Abstract
Despite of growing oncology pipeline, cancer vaccines contribute only to a minor share of total oncology-attributed revenues. This is mainly because of a limited number of approved products and limited sales from products approved under compassionate or via early access entry in smaller and less developed markets. However revenue contribution from these products is extremely limited and it remains to be established whether developers are breaking even or achieving profitability with existing sales. Cancer vaccine field is well recognized for high development costs and risks, low historical rates of investment return and high probability of failures arising in ventures, partnerships and alliances. The cost of reimbursement for new oncology agents is not universally acceptable to payers limiting the potential for a global expansion, market access and reducing probability of commercial success. In addition, the innovation in cancer immunotherapy is currently focused in small and mid-size biotech companies and academic institutions struggling for investment. Existing R&D innovation models are deemed unsustainable in current “value-for-money” oriented healthcare environment. New business models should be much more open to collaborative, networked and federated styles, which could help to outreach global, markets and increase cost-efficiencies across an entire value chain. Lessons learned from some developing countries and especially from South Korea illustrate that further growth of cancer vaccine industry will depends not only on new business models but also will heavily rely on regional support and initiatives from different bodies, such as governments, payers and regulatory bodies.
Commercial status of the oncology pipeline
The global cancer market in 2010 was valued at $54bn, an increase of 5.1% over the previous year‘s sales of $51.3bn, and is forecasted to grow at a compound annual growth rate of 6.9% from 2010–16, reaching $81bn in 2016. Collectively, the seven major markets (US, 5EU, and Japan) represented 79.1% (or $43bn) of in the global cancer market in 2010. In terms of size, the US dominated the global cancer market, with 2010 sales of $21bn and a market share of 38.5%. The global cancer market is becoming increasingly competitive, with two therapeutic classes, namely anti-neoplastics and cytostatic hormonal treatments, dominating this sector. Collectively, the leading 10 brands accounted for almost 58.2% (or $31.4bn) of the global cancer market in 2010. The major drugs that contributed to the 2010 sales in the antineoplastics category were Roche‘s Avastin (bevacizumab) at $6.2bn, Herceptin (trastuzumab) at $5.2bn, and MabThera (rituximab) at $5.1bn, with annual sales growth of 3.8%, 3.1%, and 3.3% respectively. US represent the largest oncology market (38.5%), followed by EU (28.2%), rest of the world (20.9%) and Japan (12.4%).Citation1 Therefore oncology has become an attractive area despite of growing competition, relatively high attrition rate of oncology products in phase II, crowded clinical pipeline, and reimbursement barriers.
Despite of growing oncology pipeline, cancer vaccines contribute only to a minor share of total oncology-attributed revenues. This is mainly because of a limited number of approved products and limited sales from products approved under compassionate or via early access entry in smaller and less developed markets. There are two cancer vaccine products currently registered in developed markets: Provenge (Sipuleucel-T) developed by Dendreon approved in USA in 2010 and Yervoy (Ipilimumab) developed by BMS and approved in USA and EU in 2011. Due to manufacturing, reimbursement, uptake and supply issues, reported sales of Provenge in 2011 were below initially expected $350–400 min and reached total revenues of $228 min. The upfront cost of $93,000 per patient incurred within a 4–6 week timeframe, coupled with time to physician reimbursement of approximately 30 d, has been one of the greatest barriers to uptake. In years to come, Provenge performance will be threatened by significant competition from less logistically complex new market entrants, prescribed off-label and approved in the same post-castration resistant prostate cancer indication, including once-daily, orally administered Zytiga (abiraterone; Johnson and Johnson) and MDV3100 (Medivation). There is another rival therapeutic vaccine for prostate cancer: Prostvac, is being developed by Danish biotechnology company Bavarian Nordic under a formal Collaborative Research and Development Agreement (CRADA) with the National Cancer Institute (NCI). Prostvac comprises two recombinant viral vectors, each encoding PSA, plus three immune co-stimulatory molecules called TRICOM: B7.1 (CD80), ICAM-1 (CD54), and LFA-3 (CD58). It is currently in phase III, which will be completed in 2015. Furthermore, while it is not yet possible to quantitatively evaluate the safety-efficacy profile of emerging drugs such as MDV3100 and Prostvac in relation to Provenge, it is possible to make a qualitative assumption that the launch of oral and off-the-shelf products that may be used in the pre-chemotherapy prostate cancer setting will certainly have a negative impact on the sales performance of Provenge. Sequencing confusion, difficulties in entry onto EU market and Provenge’s high price may further restrict Dendreon’s revenue growth potential.Citation2
Yervoy approved in EU and US for the treatment of patients with unresectable (inoperable) or metastatic melanoma was positioned at price of $30,000 per infusion. However, a suboptimal safety profile necessitated a risk evaluation and mitigation strategy (REMS) in the US hindering drug uptake. Currently sales are projected to reach under $100 min by 2016. Although uptake will still be limited due to high pricing and safety concerns, there are chances that revenues will climb in next ten years.Citation3
There are several other cancer vaccine products available in small developed and developing markets or under patient named schemes ().
Table 1. Cancer vaccine products approved in developed and emerging markets. MVax autologous vaccine for melanoma from Avax Technologies has been approved in Switzerland in 2005 but the company ceased to exist in 2009 and no further information of MVax status could have been obtained
However revenue contribution from these products is extremely limited and it remains to be established whether developers are breaking even or achieving profitability with existing sales. Cancer vaccine field is well recognized for high development costs and risks, low historical rates of investment return and high probability of failures arising in ventures, partnerships and alliances.Citation11 Therefore, large pharma had a limited focus in cancer vaccine field with only few large developments underway. Provided that Stimuvax (BLP25) in unresectable NSCLC from MSD-Oncothyreon and MAGE3 in melanoma and NSCLC from GSK will prove to be successful, it is expected that large pharma companies will be increasingly involved in cancer vaccine pipeline.
Nevertheless, therapeutic cancer vaccines have the potential to address several unmet needs that persist in cancer. They are most likely to prove their value as low-toxicity treatment options for early-stage disease and by enhancing the efficacy of existing regimens in late-stage disease while adding minimal toxicity. There are currently more than 140 therapeutic cancer vaccines in clinical development. It is forecasted that a combined sales of $1.3 billion might be achieved in the seven major markets by 2018. Of these products, Stimuvax (BLP-25; Merck KGaA), Provenge (sipuleucel-T; Dendreon) and MAGE-A3 ASCI (astuprotimut-r; GlaxoSmithKline) have the greatest clinical and commercial potential, and, if approved, will achieve sales of $484 min, $321 min and $195 min in the seven major markets, respectively.Citation4 However if one or more of these products will not succeed in phase III studies or obtain regulatory approval, it is likely that the total cancer vaccine market in a next five years will be in a region of $500m-$1.0 bn.
An imbalance of therapeutic indications in current cancer vaccine pipeline and limited number of vaccine candidates in clinical development threatens the future with significant gap in immunotherapeutics for a whole range of oncology indications, such as pancreatic, head and neck, brain, thyroid, ovarian and renal cancers. Considering significant gap in cancer vaccines for some indications and accounting for potentially high attrition rate in Phase II-III, it can be concluded that without efforts to reinvigorate the pipeline, there is a serious chance that we may not be able to see successful products for some forms of cancer in next 10–15 y.
Despite of limited funding, resources and expertise, small biotechnology firms and academic institutions remain a major force driving innovative and development efforts in cancer immunotherapy. While various funding issues and lack of development and regulatory expertise create numerous challenges for biotech and academia, there are clear incentives that maintain the focus of these developers on cancer vaccines regardless of past failures. The attractiveness can be potentially explained that cancer vaccine ventures allow making progress in discovery and understanding of novel immunological phenomena and sprouting into new areas of immunology and oncology. Vaccine and adjuvant technologies can have profound and broad synergies and applications across cancer and non-cancer related diseases. For instance, an approval of a novel adjuvant in oncology indication may serve as a regulatory entry into broader application for the same adjuvant in non-oncology indications, such as allergy and asthma immunotherapies; preventative vaccines against infections: HIV, malaria, and tuberculosis and therapeutic vaccines against neurodegenerative conditions.
There is a low probability of generic and follow-on threat as it is incredibly difficult to reproduce or copy vaccine-manufacturing processes. Therefore in some cases, intellectual property protection and the length of patent life are irrelevant as long as trade secrets and manufacturing expertise are well guarded. Despite of many investment and financial drawbacks, a domination of small- and mid-size biotech in cancer vaccine field can provide with numerous favorable opportunities for enhanced innovation, reduced dependency from large pharma, collaborations and partnerships with academic institutions and governments, new business models (e.g., networked businesses) and improves efficiencies in use of investment and development resources. However in order to achieve a long-term and sustained success of cancer immunotherapies, new approaches will be needed to reinvigorate the investment, innovation and engagement of a large pharma.
Crisis of pharmaceutical innovation
Pharmaceutical innovation has become increasingly risky, costly and at times inefficient, which has led to a decline in industry productivity. Estimates for the average cost of bringing a new drug to market range between $ 800 min and $ 2 bn, in which late-stage failures and the rising costs of Phase II and III trials represent key components.Citation12 Significant variations in cost figures arising from therapeutic class and individual company’s performance were previously reported with an average cost of oncology development quoted to be around $600m.Citation13-Citation16 Despite the increased investment in R&D by the industry, the number of new molecular entities achieving marketing authorization is decreasing for some therapeutic classes. R&D productivity in pharmaceuticals seems to be higher for organizations that are able to exploit the global and internationally diversified divisions of innovative labor.Citation12 In addition, the productivity decline is associated with an increasing concentration of R&D investments in areas in which the risk of failure is high, which correspond to unmet therapeutic needs and unexploited biological mechanisms. Despite of potential variations in productivity and composition of R&D portfolios between EU and US based companies, there is no evidence of any regional productivity gap.Citation12 Furthermore, despite this increase in innovation, the issue of high R&D costs has not yet been adequately addressed. It is plausible that, in addition to regulatory and commercial requirements, the increasing proportions of priority reviewed new chemical and biological entities might have contributed to these spiralling costs. The higher risk associated with such priority reviews means that many of the programmes dedicated to discovering and developing such differentiated innovations will have failed before reaching the market, thereby contributing substantially to R&D costs.Citation17 In addition, there are numerous factors affecting the productivity and those are attributed not only to development costs and rising regulatory and reimbursement barriers but also to various cost-, resource-, administrative, management and marketing inefficiencies.
Biotechnology had revolutionized but also massively complicated R&D functions demanding highly specialized knowledge and diversified skills. Crowded oncology pipeline in developed markets prompted multinationals to look more seriously at opportunities in emerging markets. To compete successfully in emerging markets one has to master a very different set of skills, especially of bringing an appropriate talent and align corporate strategy with cultural and regulatory diversity in emerging markets. The fragmentation of pharmaceutical research into myriad specialist niches and lack of in-house expertise has increased reliance on outsourcing, mergers and acquisitions, partnerships or licensing alliances. These strategies are accompanied with different advantages and pitfalls and result in different R&D yields. Outsourcing has been heavily promulgated as a method of reducing overall manufacturing, development and commercialization costs. However extensive outsourcing without managing a broad cost base may turn to be cost-inefficient. In addition, complete elimination of in-house expertise can be risky during major manufacturing, regulatory, pharmacovigilance and supply issues. Therefore outsourcing should be well balanced and blended well with existing and well maintained internal expertise.
McKinsey and David et al. (2010) have suggested that companies should streamline decision-making process during managing of earlier portfolio and eliminate potential failures earlier in order to mitigate costly attrition of phase III candidates.Citation18 Further savings can be found via reducing non-development costs. According to PricewaterhouseCoopers (2009), between 1995 and 2005, the percentage of total corporate spending accounted for by R&D rose from 15% to 17.1%, while the percentage accounted for by sales and general administration rose from 28.7% to 33.1%, with sales and marketing being by far the biggest corporate expense. In a survey of industry stakeholders conducted by the PricewaterhouseCoopers Health Research Institute, 94% of the respondents said that pharmaceutical companies spent too much money on advertising.Citation19 The increasing expenditure on sales and marketing does not commensurate with much lower demand for sales and marketing efforts around highly specialized products. Therefore further cost savings could be achieved in co-development, co-marketing and co-commercialization deals for oncology care products, such as cancer vaccines. Bundling of cancer vaccines with other core oncology care products could provide with value-increasing and cost-efficient service solutions for payers. Supply chain components can constitute up to 50% of the drug price in emerging markets (conference communication) as determined by complex distribution and transportation network. Since infrastructure in some emerging markets is seriously underdeveloped, distribution costs, cold-chain and storage requirements and various inefficiencies can create additional barriers for entry of cancer vaccines into new territories. Significant cost savings can be found from identifying distribution synergies with other industries and local food and health care suppliers.
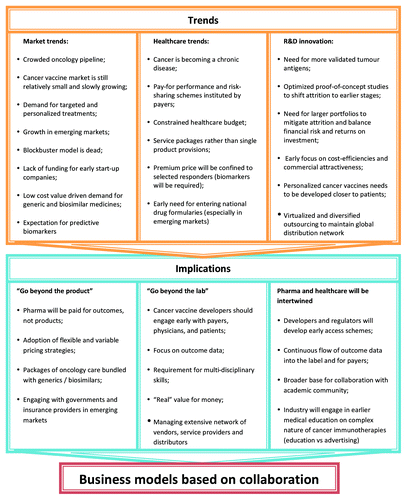
Cancer has emerged as the therapeutic area in which most of licensing deals were struck. Almost one in five of all agreements concluded during the past five years involved technologies, research programs, drug candidates or other assets with potential applications in the oncology field.Citation20 It is predicted that, by 2016, bioengineered vaccines and biologics will account for 23% of the global market (measured by value), up from 17% in 2009. The product base will become even more diverse, as advances in nanotechnology, tissue re-engineering, stem cell research and other such disciplines start to yield successes.Citation19 However, many of new cancer vaccines will require more complex development, manufacturing and distribution processes than conventional biological entities. In order to reduce R&D obsolescence and increase efficiencies, we need new business models that will support sustainable and productive innovation in cancer vaccinology ().
Federated business models
Based on dynamics of large pharma and biotech sector and inherent challenges associated with development of cancer vaccines, it is becoming clear that traditional large-pharma model will unsustainable in terms of costs, innovation productivity and commercialization of these highly targeted and specialized oncology products. In order to successfully develop a portfolio of cancer vaccine products, developers will need to reach out to numerous stakeholders, service providers and vendors across the globe.
Traditional secrecy of the pharmaceutical sector intended to protect intellectual property is a serious obstacle in creating highly innovative environment for new cancer immunotherapies. Mergers and acquisitions and licensing alliances can only partially address immediate business needs but invariably can be accompanied by considerable costs and R&D obsolescence.
Therefore, open-access collaborative partnerships will be gaining momentum in industry, and are also favored by funding agencies, venture capitalists, payers and governments. Such open-access collaborations may be a powerful alternative to closed collaborations; the sharing of early-stage research data are expected to enable scientific discovery by engaging a broader section of the scientific community in the exploration of new findings. Potentially, the sharing of data could contribute to an increased understanding of cancer immunology and a decrease in the attrition of clinical programs.Citation21 Therefore, there is a significant potential benefit in creating academia- and industry-funded cancer vaccine consortia and information banks, which could assist in exchanging and sharing new clinical and preclinical data from completed studies with cancer vaccines. Development of new personalized medicines at the global level requires learning from both success and failure stories. Access to specific genetic signatures and immunological features of highly diverse patient cancer patient population enrolled in numerous immunotherapeutic trials across the globe could shape the picture on the effect of different ethnic factors, standards of care and use of treatments on the effectiveness of cancer vaccines. Clearly fragmented industry alone will be unable to drive such an important global initiative and joint efforts with numerous cancer research bodies, oncology healthcare providers, European Commission, WHO etc. will be needed.
PriceWaterHouseCoopers (2009) has described several new R&D models including a federated approach, a company based on a network of separate entities with a common supporting infrastructure.Citation19 These might include universities, hospitals, technology suppliers, contract research organisations and manufacturing, data analysis firms and key opinion leaders from numerous countries. Federated model can include business units from within the company itself, which it places at “arm’s length.” The various participants have a mutual goal, e.g., management of clinical outcomes in a given oncology patient population. They also share funding, data, access to patients and back-office services, and this interdependence is what will maintain this entity together. All members of this model can benefit not only financially but also from innovative, outcome-related and market-creating rewards. Such a model could assist with cost-efficient development of new cancer vaccine products for the global community and drive health outcome-driven research.
There are also virtual and venture variants of federated model. In the virtual scenario, most or all of a company’s operations are outsourced and the company itself acts as a management hub, coordinating the activities of its partners. A typical example of a company that employs such a model is Shire. Strategic outsourcing can help the virtual company to concentrate on new business areas including working closely with different healthcare providers and stakeholders, reduce initial capital outlay, convert some of their fixed costs into variable costs, and become more cost and resource efficient. Importantly, the geographical expansion of such a business can be achieved without costly mergers and acquisitions. However, the virtual variant also comes with some significant drawbacks. The balance of power might shift to suppliers, and their failure can seriously jeopardise business performance. Therefore selection and management of several suppliers is often used to mitigate this risk.Citation19 Importantly, outsourcing of all value chain components for cancer immunotherapeutic might not be feasible due to shortage of high-class local providers, e.g., manufacturers of autologous cells. Variability in quality and performance of multiple local producers and distributors could drive prices up.
The venture variant of the federated model entails investing in a portfolio of companies in return for a share of the intellectual assets and/or capital growth they generate, rather than outsourcing specific tasks. Large pharma companies can set up corporate venture capital funds to invest in selected companies in return for the intellectual property or royalty payments from commercialization of the product. This model is attractive for non-interrupted funding of complex and costly clinical development program for cancer immunotherapeutics. In addition, a sustainable funding could provide with incentives for large globally diversified service providers to make commitments to development, commercialization, manufacturing and distribution efforts for the product across the globe. However the challenge will come from the way how large pharma will manage corporate funds and foster innovation. Sponsored company may have an increased risk profile due to dependency from large pharma.Citation19 Finally, it remains to be established whether a final price for the product adjusted with returns to funding source will be cost-efficient for payers. Nevertheless, some large pharma companies have started to see business potential and competitive advantage in entering into unconventional yet relatively uncontested areas, such as regenerative medicines and highly personalised medicines. Recent acquisition of Pervasis Therapeutics by Shire allowed the latter to obtain access to a new endothelial cell technology and signal that Shire is harnessing plans to become a major player in the regenerative medicines business as it matures.Citation22 Despite of daunting task of creating new environment product development, global manufacturing, distribution, sales and marketing, the attractiveness of creating new markets convinced some large companies to enter the business of cell-based technologies.
Dependently on individual portfolio and business strategy circumstances, cancer vaccine developers may opt for different business model. Open and networked models may grow from sporadic academic-industry collaborations into strategic partnerships and ultimately evolve into regional or global federated companies. Importantly that with global growth of contract research and manufacturing organisations and accumulation of suppliers’ expertise in targeted and highly specialized biotechnology products, federated companies will find greater opportunities for strategic partnerships and cost efficiencies.
Another significant challenge is a source of funding for start-up cancer vaccine developers. Increasingly, funds will be available from mixed academic and industry groups (e.g., Wellcome Trust) or large academic centers (e.g., National Cancer Research or Cancer Research UK) and venture funds operating in oncology area. During past 5 y, a buoyant growth in biotechnology ventures funds was reported in Asia and BRIC countries.Citation23 Some of these groups are focused in oncology investments, e.g., Russia based Maxwell Biotech Venture Fund or RusNano support companies developing novel vaccines and oncology treatments.
Opportunities in emerging markets
Potential opportunities for growth of cancer vaccines may come from emerging markets. Rexin-G from Epeius (replication-incompetent, pathotropic, tumor matrix (collagen)-targeted retrovector encoding an N-terminal deletion mutant of the cyclin G1 gene) has been approved in Philippines as stand-alone therapy for the treatment of all chemotherapy-resistant solid tumors and was granted with Fast Track status, as well as Orphan Drug priorities, by the US FDA.Citation9
China will be the second or third largest pharmaceutical market in the world by 2020. Total vaccine market in China is estimated in $9 bn.Citation24,Citation25 China represents one of the largest oncology markets with numerous opportunities for conventional and targeted therapies and limited number of fragmented players. Two cancer oncovirus technologies, such as Gendicine (Shenzhen SiBiono Genetech) and Oncorine (Shanghai Sunway Biotech) were approved although with relatively limited clinical data but successfully commercialized in China indicating that it is possible to achieve commercial success with premium-priced products in emerging markets ().
South Korean model for biotechnology innovation is now becoming a success story to follow. The life sciences industry in Korea consists of nearly 2,000 companies, including 580 pharma companies and 600 biotechnology companies. It was predicted that South Korea’s healthcare market will expand significantly over the next five years mainly due to the aging population. Cancer became a primary cause of morbidity and mortality in South Korea and there is significant shift in focus of R&D efforts in discovery and development of novel oncology treatments.Citation26
In 2006, Korean Government formulated “Bio-Vision 2016” (Year 2006–2016) to acquire competitive source technologies and expand industrial infrastructure. It is intended that South Korean biotechnology sector will propel the country into a position of global leadership, through strengthening of collaboration between Ministry of Health, Korean FDA, health insurance / reimbursement bodies and innovation oversight committees. As a future growth engine industry, Korean market of biotechnology industry is expected to reach $60 billion in 2015.
As a consequence of “Biovision-2016” program, South Korean government has been working very closely with Korean FDA to enhance domestic R&D innovation, manufacturing and commercialization of new biotechnology products, including cell advanced therapies and biosimilar products. The South Korean government has realized that by boosting domestic R&D, increasing investment flow and creating favorable conditions for companies to approve new biotechnology products both in South Korea and globally, it will be possible to drive economic growth, create new businesses and services. For example, South Korean government is aimed to achieve a 22% global biosimilars market share by 2020, and various incentives were enacted in order to support business operations in the sector. Direct foreign investment in free economic zones is encouraged through tax incentives and grants, and biotechnology clusters have been established to foster development of the industry.Citation27 Regulatory mechanisms were enacted to ensure emerging biotechnology industry in South Korea will be immune to hostile takeovers from foreign firms without compromising investment opportunities sourced from foreign capital and various partnership and alliance deals. The Korean Food and Drug Administration set up a series of regulatory pathways around manufacturing and clinical requirements for approval of biotechnology products with minimal data or using abbreviated and conditional routes. Some of these guidelines were inspired by framework of conditional approval established by European Medicines Agency.
The government’s promotion of the biosimilars industry is expected to contribute $2bn to South Korea’s gross domestic product and $1bn to its exports, and to foster the creation of at least five companies that will compete in the global market.Citation28 South Korea has seen several cancer immunotherapy products approved conditionally () and there are numerous products in development phase.
Table 2. Cancer vaccine products approved in South KoreaCitation29
High biotechnology R&D intensity is one of Korea’s strengths. However biotechnology firms still experience lack of some core capabilities such as global development, regulatory, and commercialization expertise. The density of scientists and engineers in Korea is not as high as in other industrialized countries, while Korea’s R&D investment intensity is in the highest rank. Therefore a pool of scientists and experts is still in high demand to achieve sustainable success of Korean R&D. A unique situation of Korean economy contrasts to economic downturn observed in EU and in lesser extent in USA, and related to re-investment of surpluses generated from booming Korean industry into growing biotechnology sector.Citation28 In addition, some elegant and unusual business models were pursued by Korean government in relation to biotechnology sector. For example, various incentives were created for electronic and digital manufacturers such as Samsung, LG and FujiFilm to diversify into biotechnology R&D and specifically into biosimilar sector. These seemingly “non-biotechnology” companies are engaging into biotechnology research for various reasons: synergies in capabilities for cost-efficient development of new products; attractiveness of governmental incentives in tax reliefs, grants and benefits of capturing sustainable growth of biotechnology sector while the remaining industries are still prone to fragile conditions of global economic recovery.
Conclusions
Clinical development of cancer vaccines is long, costly, risky and complicated. The majority of products are currently developed by small and mid-size biotech companies with large pharma keeping an “watchful eye” for new promising and approvable products. Existing R&D innovation models are deemed unsustainable in current “value-for-money” oriented healthcare environment. New business models should be much more open to collaborative, networked and federated styles which could help to outreach global markets and increase cost-efficiencies across an entire value chain. Lessons learned from some developing countries and especially from South Korea illustrate that further growth of cancer vaccine industry will depends not only on new business models but also will heavily rely on regional support and initiatives from different bodies, such as governments, payers and regulatory bodies.
References
- Insights B. The cancer market outlook to 2016, 2011; 1-55. www.business-insights.com.
- Dendreon Corporation PharmaVitae Emerging Biopharma Company Analysis. Datamonitor 2012; 1-86. www.datamonitor.com.
- Market and Product Forecasts. Targeted. Cancer Ther. Datamonitor. 2011; 5-76; www.datamonitor.com.
- Datamonitor. Pipeline Insight: Thereputic Cancer Vaccines, 2009; 1-12. www.datamonitor.com.
- Rodríguez PC, Rodríguez G, González G, Lage A. Clinical development and perspectives of CIMAvax EGF, Cuban vaccine for non-small-cell lung cancer therapy. MEDICC Rev 2010; 12:17 - 23; PMID: 20387330
- Cancer therapy China. Gene Therapy (Gendicine). 2009; Available from: http://www.cancertherapychina.com/index.php?option=com_content&view=article&id=84&Itemid=23 Accessed on April 10, 2012.
- Datamonitor. Stakeholder opinions: Bladder cancer. New drugs needed to replace ineffective 20-year old drugs. 2008; 5-76. www.datamonitor.com.
- Sievert KD, Amend B, Nagele U, Schilling D, Bedke J, Horstmann M, et al. Economic aspects of bladder cancer: what are the benefits and costs?. World J Urol 2009; 27:295 - 300; http://dx.doi.org/10.1007/s00345-009-0395-z; PMID: 19271220
- Gordon EM, Hall FL. Noteworthy clinical case studies in cancer gene therapy: tumor-targeted Rexin-G advances as an efficacious anti-cancer agent. Int J Oncol 2010; 36:1341 - 53; http://dx.doi.org/10.3892/ijo_00000619; PMID: 20428757
- Epeius Biotechnologies. Rexin-G Epeius Biotechnologies. 2009; Available from: http://www.epeiusbiotech.com/oncology-RexinG.asp Accessed on April 6, 2012.
- Shirvill J. Innovations and Opportunities in Therapeutic Vaccines. Business Insights. 2010; 5-68.
- Pammolli F, Magazzini L, Riccaboni M. The productivity crisis in pharmaceutical R&D. Nat Rev Drug Discov 2011; 10:428 - 38; http://dx.doi.org/10.1038/nrd3405; PMID: 21629293
- Adams CP, Brantner VV. Estimating the cost of new drug development: is it really 802 million dollars?. Health Aff (Millwood) 2006; 25:420 - 8; http://dx.doi.org/10.1377/hlthaff.25.2.420; PMID: 16522582
- Adams CP, Brantner VV. Spending on new drug development1. Health Econ 2010; 19:130 - 41; http://dx.doi.org/10.1002/hec.1454; PMID: 19247981
- DiMasi JA, Feldman L, Seckler A, Wilson A. Trends in risks associated with new drug development: success rates for investigational drugs. Clin Pharmacol Ther 2010; 87:272 - 7; http://dx.doi.org/10.1038/clpt.2009.295; PMID: 20130567
- PricewaterhouseCoopers Health Research Institute. Pharma 2020: Virtual R&D. Available from: www.pwc.com/pharma2020. 2008; Accessed on April 11, 2012.
- Schmid EF, Smith DA. Is pharmaceutical R&D just a game of chance or can strategy make a difference?. Drug Discov Today 2004; 9:18 - 26; http://dx.doi.org/10.1016/S1359-6446(04)02951-4; PMID: 14761802
- McKinsey & Company. Invention reinvented. McKinsey perspective on pharmaceutical R&D. 2010; Available from www.mckinsey.com Accessed on April 13, 2012.
- PricewaterhouseCoopers Health Research Institute. Pharma 2020: Challenging business models. 2009; Available from: www.pwc.com/pharma2020. Accessed on April 11, 2012.
- Datamonitor. Stakeholder Opinions: Gene Therapy. Gene therapy to deliver after 20 years of clinical development. 2009; 10-68. www.datamonitor.com
- Müller S, Weigelt J. Open-access public-private partnerships to enable drug discovery--new approaches. IDrugs 2010; 13:175 - 80; PMID: 20191434
- Faigen N. Pervasis acquisition puts Shire in regenerative medicine sweet spot. 2012; Available from: http://www.scripintelligence.com/home/Pervasis-acquisition-puts-Shire-in-regenerative-medicine-sweet-spot-329259 Accessed on April 13, 2012.
- Ernst & Young. Beyond borders: Global biotechnology report. 2011; 4-74. www.ey.com/Publication/vwLUAssets/Global_Biotechnology_Report_2011/$FILE/Biotech_BeyondBorders_2011.pdf
- Datamonitor. Oncology Market Access in China Market bolstered by rising incidence and improved regulatory environment. 2011; 2-61. www.datamonitor.com
- PharmaLive. Oncology Market Soars in China. 2011; Available from: http://pharmalive.com/magazines/medad/view. cfm?articleID=9544 Accessed April 13, 2012.
- Landsdell M. Innovative drug discovery in emerging markets. Business Insights. 2010; 4-51.
- Chung S. Technology Innovation and Economic Growth Korean Experiences. World Bank Workshop, 2005; 3-22.
- World Bank. Korea as a Knowledge Economy: Evolutionary Process and Lessons Learned. The World Bank, Washington D.C. 2006; 2-15.
- KFDA. Recent trends and Korea's cases in the development of gene therapy products KFDA. 2008; Available from: http://www.bioin.or.kr/upload.do?cmd=download&seq=7676&bid=industry. Accessed on April 5, 2012.