Abstract
Cancer immunotherapy has seen a tremendous number of failures and only few recent regulatory successes. This review is a first in a series dedicated to evaluate the status of current global clinical pipeline for cancer vaccines. Apart from specific areas of medical need which can be addressed by cancer vaccines, the analysis of the pipeline by clinical indication suggests that a disproportionately large number of candidates is currently developed in prostate, breast, lung cancer and melanoma with significant gap of candidates in remaining oncology indications. With potential offering and benefits that cancer immunotherapy could bring to patient community and society as a whole, we require new innovative R&D, improved Antigen Discovery programs and business models to fill serious gaps in cancer vaccine R&D pipeline.
Keywords: :
Epidemiology of Cancers
The National Cancer Institute estimates that nearly 12 million Americans with a history of cancer were alive in January 2008. Some of these individuals were cancer free, while others still had evidence of cancer and may have been undergoing treatment. In the US, around 1,638,910 new cancer cases are expected to be diagnosed in 2012.Citation1-Citation3 In the EU, it is estimated that nearly 1.3 million people (717,398 men and 565,703 women) will die from cancer in 2012.Citation4 Despite that cancer remain a second leading cause of death, the incidence and mortality rates for four most common types of cancer: prostate, breast, lung and colorectal cancers were steadily decreasing due to a variety of factors, namely improved early diagnosis, enhanced treatment paradigm and expedited health care access.
However due to population growth and aging, the number of new cancer patients in the USA is expected to double to 2.6 million people by the 2050s. This number could further increase due to some forms of cancer which are often associated with dismal prognosis and sub-optimal treatments, e.g., esophageal, pancreatic, hepatocellular and hematological tumors. Further, as survival from some of the cancers will continue to improve (in particular, breast, thyroid cancer and melanoma of the skin), this will add to the growing population of cancer survivors with complex health care and societal needs, including reduced income and productivity due to a prolonged illness, economic stress and limited or diminishing social support. In addition, as cancer survivors’ age, some will be at increased risk for second cancers, requiring additional medical surveillance. The need will also grow for access to comprehensive cancer centers, and for health officials to develop appropriate plans to meet these needs.
Therefore, some forms of cancer will eventually manifest as a chronic disease requiring oncology research to develop much more sophisticated therapeutic armamentarium that will be suitable for long-term maintenance treatment of cancer without compromising quality of life, and with minimal burden on health care in terms of treatment delivery and monitoring costs. Importantly, conventional treatments have failed so far to optimise long-term outcomes in lung, pancreatic, esophageal and hepatocellular cancers (HCC) with desperate need for new therapeutic approaches.
Medical Need for Cancer Immunotherapy
Cancer immunotherapy has always been an attractive strategy for treatment of cancer promising to restore own specific immunological responses against the tumor and providing with safe, tolerable and long-lasting therapeutic regimen. For more than 100 years, the hallmark of medical treatment for cancer has been intravenous cytotoxic chemotherapy. These drugs non-selectively target rapidly dividing cells, including cancer cells and certain rapidly renewed normal tissues. As a result, many patients experience the classic toxicities of alopecia, gastrointestinal symptoms and myelosuppression. Profound immunosuppressive effects of conventional chemotherapy might have been partially responsible for numerous failures reported in cancer vaccines trials over past 20 years because immunotherapy efforts are not compatible with long-lasting immune ablative effects of chemotherapy regimens. In the past decade, however, a dramatic shift in cancer therapy has occurred. Although traditional cytotoxic chemotherapy remains a treatment backbone for many malignancies, targeted therapies are now a component of treatment for many types of cancer, including breast, colorectal, lung, and pancreatic cancers, as well as lymphoma, leukemia and multiple myeloma. Of the new anticancer drugs approved by the US Food and Drug Administration (FDA) since January 2012, 39 have been targeted therapies based on entities of monoclonal antibodies, antibody-drug conjugates, fusion proteins and small molecule drugs. Specifically in 2011, among 35 new medicines approved by FDA in 2011, seven have delivered major breakthroughs in cancer treatments and two personalized therapies for lung cancer and melanoma approved with diagnostic companion tests.
Targeted or personalized therapies of cancer promise to deliver benefits of more selective targeting of tumor without detrimental impact on healthy organs and tissues, improve patient reported outcomes, adherence and compliance. In addition, the use of companion diagnostic tests can serve as a commercial barrier for protecting a target-based market. However the drawbacks of some of these therapies include a small proportion of patients who may qualify for a targeted approach, high development costs that are subsequently displaced by industry onto health care providers, cost and access of diagnostic tests etc.
There are two distinct types of cancer immunotherapy using biologically derived agents: (1) passive therapy using monoclonal antibodies against non-immunologically related cancer specific surface antigens (e.g., trastuzumab against HER2 receptor in breast and gastric carcinoma or cetuximab against epidermal growth factor receptor (EGFR) in colorectal cancer) and (2) active immunotherapy based on boosting the immunity against cancer specific antigens (e.g., sipuleucel-T in castrate-resistant prostate cancer). The latter also includes monoclonal antibodies directed against immunologically related targets that promote tumor shrinkage via immunologically driven pathways, e.g., ipilimumab against CTLA4 antigen in advanced melanoma.
Growth in targeted therapies has reinvigorated interest in cancer immunotherapy because: (1) therapeutic vaccines employed as a monotherapy could deliver targeted immune-mediated effect on tumors; (2) the advantage of cancer vaccines compared with passive immunotherapy is that T-cell driven responses and memory associated with those could assist with immune response during disease recurrence; (3) combination regimens composed of vaccines and small molecule and/or biological anticancer agents could synergistically enhance the effect on tumor microenvironment, metastatic growth and tissue remodelling; (4) effect on tumor “evasion” via immunological bypassing of accumulated tumor defects resulting in theoretical possibility of reduced failure of a backbone targeted therapy agent (e.g., anti-HER2 antibody); (5) sparing effect on the use of other chemotherapy agents that may translate in safety and cost benefits; (6) improvements in quality of life, tolerability, compliance and patient reported outcome profiles; (7) there is a remote theoretical possibility that cancer immunotherapies may reduce long-term risk of secondary cancers in surviving patients and (8) cancer vaccines are less prone to genericisation and biosimilar rivalry.
From clinical perspective, cancer vaccines could further prolong overall survival (OS), reduce the rate of disease recurrence and expand patient population which is excluded from biomarker-stratified groups (e.g., KRAS-mutant careers, triple-negative breast cancer or HER2-receptor-negative breast and gastric cancer) (). Overall, cancer vaccines could deliver a number of different therapeutic values which could be integrated by potential developers into target product profiles.
Table 1. Therapeutic cancer vaccines have the potential to address a number of unmet needs across different types of cancers (modified from Datamonitor, 2009Citation5)
Pipeline Review
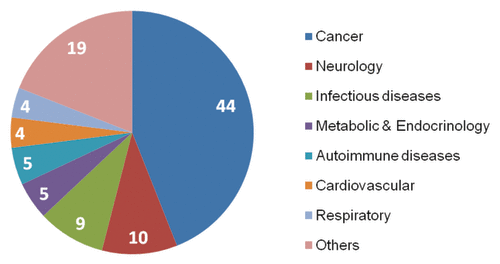
Cancer continues to dominate the US pipeline as companies increasingly pursue indications with high unmet need that do not require overly large clinical trial populations—thus requiring lower amounts of capital. Many companies are following a strategy of attempting to receive approval for a narrow, well-defined indication, with the intention of conducting post-approval trials in other indications with the same mechanism of action. Consistent with the US, cancer is the dominant indication in European pipelines, growing steadily over the last three years.Citation6 Therefore the distribution of clinical pipeline by therapeutic area is very similar between US and EU ().
Figure 1. US clinical pipeline by indication in 2010 (%).Citation6
According to Datamonitor and Business Insights findings in 2009–2010, there are approximately 150 therapeutic cancer vaccines in phase I–III clinical development across seven major markets. Approximately 70% of these products are standardized (“off-shelf”) vaccines. The remaining proportion is accounted by personalized vaccines.
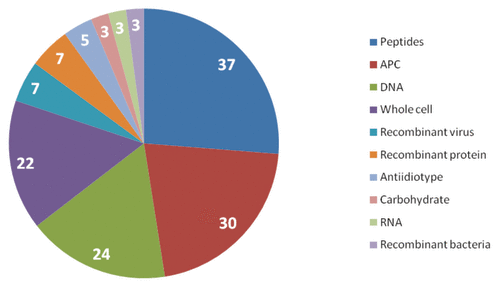
In terms of the platform technologies, the pipeline is dominated by candidate products composed of peptides, antigen-presenting cells and DNA based technologies (see ).
Figure 2. Analysis of the therapeutic vaccines clinical pipeline by class of platform technology.Citation5,Citation7 APC, antigen-presenting cell.
The analysis of the pipeline by clinical indication suggests that a disproportionately large number of candidates (almost 60%) is currently developed in prostate, breast, lung cancer and melanoma (). Such an imbalance can be explained by recent regulatory successes of Provenge (sipuleucel-T) in prostate cancer (US approval) and Yervoy (ipilimumab) in advanced melanoma (EU and US approvals). In addition, the number of antigens discovered and validated in these indications exceeded the number of antigens characterized in other types of tumors and this factor enabled to drive the innovation in these clinical indications. The finding from this analysis illustrates that a large number of oncology indications are currently underrepresented in the pipeline potentially increasing the reserve for unmet medical need which might be met only by small-molecule and non-vaccine biological therapies.
Figure 3. Analysis of the therapeutic vaccines by clinical indication.Citation5
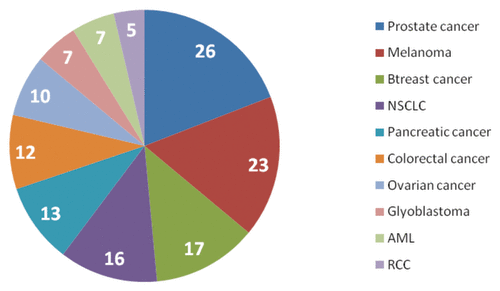
In average, it may take up to 5–10 years to see whether the landscape of the pipeline will change and new indications will increase the contributory weight in the overall cancer vaccine portfolio. Recently emerged novel targeted therapies which left a footprint in treatment paradigm of prostate (sipuleucel-T, abiraterone), breast (herceptin, trastuzumab-DM1), NSCLC (crisotinib) and melanoma (vemurafenib and ipilimumab) will eventually raise barriers for entry of new developers and this will potentially influence the pattern of early vaccine candidates. Considering significant gap in cancer vaccines for hematological, brain, thyroid, ovarian, pancreatic and colorectal tumors and accounting for potentially high attrition rate in Phase II–III, it can be concluded that without efforts to reinvigorate the pipeline, there is a serious chance that we may not be able to see successful products for some forms of cancer in next 10–15 years.
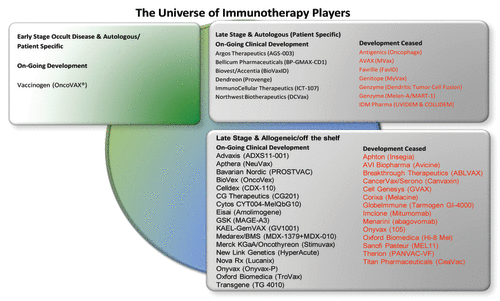
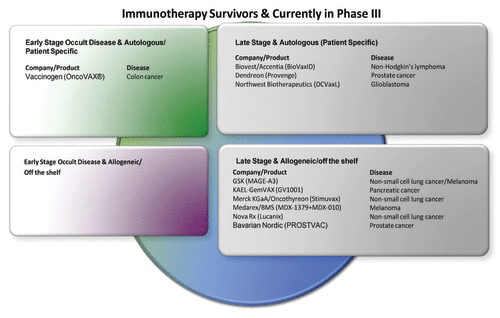
The pattern of cancer vaccine candidates, on-going clinical development, ceased development, specific or nonspecific therapies and stage of disease is represented in . Similarly, the majority of currently open phase III studies are shown in .
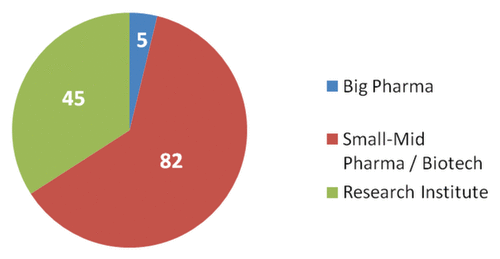
The cancer vaccine field is well recognized for high development costs and risks, low historical rates of investment return and high probability of failures arising in ventures, partnerships and alliances.Citation7 Therefore, large pharma had a limited focus in the cancer vaccine field with only a few large developments underway (). It is likely that high-profile setbacks of cancer vaccines such as Sanofi’s experience with the development partnership with Oxford Biomedica on TroVax for metastatic renal carcinoma (mRCC)Citation8 and Takeda’s experience with Cell Genesys prostate cancer programCitation9 discouraged large pharma companies to commit investment into cancer vaccine field ()
Figure 6. Analysis of the therapeutic vaccine pipeline by type of developing company.Citation5
One very important reason for the failures of cancer vaccines has been the lack of recognition that cancer is a genetic disease and that a multitude of mutations in a developing tumor requires a newer and more immunologically critical approach in the area of Antigen Discovery. In the past 15 y, the majority of cancer immunology platforms was based the consideration of tumors being homogeneous. Antigen discovery (one size fits all) was directed by this false premise. Today with the rapid and efficient advances of DNA sequencing a new picture of the cancer genome is emerging. There is now recognition of the fact that there is both intertumoral heterogeneityCitation10 and intratumoral heterogeneityCitation11 of tumors, based on the random and diverse point mutations that drive, characterize and constitute tumors. So with cancer being a heterogeneous disease, it is no longer reasonable to use homogeneous treatments or platforms for specific cell mediated immunotherapy. The clinically relevant approach to antigen discovery is the use of the patient’s primary tumor which will provide an adequate source of the polyvanlent immunogens created by those mutations that give rise to the prevalent private antigens. This new paradigm defeats the preference of large pharma for off the shelf antigen use for homogeneous treatments. The current understanding of the cancer genome also diminishes the feasibility of single agent targeted therapy due to acquired resistance and adds a risk to marker driven therapies based and derived on single biopsies of tumors.Citation12
Despite of limited funding and numerous other hurdles, small and mid-size pharma companies and academic institutions remain a major force which drives innovative and development efforts in cancer immunotherapy. While various funding issues, the impact of the contemporary knowledge of tumor cell biology and lack of development and regulatory expertise create numerous challenges for biotech and academia, there are clear incentives which maintain the focus of these developers on cancer vaccines regardless of past failures. The attractiveness can be potentially explained that cancer vaccine ventures allow making progress in discovery and understanding of fundamental immunological phenomena and sprouting into new areas of immunology and oncology. Vaccine technologies can have profound and broad application across cancer and non-cancer related diseases. There is low probability of generic and follow-on threat as it is incredibly difficult to reproduce or copy vaccine manufacturing processes. Therefore, in some cases intellectual property protection and the length of patent life are irrelevant as long as trade secrets and manufacturing expertise are well maintained. Despite of many investment and financial drawbacks, a domination of small- and mid-size biotech in cancer vaccine field can provide with numerous favorable opportunities for enhanced innovation, reduced dependency from large pharma, collaborations and partnerships with academic institutions and governments, new business models (e.g., networked businesses) and improve efficiencies in use of investment and development resources.
However in order to achieve a long-term and sustained success of cancer immunotherapies, new approaches will be needed to reinvigorate investment, engagement of large pharma, and foster R&D innovation across all phases of development. Potential opportunities for growth may come from more relevant or patient-tumor specific antigen discovery, emerging markets.Citation13 Rexin-G from Epeius (replication-incompetent, pathotropic, tumor matrix (collagen)-targeted retrovector encoding an N-terminal deletion mutant of the cyclin G1 gene) has been approved in the Philippines as stand-alone therapy for the treatment of all chemotherapy-resistant solid tumors and was granted with Fast Track status, as well with clearly unmet medical needs in oncology, potential offering and benefits that cancer immunotherapy can bring to patient community and society as a whole, we require new models to fill serious gaps in cancer vaccine R&D pipeline so we can ensure that new products will be seamlessly developed and commercialized in underserved oncology markets benefiting cancer patient care and future treatment paradigm.
References
- American Cancer Society. Cancer Facts and Figures, 2011. Atlanta, American Cancer Society, 2011: 1-10.
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin 2010; 60:277 - 300; http://dx.doi.org/10.3322/caac.20073; PMID: 20610543
- Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 2011; 61:212 - 36; http://dx.doi.org/10.3322/caac.20121; PMID: 21685461
- World Health Organization. Cancer mortality database, 2010; Available from: http://www.dep.iarc.fr/WHOdb/WHOdb.htm Accessed April 13, 2012.
- Insight P. Therapeutic Cancer Vaccines. Datamonitor, 2009: 1-50.
- Ernst & Young. Beyond borders: Global biotechnology report, 2011: 1-25. Available from http://www.ey.com/GL/en/Industries/Life-Sciences/Beyond-borders--global-biotechnology-report-2012
- Shirvill J. Innovations and Opportunities in Therapeutic Vaccines. Business Insights, 2010: 1-100.
- Oxford Biomedica. Oxford Biomedica press release (April 29, 2009) Oxford Biomedica Regains Rights to its Trovax Cancer Vaccine, 2009; Available from: http://www.oxfordbiomedica.co.uk/page.asp?pageid =59&newsid=72 Accessed on April 13, 2012.
- Fiercebiotech. Cell Genesys and Takeda Announce Termination of Collaboration Agreement for GVAX Immunotherapy for Prostate Cancer, 2008; Available from: http://www.fiercebiotech.com/press-releases/cell-genesys- and-takeda-announce-termination-collaboration-agreement-gvax-mmunothera Accessed on April 13, 2012.
- Wood LD, Parsons DW, Jones S, Lin J, Sjöblom T, Leary RJ, et al. The genomic landscapes of human breast and colorectal cancers. Science 2007; 318:1108 - 13; http://dx.doi.org/10.1126/science.1145720; PMID: 17932254
- Gerlinger MR, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 2012; 366:883 - 92; http://dx.doi.org/10.1056/NEJMoa1113205; PMID: 22397650
- Wagle N, Emery C, Berger MF, Davis MJ, Sawyer A, Pochanard P, et al. Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling. J Clin Oncol 2011; 29:3085 - 96; http://dx.doi.org/10.1200/JCO.2010.33.2312; PMID: 21383288
- PharmaLive. Oncology Market Soars in China. PharmaLive. 2011; Available from: http://pharmalive.com/magazines/medad/view. cfm?articleID=9544 Accessed April 13, 2012.