Pediatr Infect Dis J 2009; 28:220 - 4; http://dx.doi.org/10.1097/INF.0b013e31819040e7; PMID: 19209096
- Trotter CL, Andrews NJ, Kaczmarski EB, Miller E, Ramsay ME. Effectiveness of meningococcal serogroup C conjugate vaccine 4 years after introduction. Lancet 2004; 364:365 - 7; http://dx.doi.org/10.1016/S0140-6736(04)16725-1; PMID: 15276396
- Campbell H, Andrews N, Borrow R, Trotter C, Miller E. Updated postlicensure surveillance of the meningococcal C conjugate vaccine in England and Wales: effectiveness, validation of serological correlates of protection, and modeling predictions of the duration of herd immunity. Clin Vaccine Immunol 2010; 17:840 - 7; http://dx.doi.org/10.1128/CVI.00529-09; PMID: 20219881
- Maiden MC, Stuart JM, UK Meningococcal Carraige Group. Carriage of serogroup C meningococci 1 year after meningococcal C conjugate polysaccharide vaccination. Lancet 2002; 359:1829 - 31; http://dx.doi.org/10.1016/S0140-6736(02)08679-8; PMID: 12044380
- Sfadi MA, McIntosh ED. Epidemiology and prevention of meningococcal disease: a critical appraisal of vaccine policies. Expert Rev Vaccines 2011; 10:1717 - 30; http://dx.doi.org/10.1586/erv.11.159; PMID: 22085175
- Zangwill KM, Stout RW, Carlone GM, Pais L, Harekeh H, Mitchell S, et al. Duration of antibody response after meningococcal polysaccharide vaccination in US Air Force personnel. J Infect Dis 1994; 169:847 - 52; http://dx.doi.org/10.1093/infdis/169.4.847; PMID: 8133100
- Bilukha O, Messonnier N, Fischer M. Use of meningococcal vaccines in the United States. Pediatr Infect Dis J 2007; 26:371 - 6; http://dx.doi.org/10.1097/01.inf.0000259996.95965.ef; PMID: 17468644
- Hassan-King MK, Wall RA, Greenwood BM. Meningococcal carriage, meningococcal disease and vaccination. J Infect 1988; 16:55 - 9; http://dx.doi.org/10.1016/S0163-4453(88)96117-8; PMID: 3130424
- Gold R, Lepow ML, Goldschneider I, Draper TL, Gotschlich EC. Clinical evaluation of group A and group C meningococcal polysaccharide vaccines in infants. J Clin Invest 1975; 56:1536 - 47; http://dx.doi.org/10.1172/JCI108235; PMID: 1202084
- Jokhdar H, Borrow R, Sultan A, Adi M, Riley C, Fuller E, et al. Immunologic hyporesponsiveness to serogroup C but not serogroup A following repeated meningococcal A/C polysaccharide vaccination in Saudi Arabia. Clin Diagn Lab Immunol 2004; 11:83 - 8; http://dx.doi.org/10.1128/CDLI.11.1.83-88.2004; PMID: 14715549
- Committee on Infectious Diseases. Meningococcal conjugate vaccines policy update: booster dose recommendations. Pediatrics 2011; 128:1213 - 8