Abstract
To compare the long-term immunogenicity and seroprotection rates in healthy children following 23 years of vaccination with 10 μg or 20 μg doses of plasma-derived hepatitis B vaccine, we revisited all participants from our previous randomized controlled trial. At year 23, 81 participants were tested for HBV serological markers and HBV-DNA, and a booster dose was given to those with anti-HBs titer < 10 mIU/mL. After eliminating the interference of a Year 11 booster dose and vaccines received outside of the trial, around 50% of participants still maintained anti-HBs titers ≥ 10 mIU/mL in both 10 μg and 20 μg groups (p > 0.05). The peak immune response of vaccination (anti-HBs antibody levels at 12 mo after 1st vaccine dose) and Year 11 anti-HBs levels were significantly associated with Year 23 seroprotection rates. Most of the participants in both groups, regardless of their prior immune status, developed a rapid and robust anamnestic antibody response after the booster dose at year 23. No case of clinically significant HBV infection was observed during the entire study period of 23 y with only one transient HBsAg seroconversion in 10 μg vaccine group. We concluded that seroprotection provided by 10μg or 20 μg doses of hepatitis B vaccine persists for 23 years in more than half of vaccinated individuals in highly HBV-endemic areas, irrespective of 10 μg or 20 μg vaccine doses. Future studies with larger sample sizes comparing long-term efficacy of various doses of plasma-derived and recombinant HBV vaccines are recommended.
Introduction
Hepatitis B virus (HBV) infection is still a major public health problem all over the world.Citation1 Immunization with hepatitis B vaccine is the best way to prevent HBV infection.Citation2,Citation3 The immunogenicity of hepatitis B vaccine has been a subject of intense research ever since HBV vaccine was adopted in most areas of the world.Citation4 Although high levels of seroprotection rates provided by HBV vaccine (both early plasma-derived and current recombinant) have been adequately confirmed, the duration of protection remains largely unknown.Citation5,Citation6 In addition, the high cost of adopting vaccination programs still remains one of the main obstacles for the promotion of mass immunization against HBV infection in most developing countries.
To lower the cost of vaccination programs, researchers have performed several studies to explore the immunogenicity and efficacy of different doses of HBV vaccine in various groups of population.Citation7,Citation8 It has been reported that different doses of vaccine could offer similar levels of seroprotection rates within five years after immunization.Citation9,Citation10 However, participants receiving 20 μg of vaccine have been found to have higher antibody levels than those receiving 10 μg vaccine in some studies.Citation11 Despite several research endeavors, there is very little knowledge about the long-term seroprotection following different doses of vaccine.
For the current study, we recruited participants vaccinated with plasma-derived HBV vaccine during our previous randomized, double-blind, vaccine intervention trial after 23 y of primary vaccination. We aimed to compare the long-term immunogenicity and seroprotection rates for healthy children who had received 10 μg and 20 μg doses of a plasma-derived hepatitis B vaccine during the previous trial. Additionally, we investigated the host and vaccine factors associated with long-term seroprotection (23 y) following plasma-derived HBV vaccination.
Results
Of the 126 participants approached for the current study, 105 (83.3%) completed the questionnaire, and 90 (71.4%) took part in the physical examination along with blood sample collection. From these, 9 participants had received hepatitis B vaccines outside the study and were excluded from further analysis. The remaining 81 participants included 35 and 46 participants who had received 10 μg and 20μg doses of HBV vaccines during the vaccination trial, respectively. None of these participants had reported any liver-associated illnesses experienced during the past 12 y.
Anti-HBs levels at Year 23 after primary vaccination
Seroprotection rates and geometric mean titers (GMTs) for anti-HBs antibody levels for participants at key time points following HBV vaccination (10 μg and 20 μg doses) have been represented in . At Year 23, anti-HBs seroprotection rates for the group who received 10 μg HBV vaccine doses was almost identical to the group who received 20 μg HBV vaccine doses (60.0% (21/35) vs. 58.7% (27/46); χ2 = 1.639, p > 0.05). No statistically significant differences between either of these groups were observed during the entire 23-y study period.Citation23,Citation24 Seroprotection rates and GMTs in 20 μg group were slightly higher than those in 10 μg group at months 7 and 12; however, this was not statistically significant (p > 0.05).
Table 1. Anti-HBs seroprotection rates and GMTs for the original study sample and the current study sample at key time points, stratified by 10 μg and 20 μg vaccine dose groups
Effect of booster dose at Year 11
Participants in each dose group were stratified into 4 subgroups according to their characteristics at Year 11 (). No signifcant differences were observed for seroproection rates between 10 μg and 20 μg vaccine dose groups after this stratification. Of the 20 participants from the 20 μg vaccine dose group who lost their seroprotection and received a booster at Year 11, 8 (40.0%) maintained their anti-HBs titers ≥ 10 mIU/mL at Year 23. Assuming if these 8 participants had not received the Year 11 booster dose and eventually lost all the seroprotective anti-HBs levels by Year 23, the total seroprotection rate at Year 23 would have been 41.3% (19/46). Similarly, a seroprotection rate of 57.1% (20/35) would be assumed for 10 μg vaccine dose group at Year 23, after discounting participants who received the Year 11 booster dose. These seroprotection rates were not significantly different between the two groups (χ2 = 1.997, p = 0.16). In other words, after eliminating the interference of Year 11 booster dose and vaccines outside the study, around 50% of the participants still maintained anti-HBs titers ≥ 10 mIU/mL in both groups.
Table 2. Anti-HBs seroprotection rates and GMTs at Year 23 for10 μg and 20 μg vaccine dose group participants, stratified by participant characteristics at Year 11
Factors associated with Year 23 seroprotection rates
Multivariate logistic regression modeling identified that anti-HBs level at 12 mo after the 1st dose was significantly associated with seroprotection rates at Year 23 (OR = 4.63, 95%CI 1.22–17.52). Another significant factor associated with Year 23 seroprotection rates was anti-HBs level at Year 11(OR = 8.55, 95%CI 2.32–31.58). Sex of the participant and dosage of primary vaccination were not significantly associated with seroprotection rates at Year 23, as shown in . Year 11 booster dose was not significantly associated with Year 23 seroprotection rates as per univariate analysis. Additionally, very few participants had received Year 11 booster dose, so this variable was not entered in the final logistic regression model due to its correlation with Year 11 anti-HBs levels and the small numbers.
Table 3. Factors independently associated with Year 23 seroprotection rates (anti-HBs > 10mIU/mL)
Anamnestic response to booster dose at Year 23
Of all 33 participants who had anti-HBs titers < 10 mIU/ml and were negative for HBsAg, anti-HBc and HBV-DNA at Year 23, 28 (11 in 10 μg group and 17 in 20 μg group) received a booster dose and a subsequent test. The five participants could not be evaluated for anamnestic response owing to reasons such as working in cities far away from Xi’an or refusing to provide blood samples. Seroprotection levels of anti-HBs were observed in 23 participants after 1–2 weeks following after the booster dose: nine 9 (81.8%) and 14 (82.4%) from 10 μg and 20 μg vaccine dose groups, respectively.. No statistically significant difference was observed in the anamnestic response to the booster dose between the two groups (χ2 = 0.001, p = 0.97). Rise in anti-HBs titers before and after the Year 23 booster dose for these 23 participants are shown in . A 4-fold or greater increase in anti-HBs titer was observed in 18 participants (64.3%), including 7 (63.6%) and 11 (64.7%) for 10 μg and 20 μg vaccine dose groups, respectively.
Figure 1. Rise in anti-HBs titers before and after Year 23 booster dose in booster vaccine responsive participants.
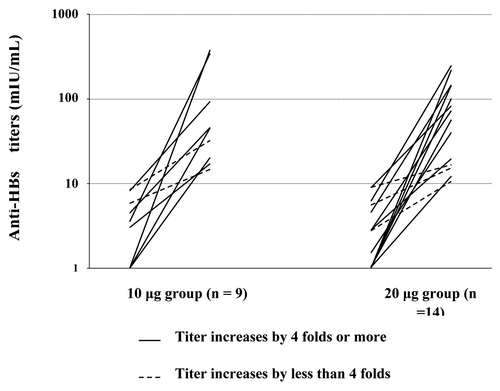
Including 23 participants who responded to the Year 23 booster dose with 48 participants who had anti-HBs levels ≥ 10 mIU/ml at Year 23, there were a total of 71 (87.7%) participants who were seroprotected at the end of the current study. These rates were also identical for 10 μg (85.7% [30/35]) and 20 μg (89.1% [41/46]) vaccine dose groups.
HBV infection rates at Year 23
Ninety participants who participated in the physical examination demonstrated good overall health status with normal liver function tests and normal liver appearance on ultrasonogram. None of them tested positive for HBsAg or HBV-DNA. From the 81 participants who permitted blood sampling, anti-HBc test was positive in 20.0% (7/35) of 10 μg group participants and 13.0% (6/46) of 20 μg group participants; however, this difference was not statistically significant (χ2 = 0.714, p = 0.39). From these 13 anti-HBc positive participants, only three did not develop seroprotective anti-HBs titers. No comparisons between anti-HBc positive and anti-HBc negative subgroups were performed due to small numbers.
Discussion
To our knowledge, this is the longest period of observation that investigated long-term immunogenicity and efficacy of primary vaccinations with different doses of a plasma-derived hepatitis B vaccine in healthy children.Citation12 Our results suggest that both doses of HBV vaccine provided similar and long-term protective immunity. After eliminating the interference of the booster dose at Year 11, participants in both 10 μg and 20 μg vaccine dose groups still maintained reasonable anti-HBs seroprotection rates at Year 23. A vast majority of the participants in both vaccine dose groups with anti-HBs < 10 mIU/mL, regardless of their prior immune status, developed a rapid and robust anti-HBs anamnestic response after a booster dose at Year 23.
Conflicting reports from previous studies comparing the efficacy of HBV vaccine have been observed. Two studies reported that participants receiving 20 μg dosage developed a higher antibody level after a month of last dose of vaccination than those receiving 10 μg dosage.Citation11,Citation13 However, Gilbert et al. observed that vaccination with 10 μg of recombinant HBV vaccine may provide a clinically effective alternative to the standard 20 μg dose in healthy adolescents.Citation14 Our previous vaccine intervention trial also demonstrated that both 10 μg and 20 μg doses could elicit similar protective response lasting for 11 y.Citation15 In the current study, it was confirmed that similar seroprotection rates were achieved that lasted for 23 y. No significant differences between these vaccinated groups were identified during this study. This result was consistent with the findings from previous research studies.Citation16,Citation17
Maintaining seroprotection rates at Year 23 were significantly associated with vaccine response at early stages (i.e., anti-HBs levels at Year 1 and Year 11) as identified by multivariate logistic regression. This can be explained by the possibility that the anti-HBs value peaks at 12 mo after the primary vaccination and thus predicts persistent protective anti-HBs levels for as long as 23 y. This finding was consistent with previous studies reporting that early immune response was highly correlated with subsequent long-term seroprotection rates in healthy children and adolescentsCitation18,Citation19 Additionally, we also observed that 10 μg and 20 μg vaccine doses did not differentially influence the persistence of the protective anti-HBs level.
No acute or chronic HBV infections were identified during 23 y post vaccination. Participants in either groups were free of clinically significant HBV infection during the entire follow-up period. A possible reason may be the relatively small sample size of our study; however, But et. al. also observed similar lack of infections at 22 y after vaccination.Citation19 The prognosis of HBV infection is highly correlated with age.Citation20 The natural history of HBV infection has been confirmed to cause asymptomatic chronic infections in almost all of HBV-infected neonates due to their immature immune systems, whereas, only about 5% of population above 4 y of age remain chronically infected.Citation21 This evidence supports our finding since our study was conducted in children above 5 y of age. From 1986 to 2009, only one subject in the two groups was found to have transient HBsAg seroconversion and a very small fraction of the participants were anti-HBc positive at Year 23. These results suggested that 10 μg and 20 μg doses of hepatitis B vaccine both could equally provide a long-term protection against chronic HBV infection.
One of the potential limitations of the study was its small sample size given the attrition due to a very long-term follow-up. This limitation is encountered by other similar studies aiming to study the vaccine efficacy after almost two decades of primary vaccination. The seroprotection rates reported by these long-term follow-up studies ranged from 34% to 52%.Citation22 We observed that almost 40% of parttictipants remained under the threshold for seroprotective anti-HBs levels after 23 y following vaccination with at least 3 doses. Seroprotection rates reported in our study are consistent with the observed range. During this long follow-up period, there have been changes in the standards for expressing anti-HBs titers from S/N ratio to mIU/ml units, which may make the comparison of anti-HBs levels over time a bit challenging. For the current study, we used same RIA test kits for all study time points and focused on comparison of seroprotection rates between the two vaccine dose groups to avoid this potential bias.
The seroprotection rates after a month of completing vaccination was lower than expected for this population (84% to 90%). We can only speculate this to be an effect of malnutrition, high HBV endemicity or plasma-derived vaccine. However, a recent meta-analysis on long-term protection of various HBV vaccine doses indicated that three shots of HBV vaccine could provide 20 y’ protection against infection for the healthy general population.Citation12 The plasma-derived vaccine used in the current study has been replaced by recombinant in the current vaccination programs. However, it has been suggested that both the plasma-derived and recombinant hepatitis B vaccines offer equal long-term protective immunity.Citation15,Citation20, Hence, the findings from this study provide valuable evidence to the long-term efficacy of plasma-derived vaccinations, irrespective of 10 or 20 μg doses.
In conclusion, the long-term immunogenicity and seroprotection rates in both 10 μg and 20 μg dose plasma-derived hepatitis B vaccine groups were similar during 23 y of follow-up in participants from highly HBV-endemic areas. Participants were protected against HBV infection for 23 y post primary vaccination. The peak immune response of vaccination (anti-HBs antibody levels at 12 mo after 1st vaccine dose) and Year 11 anti-HBs levels were significantly associated with Year 23 seroprotection rates. Future studies with larger sample sizes comparing long-term efficacy of various doses of plasma-derived and recombinant HBV vaccines are recommended.
Materials and Methods
Study population
In 1986, a randomized, double-blind, placebo-controlled trial was conducted with an aim to evaluate the safety, immunogenicity and efficacy of a new plasma-derived hepatitis B vaccine (Lanzhou Institute of Biological Product, China) to prevent HBV infection.Citation23,Citation24 For this trial, 126 children aged 5–9 y received three vaccine doses (0, 1, and 6 mo) by intramuscular route in the deltoid region, including 64 and 62 children receiving 10 μg and 20 μg doses, respectively. During 11 follow-up years from 1986 to 1997, blood samples were collected at 12 study time points to detect hepatitis B surface antigen (HBsAg), antibody to HBsAg (anti-HBs), and antibody to hepatitis B core antigen (anti-HBc). Radioimmunoassay (RIA) was used to detect HBsAg and anti-HBs whereas enzyme-linked immunosorbent assay (ELISA) was used to detect anti-HBc (Beijing North Institute of Biological Technology Kits). For these tests, radioimmunological count per minute (cpm) of sample over mean cpm of negative controls (S/N ratio) was used to indicate anti-HBs antibody level. Seroconversion for HBsAg or anti-HBc were determined according to the instructions from the kit manufacturer. A booster dose of 5 μg recombinant yeast-derived vaccine (Beijing Tiantan Biological Products Company) was given to a majority of participants at the end of this trial due to an anti-HBs S/N ratio < 10. However, no cases of acute or chronic HBV infections were observed duting the entire study period. Receiving HBV vaccine doses outside of the study were not reported by any participant. Detailed protocol for this trial has been described earlier.Citation23,Citation24
After 23 y following the primary vaccination (Year 23), 126 participants were approached again in 2009 for the current study. The current study was approved by the Ethics Committee of the Xi’an Jiaotong University and written informed consent was obtained from all the participants. They were asked to complete a questionnaire to collect information on their socio-demographic characteristics, vaccination experience, and liver-associated illnesses during the 12 y following the end of vaccination trial (from 1997 - 2009). For those who suffered hepatitis B related symptoms during this time period, medical records were checked to collect further information.
A free physical examination and basic laboratory testing such as alanine transaminase tests (ALT), ultrasonogram of the abdomen and electrocardiogram for these participants were conducted in the university-affiliated hospital. Blood samples (3–5 mL per person) were also collected from each participant to detect HBsAg, anti-HBs, anti-HBc, and HBV-DNA. The samples were centrifuged and sera were stored at -70°C until serological testing. Anti-HBs and HBsAg were measured by RIA and anti-HBc was measured by both RIA and ELISA using kits from Beijing Institute of Biological Technology.
Immediately after the test, a booster dose (5 μg recombinant yeast-derived vaccine produced by Beijing Tiantan Biological Products Company) was given to all those who were susceptible to HBV infection. A blood sample was collected 1–2 weeks after the booster for testing anti-HBs anamnestic response. In case of positive HBsAg or HBV DNA tests, an additional blood sample was collected after 3–6 mo to determine chronic HBV infection. The field and laboratory work were accomplished within 6 mo of recruitment.
Definitions
Seroprotection was defined as anti-HBs titer ≥ 10 mIU/mL. HBsAg seroconversion was defined as an S/N ratio ≥ 2.1. Anti-HBc seroconversion was defined if both RIA and ELISA tested positive. A participant was considered susceptible to HBV infection if anti-HBs antibody titer < 10 mIU/mL and negative for HBsAg, HBV-DNA and anti-HBc. An anamnestic response to booster was defined as a 4-fold rise in anti-HBs titer and anti-HBs ≥ 10 mIU/mL following a booster vaccine dose. Chronic HBV infection was determined if either HBsAg or HBV DNA test remained positive even after retesting at 3–6 mo from the initial positive test.
Statistical analysis
Geometric mean titers (GMT) for anti-HBs antibody levels were calculated. Group comparisons for the differences in seroprotection rates were performed by independent-samples t-test for continuous variables and Pearson’s chi-square test or Fisher’s exact test for categorical variables. Multivariate logistic regression analysis was used to identify factors associated with seroprotection rates at at Year 23. Two-tailed P-value of < 0.05 was chosen to indicate statistical significance. All the statistical analyses were performed using the SPSS Version 13.0 software package (SPSS Inc., Chicago, IL, USA).
A multiple logistic regression was performed to identify the factors associated with a seroprotection. The anti-HBs level at Year 23 was defined as the dependent variable (≥ 10 mIU/mL = 1; < 10 mIU/mL = 0). Potential influencing factors such as sex (man = 1; female = 0), dosage of primary vaccination (20 μg = 1; 10 μg = 0), anti-HBs level at 12 mo after 1st vaccine dose (anti-HBs S/N ratio: ≥ median = 1; < median = 0), anti-HBs level at Year 11 (S/N value ≥ 10 = 1; S/N value < 10 = 0) and Year 11 booster dose (received = 1; not received = 0), were defined as independent variables. Model building was done by forward and backward selection methods to identify significant variables associated with seroprotection. Likelihood Ratio test and Wald test were used to assess the fit of the final models.
| Abbreviations: | ||
| HBV | = | hepatitis B virus |
| HBsAg | = | hepatitis B surface antigen |
| Anti-HBs | = | antibody to hepatitis B surface antigen |
| Anti-HBc | = | antibody to hepatitis B core antigen |
| RIA | = | radioimmunoassay |
| ELISA | = | enzyme-linked immunosorbent assay |
| cpm | = | count per minute |
| S/N ratio | = | sample over mean cpm of negative controls |
| GMT | = | geometric mean titer |
| OR | = | odds ratio and CI, confidence interval |
Acknowledgments
The authors gratefully acknowledge all the study participants. We also indebted to the participating nurses and laboratory technician at the study sites and the sponsor’s project staff for their support.
Funding
This research was supported by a grant from the National Natural Science Foundation of China (no. 309725518) and a grant from National T&S Major Project of China (no. 2008ZX1002–001).
Disclosure of Potential Conflicts of Interest
The authors have no conflicts of interests to declare.
References
- Hammitt LL, Hennessy TW, Fiore AE, Zanis C, Hummel KB, Dunaway E, et al. Hepatitis B immunity in children vaccinated with recombinant hepatitis B vaccine beginning at birth: a follow-up study at 15 years. Vaccine 2007; 25:6958 - 64; http://dx.doi.org/10.1016/j.vaccine.2007.06.059; PMID: 17714836
- Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, et al, Taiwan Childhood Hepatoma Study Group. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. N Engl J Med 1997; 336:1855 - 9; http://dx.doi.org/10.1056/NEJM199706263362602; PMID: 9197213
- Lin HH, Wang LY, Hu CT, Huang SC, Huang LC, Lin SS, et al. Decline of hepatitis B carrier rate in vaccinated and unvaccinated subjects: sixteen years after newborn vaccination program in Taiwan. J Med Virol 2003; 69:471 - 4; http://dx.doi.org/10.1002/jmv.10333; PMID: 12601753
- Lu CY, Ni YH, Chiang BL, Chen PJ, Chang MH, Chang LY, et al. Humoral and cellular immune responses to a hepatitis B vaccine booster 15-18 years after neonatal immunization. J Infect Dis 2008; 197:1419 - 26; http://dx.doi.org/10.1086/587695; PMID: 18444799
- van der Sande MA, Waight P, Mendy M, Rayco-Solon P, Hutt P, Fulford T, et al. Long-term protection against carriage of hepatitis B virus after infant vaccination. J Infect Dis 2006; 193:1528 - 35; http://dx.doi.org/10.1086/503433; PMID: 16652281
- Zanetti AR, Van Damme P, Shouval D. The global impact of vaccination against hepatitis B: a historical overview. Vaccine 2008; 26:6266 - 73; http://dx.doi.org/10.1016/j.vaccine.2008.09.056; PMID: 18848855
- Elavia AJ, Marfatia SP, Banker DD. Immunization of hospital personnel with low-dose intradermal hepatitis B vaccine. Vaccine 1994; 12:87 - 90; http://dx.doi.org/10.1016/0264-410X(94)90015-9; PMID: 8303946
- Egemen A, Aksit S, Kurugöl Z, Erensoy S, Bilgiç A, Akilli M. Low-dose intradermal versus intramuscular administration of recombinant hepatitis B vaccine: a comparison of immunogenicity in infants and preschool children. Vaccine 1998; 16:1511 - 5; http://dx.doi.org/10.1016/S0264-410X(98)80006-6; PMID: 9711797
- Duval B, Boulianne N, De Serres G, Laflamme N, De Wals P, Massé R, et al. Comparative immunogenicity under field conditions of two recombinant hepatitis B vaccines in 8-10-year-old children. Vaccine 2000; 18:1467 - 72; http://dx.doi.org/10.1016/S0264-410X(99)00422-3; PMID: 10618544
- Goh KT, Tan KL, Kong KH, Oon CJ, Chan SH. Comparison of the immune response of four different dosages of a yeast-recombinant hepatitis B vaccine in Singapore children: a four-year follow-up study. Bull World Health Organ 1992; 70:233 - 9; PMID: 1600584
- Chiaramonte M, Majori S, Ngatchu T, Moschen ME, Baldo V, Renzulli G, et al. Two different dosages of yeast derived recombinant hepatitis B vaccines: a comparison of immunogenicity. Vaccine 1996; 14:135 - 7; http://dx.doi.org/10.1016/0264-410X(95)00148-T; PMID: 8852410
- Poorolajal J, Mahmoodi M, Majdzadeh R, Nasseri-Moghaddam S, Haghdoost A, Fotouhi A. Long-term protection provided by hepatitis B vaccine and need for booster dose: a meta-analysis. Vaccine 2010; 28:623 - 31; http://dx.doi.org/10.1016/j.vaccine.2009.10.068; PMID: 19887132
- ul-Haq N, Hasnain SS, Umar M, Abbas Z, Valenzuela-Silva C, Lopez-Saura P. Immunogenicity of 10 and 20 microgram hepatitis B vaccine in a two-dose schedule. Vaccine 2003; 21:3179 - 85; http://dx.doi.org/10.1016/S0264-410X(03)00232-9; PMID: 12804846
- Schiff GM, Sherwood JR, Zeldis JB, Krause DS. Comparative study of the immunogenicity and safety of two doses of recombinant hepatitis B vaccine in healthy adolescents. J Adolesc Health 1995; 16:12 - 7; http://dx.doi.org/10.1016/1054-139X(94)00105-N; PMID: 7742331
- Xu HW, Zhuang GH, Wang XL, Chen QJ, Chen ZJ. Efficacy and immune memory of plasmaderived hepatitis B vaccine 11 years after primary immunization. Chin J Prev Med 2000; 34:113 - 5
- Möst J, Larcher C, Vogetseder W, Prodinger WM, Huemer HP, Ebenbichler CF, et al. Recombinant versus plasma-derived hepatitis B vaccine: comparison of immunogenicity in medical students. Vaccine 1992; 10:740 - 1; http://dx.doi.org/10.1016/0264-410X(92)90507-G; PMID: 1308114
- Chiaramonte M, Majori S, Ngatchu T, Moschen ME, Baldo V, Renzulli G, et al. Two different dosages of yeast derived recombinant hepatitis B vaccines: a comparison of immunogenicity. Vaccine 1996; 14:135 - 7; http://dx.doi.org/10.1016/0264-410X(95)00148-T; PMID: 8852410
- McMahon BJ, Dentinger CM, Bruden D, Zanis C, Peters H, Hurlburt D, et al. Antibody levels and protection after hepatitis B vaccine: results of a 22-year follow-up study and response to a booster dose. J Infect Dis 2009; 200:1390 - 6; http://dx.doi.org/10.1086/606119; PMID: 19785526
- But DY, Lai CL, Lim WL, Fung J, Wong DKH, Yuen MF. Twenty-two years follow-up of a prospective randomized trial of hepatitis B vaccines without booster dose in children: final report. Vaccine 2008; 26:6587 - 91; http://dx.doi.org/10.1016/j.vaccine.2008.09.034; PMID: 18835318
- Lai CL, Yuen MF. The natural history of chronic hepatitis B. J Viral Hepat 2007; 14:Suppl 1 6 - 10; PMID: 17958636
- Lee WM. Hepatitis B virus infection. N Engl J Med 1997; 337:1733 - 45; http://dx.doi.org/10.1056/NEJM199712113372406; PMID: 9392700
- Lin YC, Chang MH, Ni YH, Hsu HY, Chen DS. Long-term immunogenicity and efficacy of universal hepatitis B virus vaccination in Taiwan. J Infect Dis 2003; 187:134 - 8; http://dx.doi.org/10.1086/345871; PMID: 12508157
- Xu HW, Li DS, Xu JW, Zhuang GH, Wang XL. [Evaluation of long-term efficacy on plasma-derived hepatitis B vaccine]. Zhonghua Liu Xing Bing Xue Za Zhi 1992; 13:362 - 5; PMID: 1303319
- Wu Q, Zhuang GH, Wang XL, Wang LR, Li N, Zhang M. Antibody levels and immune memory 23 years after primary plasma-derived hepatitis B vaccination: results of a randomized placebo-controlled trial cohort from China where endemicity is high. Vaccine 2011; 29:2302 - 7; http://dx.doi.org/10.1016/j.vaccine.2011.01.025; PMID: 21277403