Abstract
Immunization is one of the most important public health interventions and a cost effective strategy to control the infectious diseases especially in children. Complete immunization coverage in India has increased from below 20% in the 1980s to nearly 61% at present, but still more than 1/3rd children remain un-immunized. Advent of combination vaccines has facilitated incorporation of additional vaccines into immunization schedule. Pentavalent vaccine, against five killer diseases–diphtheria, pertussis, tetanus, hepatitis B and Hemophilus influenza type B (Hib), has been introduced in almost all GAVI eligible countries by 2011. Government of India introduced the vaccine in two states in pilot phase and has given green signal to six more states. The use of pentavalent vaccine automatically raises the coverage level of hepatitis B and Hib vaccines. If the vaccines are provided individually, the coverage of hepatitis B and Hib vaccines usually lags behind DPT coverage. This gap can be filled by using pentavalent vaccine in routine immunization programmes.
Introduction
In 1974, WHO launched expanded program on immunization (EPI) with the aim of controlling these six childhood diseases: tuberculosis, diphtheria, pertussis, tetanus, polio and measles. Government of India (GoI) in 1978 adapted EPI with slight modification by replacing measles with typhoid. Later on typhoid was discontinued and measles included in the program and renamed as universal immunization program (UIP) in 1985.Citation1
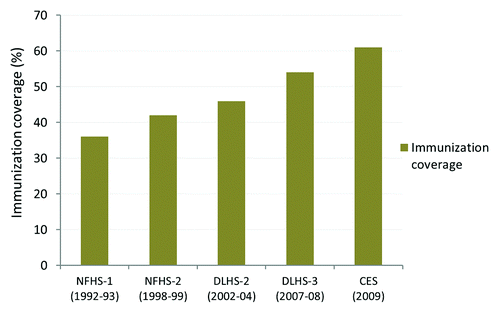
India is a developing country with a population of 1,210 million and high infant and under 5 mortality rate of 47 and 59 per 1,000 live births, respectively.Citation2,Citation3 Immunization is one of the most important public health interventions and a cost effective strategy to control the infectious diseases especially in children. Complete immunization coverage in India has increased from below 20% in the 1980s to nearly 61% at present, but still more than 1/3rd of the children remain un-immunized ().Citation4-Citation9 Immunization coverage for third dose of diphtheria, pertussis and tetanus (DPT3) and hepatitis B (Hep B) vaccine is 71.5 and 58.9% respectively.Citation9 No data regarding Hemophilus influenzae type b (Hib) vaccine coverage is available in the country. US$10–20 is the estimated cost to fully vaccinate a child and further by significantly reducing the total cost of treatment of diseases it offers opportunities for poverty reduction and social and economic development of the country.Citation10
The advent of combination vaccines significantly reduced the volume of immunization activities by decreasing the number of injections, amount of pain and cumulative exposure to preservatives and stabilizers that may contribute to adverse events. In addition, it has facilitated incorporation of additional vaccines into immunization schedule, thereby higher immunization coverage.Citation11,Citation12 Pentavalent vaccines (a combination vaccine which protects against five killer diseases: diphtheria, pertussis, tetanus, hepatitis B and Hib) have been launched in 65 out of 67 GAVI eligible countries by 2011.Citation13,Citation14 The pentavalent vaccines provide a golden opportunity to curb Hib disease and hepatitis B along with diphtheria, pertussis and tetanus in the developing countries.
Disease Burden
During 2010, there were 3,129 reported cases of diphtheria with 177 deaths in India. Pertussis also contributed with 38,493 reported cases and 6 deaths in the same year. There were 1,574 reported cases and 136 deaths due to tetanus other than neonatal tetanus in the country.Citation9 The disease burden of diphtheria, pertussis and tetanus decreased by 85, 95 and 75% respectively in last three decades after introduction of DPT vaccine in India.Citation4,Citation9
Hib disease is a common infectious disease which can lead to serious morbidity and also mortality in pediatric age group. Hepatitis B infection in children results in significant mortality and morbidity later in life.
Hib is a leading cause of bacterial meningitis among infants (357/100,000) and young children (0–4 y; 86/100,000) with case fatality ranging from 20 to 29%. Nearly 30% survivors of Hib meningitis suffer from major disabilities. A study estimated 72,000 deaths per year due to Hib disease among children under five with a case fatality rate of 16% among invasive cases in India.Citation15-Citation21
It has been estimated that, of the 25 million infants born every year in India, over one million run the lifetime risk of developing chronic hepatitis B infection. Moreover, every year > 100,000 Indians die due to illnesses related to hepatitis B infection.Citation22
Immunogenicity, Safety and Efficacy of Pentavalent Vaccine
Numerous immunogenicity studies have been performed in India, and have shown that immunogenicity against each of the vaccine component and reactogenicity is same as that of simultaneous but separate site administration of DPT, Hep B and Hib vaccines.Citation23-Citation27 In a study from India, on post-primary immunization with Serum Institute of India’s (SII) and GSK’s pentavalent vaccine, 100% seroprotection was detected for diphtheria, tetanus, hepatitis B and Hib components in both SII and GSK groups and for pertussis, efficacy was 96.1% in SII group and 95.4% in GSK group.Citation23
The vaccines have excellent tolerability profile with only minor adverse events. All the infants who reported common systemic reactions i.e., fever, irritability and unusual crying, recovered with symptomatic treatment. No vaccine-related neurological, hypersensitivity reactions or other serious adverse events were reported in infants.Citation23-Citation27
Cost Effectiveness
A preliminary cost-effectiveness analysis from India indicated that Hib vaccine introduction into the UIP is highly cost-effective. With conservative burden of disease assumptions and the UNICEF price (2008) of US$3.60 per Hib vaccine dose, this analysis showed that costs per DALY averted is US$254. Based on trends of other vaccines in national programs, prices would be expected to fall significantly from current levels. With a price of US$2 per dose of pentavalent vaccine, the cost per DALY averted will be US$26.Citation28 The SII has announced in June 2011 to sell the vaccine at US$1.75 per dose, it is expected that the other manufacturers will follow suit.Citation29
A cost-effectiveness analysis study in Haryana State for Hib vaccine from 2010 to 2024 concluded that using UNICEF pricing, the incremental cost of Hib vaccine introduction from a government and a societal perspective was estimated to be US$81.4 and US$27.5 million, respectively. Vaccination of 73.3, 71.6 and 67.4 million children with first, second and third dose of pentavalent vaccine, respectively, would avert 7,067,817 cases, 31,331 deaths and 994,564 DALYs. Incremental cost per DALY averted from a government (US$819) and a societal perspective (US$277) was found to be less than the per capita gross national income of India in 2009.Citation30
Progress Toward Implementation
In 2008, National Technical Advisory Group on Immunization recommended the inclusion of pentavalent vaccine in UIP, depending on the availability of vaccines.Citation31 Following the recommendation, the GoI decided to launch Hib containing pentavalent vaccines in ten states. The decision was hailed internationally by public health practitioners as India constitutes 34% of the birth cohort in GAVI-eligible countries and even in the absence of population- based data, the country is estimated to have the highest number of deaths due to Hib in children under 5 y of age.Citation29
After initial controversies, India has introduced pentavalent vaccine in two states (Tamilnadu and Kerala) through routine immunization program in the year 2011. Acknowledging the significance of Hib vaccination, now the GoI has decided to introduce Hib vaccine in UIP as liquid pentavalent vaccine in six states, namely, Gujarat, Haryana, Karnataka, Goa, Jammu, Kashmir and Puducherry from October 2012 to December 2014.Citation32
Scope
The use of pentavalent vaccine automatically raises the coverage level of hepatitis B and Hib vaccines. If the vaccines are provided individually, the coverage of hepatitis B and Hib vaccines usually lags behind DPT coverage. This gap can be filled by using pentavalent vaccine in routine immunization programs. This will help India in combating a large but preventable burden of Hib disease as well as hepatitis B and achieving Millenium Development Goal 4 of reducing child mortality.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Ministry of health and family welfare. Immunization handbook for medical officers. New Delhi: Government of India, 2009.
- Registrar General of India. Provisional Population Totals Paper 1 of 2011 India Series 1. New Delhi: Office of the Registrar General and Census Commissioner of India, 2011.
- Registrar General of India. Sample registration system: measures of fertility and mortality in India. New Delhi: Government of India, 2011.
- Sharma S. Immunization coverage in India. New Delhi: Institute of Economic Growth; 2007. Working paper series No. E/283/2007.
- International Institute of Population Sciences. NFHS-1, 1992-93. Summary of findings. Mumbai;IIPS, 1995.
- International Institute of Population Sciences. ORC Macro. NFHS-2, 1998-99. Summary of findings. Mumbai:IIPS, 2000.
- International Institute for Population Sciences. Reproductive and Child Health. District Level Household Survey (DLHS-2) 2002-04 India. Mumbai:IIPS, 2006.
- International Institute for Population Sciences. District Level Household and Facility Survey 200708. Mumbai:IIPS, 2010.
- UNICEF. Coverage Evaluation Survey, 2009. All India Report. New Delhi: UNICEF, 2010. Available from: http://www.unicef.org/india/National_Fact_Sheet_CES_2009.pdf.
- Shea B, Andersson N, Henry D. Increasing the demand for childhood vaccination in developing countries: a systematic review. BMC Int Health Hum Rights 2009; 9:Suppl 1 S5; http://dx.doi.org/10.1186/1472-698X-9-S1-S5; PMID: 19828063
- Nakayama T, Aizawa C, Kuno-Sakai H. A clinical analysis of gelatin allergy and determination of its causal relationship to the previous administration of gelatin-containing acellular pertussis vaccine combined with diphtheria and tetanus toxoids. J Allergy Clin Immunol 1999; 103:321 - 5; http://dx.doi.org/10.1016/S0091-6749(99)70508-7; PMID: 9949325
- Halsey NA. Limiting infant exposure to thimerosal in vaccines and other sources of mercury. JAMA 1999; 282:1763 - 6; http://dx.doi.org/10.1001/jama.282.18.1763; PMID: 10568650
- Available from. . http://www.gavialliance.org/support/nvs/hib/
- Available from. . http://www.unicef.org/supply/files/8._DTP-containing_vaccines_including_pentavalent_and_hexavalent.pdf
- Minz S, Balraj V, Lalitha MK, Murali N, Cherian T, Manoharan G, et al. Incidence of Haemophilus influenzae type b meningitis in India. Indian J Med Res 2008; 128:57 - 64; PMID: 18820360
- Watt JP, Wolfson LJ, O’Brien KL, Henkle E, Deloria-Knoll M, McCall N, et al, Hib and Pneumococcal Global Burden of Disease Study Team. Burden of disease caused by Haemophilus influenzae type b in children younger than 5 years: global estimates. Lancet 2009; 374:903 - 11; http://dx.doi.org/10.1016/S0140-6736(09)61203-4; PMID: 19748399
- Singhi P, Bansal A, Geeta P, Singhi S. Predictors of long term neurological outcome in bacterial meningitis. Indian J Pediatr 2007; 74:369 - 74; http://dx.doi.org/10.1007/s12098-007-0062-6; PMID: 17476082
- Kabra SK, Kumar P, Verma IC, Mukherjee D, Chowdhary BH, Sengupta S, et al. Bacterial meningitis in India: an IJP survey. Indian J Pediatr 1991; 58:505 - 11; http://dx.doi.org/10.1007/BF02750932; PMID: 1800332
- Chinchankar N, Mane M, Bhave S, Bapat S, Bavdekar A, Pandit A, et al. Diagnosis and outcome of acute bacterial meningitis in early childhood. Indian Pediatr 2002; 39:914 - 21; PMID: 12428036
- Invasive Bacterial Infections Surveillance (IBIS) Group of the International Clinical Epidemiology Network. Are Haemophilus influenzae infections a significant problem in India? A prospective study and review. Clin Infect Dis 2002; 34:949 - 57; http://dx.doi.org/10.1086/339327; PMID: 11880961
- Das BK, Arora NK, Mathur P, Ostwal P, Mandal S, Kabra SK, et al. Nasopharyngeal carriage of Haemophilus influenzae. Indian J Pediatr 2002; 69:775 - 7; http://dx.doi.org/10.1007/BF02723690; PMID: 12420910
- Verma R, Khanna P, Prinja S, Rajput M, Chawla S, Bairwa M. Hepatitis B Vaccine in national immunization schedule: a preventive step in India. Hum Vaccin 2011; 7:1387 - 8; http://dx.doi.org/10.4161/hv.7.12.17878; PMID: 22134433
- Sharma HJ, Yadav S, Lalwani SK, Kapre SV, Jadhav SS, Chakravarty A, et al. Immunogenicity and safety of an indigenously manufactured reconstituted pentavalent (DTwP-HBV+Hib) vaccine in comparison with a foreign competitor following primary and booster immunization in Indian children. Hum Vaccin 2011; 7:451 - 7; http://dx.doi.org/10.4161/hv.7.4.14208; PMID: 21403463
- Bavdekar SB, Maiya PP, Subba Rao SD, Datta SK, Bock HL. Immunogenicity and safety of combined diphtheria tetanus whole cell pertussis hepatitis B/ Haemophilus influenzae type b vaccine in Indian infants. Indian Pediatr 2007; 44:505 - 10; PMID: 17684303
- Ali SS, Chandrashekar SR, Singh M, Bansal RK, Sharma DR, Arora D. A multicenter, prospective, open-label, non-comparative study to evaluate the immunogenicity and tolerance of a new, fully liquid pentavalent vaccine (DTwP-HepB-Hib vaccine). Hum Vaccin 2007; 3:116 - 20; http://dx.doi.org/10.4161/hv.3.4.4061; PMID: 17617743
- Rao R, Dhingra MS, Bavdekar S, Behera N, Daga SR, Dutta AK, et al. A comparison of immunogenicity and safety of indigenously developed liquid (DTwPHB-Hib) pentavalent combination vaccine (Shan 5) with Easyfive (liq) and TritanrixHB + Hiberix (lyo) in Indian infants administered according to the EPI schedule. Hum Vaccin 2009; 5:425 - 9; PMID: 19333002
- Chatterjee S, Rego SJ, D’Souza F, Bhatia BD, Collard A, Datta SK, et al. The immunogenicity and safety of a reduced PRP-content DTPw-HBV/Hib vaccine when administered according to the accelerated EPI schedule. BMC Infect Dis 2010; 10:298; http://dx.doi.org/10.1186/1471-2334-10-298; PMID: 20950457
- Subcommittee on Introduction of Hib Vaccine in Universal Immunization Program, National Technical Advisory Group on Immunization, India. NTAGI subcommittee recommendations on Haemophilus influenzae type B (Hib) vaccine introduction in India. Indian Pediatr 2009; 46:945 - 54; PMID: 19955578
- Nair H, Hazarika I, Patwari A. A roller-coaster ride: Introduction of pentavalent vaccine in India. J Glob Health 2009; 1:32 - 5
- Gupta M, Prinja S, Kumar R, Kaur M. Cost-effectiveness of Haemophilus influenzae type b (Hib) vaccine introduction in the universal immunization schedule in Haryana State, India. Health Policy Plan. 2012 Mar 8. [Epub ahead of print]
- Government of India. Meeting of the National Technical Advisory Group on Immunization 16 June, 2008 – Minutes and Recommendations. New Delhi: Government of India, 2008.
- Press Information Bureau, Government of India. http://pib.nic.in/newsite/erelease.aspx?relid=79191. English Release 17-April 2012.