Abstract
Virus specific, non-neutralizing antibodies such as complement dependent lytic (CDL) antibodies may reduce morbidity following infection through the clearance of infectious virus particles and infected cells. We examined hemagglutination inhibition (HAI), microneutralization (MN) and CDL antibody titers to influenza A H1 and H3 virus strains in 23 healthy young adults who received the 2005–2006 trivalent inactivated influenza vaccine. Post vaccination, we detected statistically significant increases in MN and CDL antibodies but not in HAI antibodies. Statistically significantly higher fold increases in CDL antibodies post vaccination were seen compared with MN and HAI antibodies post vaccination. However, the overall fold increases were modest, likely related to the fact that most of the subjects had received influenza vaccination previously. This study showed that influenza vaccination is not only capable of increasing the level of antibodies that neutralize virus but also antibodies that can cause lysis of infected cells. The biological significance of these CDL antibodies merits further investigation in clinical studies.
Introduction
Current influenza vaccine approaches depend primarily on the induction of antibodies to the viral surface protein hemagglutinin (HA), which are subtype specific and vulnerable to antigenic drift. The hemagglutination inhibition (HAI) test is widely used by vaccine manufacturers and regulatory authorities to determine responses post influenza vaccination because of its correlation with protection as well as its ease of performance and low cost.Citation1 However, virus specific, non-neutralizing antibodies such as complement dependent lytic (CDL) antibodies may also contribute to influenza specific immunity through the clearance of infectious virus particles and infected cells. The binding of these antibodies to viral epitopes (primarily on the HA protein) on the surface of infected cells initiates a cascade mediated by a series of complement proteins resulting in the formation of a membrane attack complex that perforates the cell membrane resulting in the lysis of the infected cell.Citation2,Citation3
Under an IRB approved protocol, 30 healthy subjects were immunized with the licensed 2005–2006 trivalent inactivated influenza vaccine comprised of H1N1 A/New Caledonia/20/99 and H3N2 A/California/07/2004 and the B/Shanghai/361/2002-like virus components. In a previously published report on T cell and MN antibody responses in this group, we showed that Log10 MN antibody titers increased significantly after vaccination in these 30 subjects for the influenza A viruses tested (p < 0.05) although the fold increases were moderate (about 2-fold).Citation4
Given the variability in the collection times for these samples, we decided that further analysis of antibody responses would be limited to a subset of 23 subjects (median age 44.5, range 26–55) whose collection times were more similar. Blood samples were obtained three times: before vaccination, at approximately 2–3 weeks (13–21days) post-vaccination and at approximately 9–10 weeks (63–70 d) post-vaccination. We measured CDL and HAI antibody titers using influenza A virus strains antigenically similar to the 2005–2006 vaccine strains: Influenza A/New Caledonia/20/99 IVR-166 (5.5 × 107 PFU/ml) and A/Wisconsin/67/2005X–161B (2.0 × 108 PFU/ml) vaccine virus strains.
Statistical analysis consisted of geometric mean Log10 comparisons between prevaccination and postvaccination HAI, MN and CDL antibody titers, comparisons of fold increases in antibody titers post vaccination and correlations between these antibody titers using GraphPad Prism software version 5.04 for Windows (GraphPad Software, www.graphpad.com). The ANOVA test was used for prevaccination and post vaccination comparisons of CDL, MN and HAI responses and for comparisons of fold increases between these three antibody assays. If the result of the ANOVA test was significant, then either the paired t test (for comparisons between timepoints) or the unpaired t test (for comparisons of fold increases) was performed. A p value < 0.05 was considered statistically significant.
and show pre and post vaccination MN, HAI, and CDL antibody responses to the A/H1N1 New Caledonia and the A/H3N2 Wisconsin virus for the 23 subjects. Determination of HAI assays were performed using a standard protocol with some modifications.Citation5 Sera were incubated overnight at 37°C with Receptor Destroying Enzyme II (Accurate Chemical and Scientific Corporation), and then heat-inactivated at 56°C for 30 min. Two-fold dilutions of serum from 1:5 to 1:5120 were prepared, an equal volume of standardized antigen (4 HA units) was added and incubated for 20 min at room temperature, after which an equal volume of 0.5% turkey red blood cells (Bio Link Inc.) was added and then incubated for 45 min at room temperature. HAI titer was defined as the highest serum dilution which completely prevented hemagglutination. Serum samples were available for testing in only 22/23 subjects.
Figure 1. Serum HAI, MN and CDL A/ New Caledonia antibody responses following receipt of influenza vaccine. The mean Log 10 HAI, MN, and CDL titers for the 23 subjects in this study are shown before vaccination, at 2–3 weeks and at 9–10 weeks after vaccination. MN data presented here is a subset of data previously published.Citation4 X axis represents the prevaccination and post vaccination timepoints tested and the Y axis represents the mean log10 antibody titer. Statistically significant (p < 0.05) increases between prevaccination to either of the post vaccination timepoints are denoted by * and were calculated using paired t test.
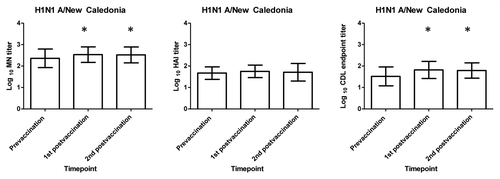
Figure 2. H3N2 A/ Wisconsin serum HAI, MN and CDL antibody responses following receipt of influenza vaccine. The mean Log 10 HAI, MN, and CDL HAI, MN, and CDL titers for the 23 subjects in this study are shown before vaccination, at 2–3 weeks and at 9–10 weeks after vaccination. MN data presented here is a subset of data previously publishedCitation4 X axis represent the prevaccination and post vaccination timepoints tested and the Y axis represents the mean log10 antibody titer. Statistically significant (p < 0.05) increases between prevaccination to either of the post vaccination timepoints are denoted by * and were calculated using paired t test.
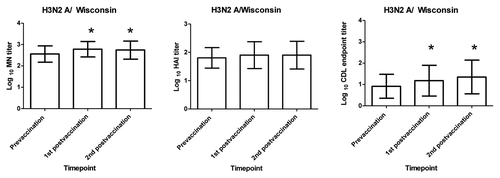
In general, MN titers were higher than HAI titers at all timepoints tested. Prevaccination HAI and MN titers of ≥ 40 to the A/H1N1 New Caledonia and the A/H3N2 Wisconsin virus were seen in the majority of subjects which was not surprising given that most of these subjects had previously been immunized. We found modest but still statistically significant increases in Log10 MN but not HAI antibodies titers from the prevaccination to each of the post vaccination timepoints (paired t test p < 0.05) for the influenza A viruses tested (, and ).
Table 1. Geometric mean (95% CI) of log10 antibody titers against the influenza viral strains at baseline, 2–3 weeks post and 9–10 weeks post vaccination
We modified a previously developed assay in order to measure CDL responses in these subjects.Citation3,Citation6A549 cells, a human lung cancer cell line (type II alveolar epithelial cells), were infected at multiplicities of infection of 1–10 with the H1N1 A/New Caledonia/20/99 and the H3N2 A/Wisconsin/67/2005X–161B viruses and used as target cells. We tested heat inactivated sera at 4 fold dilutions (1:8–1:512) in the presence of Low-Tox® Guinea Pig Complement (GPC) (Cedarlane Laboratories),and lysis of influenza virus -infected target cells was calculated. Percent specific immune lysis (SIL) was calculated as the [(% lysis by Ab + complement) – (% lysis by complement only) / (% maximum lysis) – (% lysis by complement only)]. Using these values, we determined the serum dilution at which the addition of complement would no longer mediate cytotoxicity of target cells in the presence of the tested serum. The highest serum dilution at which 50% or greater of the peak specific immune lysis (SIL) was seen was defined as the endpoint titer.
In general, CDL endpoint titers were lower than MN titers but comparable to HAI titers (). CDL antibody responses were lower to the H3N2 A/Wisconsin virus compared with the A/ H1N1/ New Caledonia virus. A contributing factor may be a higher level of background lysis of influenza A H3N2 virus-infected target cells by Low-Tox® GPC alone compared with influenza A H1N1 or influenza B virus-infected cells (data not shown). GPC alone can cause lysis of target cells infected with some H3N2 virus strains (data not shown), and this lytic activity has been shown to be inhibited by the addition of mannose or anti-mannose binding lectin (MBL) antibody.Citation7 When we added mannose with GPC to H3N2 infected cells, SIL of H3N2-infected target cells approached the level of H1N1-infected target cells (data not shown). Despite this background lysis of GPC of H3N2 infected targets, statistically significant increases in CDL antibody titers were seen between the prevaccination and each of the post vaccination timepoints for both influenza viruses tested (paired t test p < 0.0.05) ( and ; ).
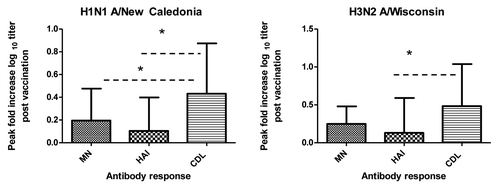
Our study examined the pattern of antibody responses in influenza vaccinated adults using three different assays: HAI assays, that measure antibodies that bind near the HA receptor site and block the interaction of HA with sialic acid residues on erythrocytes and inhibit their agglutination, which has been used as a common method to evaluate immunogenicity of influenza vaccines, MN assays which detect functional virus neutralizing antibodies and CDL assays which detect antibodies that are capable of lysing influenza infected cells. Assays for neutralizing antibodies have been found to be more sensitive than for HAI both in the detection of low level of antibodies and in the diagnosis of influenza infections.Citation6,Citation8 Statistically significant differences in fold increases were seen when comparing HAI and CDL responses for both viruses (unpaired t test p < 0.05) and when comparing MN and CDL responses for the H1N1 virus (unpaired t test p < 0.05) ().This was not surprising given the fact that HAI, MN and CDL antibody assays do not measure the same antibody. We also found only moderate though statistically positive correlations (Spearman’s rank correlation r = 0.56–0.59) (data not shown) between HAI and CDL titers as well as between MN and CDL titers for the influenza A/H3N2 virus and weak correlations (Spearman’s rank correlation r = 0.36–0.42) for the influenza A/H1N1influenza virus tested (data not shown).
Figure 3. Comparison of HAI, MN and CDL endpoint dilution fold increases post vaccination. Peak fold increases in log10 HAI and MN titers and CDL endpoint dilution antibody titers were calculated from the prevaccination timepoint to the peak post vaccination timepoint for the H1N1 A/New Caledonia virus and the H3N2 A/Wisconsin virus. X axis represents the antibody assay tested and the Y axis represents the fold increase in the log10 antibody response. Statistically significant (p < 0.05) differences in fold antibody titers post vaccination are denoted by * and were calculated using unpaired t test.
The role of complement dependent lysis has been investigated in such viral systems as HIV, hepatitis B virus and herpes virus.Citation9-Citation12 Depending upon whether or not heterologous sera is used as a source of complement, studies have reported a dependence on the alternate pathway for antibody and complement dependent lysis and a negligible role for the classical pathway.Citation10,Citation13,Citation14 The main mechanism of clearance of virus by CDL antibodies is through the lysis of infected cells before maximal replication and release of infectious virus particles are generated. CDL antibodies can also neutralize free virus particles released from infected cells which are then removed by phagocytosis.Citation15,Citation16
Though there have been numerous human studies on the MN and HAI responses induced by influenza vaccines, there has, in contrast, only been one vaccine study that has measured CDL responses. In a small clinical influenza vaccine study conducted by Quinnan et al., after the 1977 H1N1 widespread outbreak, 10 subjects received a monovalent A/USSR/90/77 (H1N1) vaccine followed by trivalent A/USSR/77, A/Texas/77 and B/Hong Kong vaccine four weeks later. Antibody titers were determined by HAI, neutralization and CDL antibody assays. Similar to what we found, CDL antibody titers were statistically significant higher than HAI titers. Though subjects who were enrolled were unlikely to have previously experienced H1N1 influenza viruses, prevaccination sera from 3/10 volunteers showed low levels of CDL activity against target cells infected with A/USSR/90/77 (H1N1), suggesting the presence of pre-existing subtype-cross-reactive CDL antibodies. In addition, all volunteers developed a subtype specific response to the monovalent A/USSR/90/77 (H1N1) vaccine post vaccination.Citation6
The potential for cross reactive responses seen in vitro by human monoclonal CDL antibodies is of great interest given the critical issues of antigenic drift in which small changes in the HA and the neuraminadase protein can reduce the efficacy of seasonal influenza vaccines and antigenic shift in which completely new virus strains emerge as in the recent 2009 H1N1 influenza pandemic.Citation17,Citation18 Using human monoclonal antibodies cloned from plasmablasts obtained from patients infected with the 2009 pandemic H1N1 strain and from healthy adults vaccinated with seasonal influenza vaccine,Citation19,Citation20 we found that HA stalk- specific monoclonal antibodies that do not inhibit hemagglutination could neutralize influenza virus particles and eliminate virus-infected cells through CDL.Citation15 Two of these three stalk-specific neutralizing MAbs tested were found to be more cross-reactive to temporally distant H1N1 strains than the conventional hemagglutination-inhibiting and neutralizing MAbs; one of the stalk-specific MABs was found to be subtype cross-reactive to H1 and H2 hemagglutinins. These studies demonstrated that CDL antibodies can mediate subtype cross reactive responses to the HA protein. In addition, studies using human and murine monoclonal antibodies suggest that influenza virus proteins neuraminadase, matrix protein 2 (M2) and nucleoprotein (NP) may be targets in addition to the HA protein for these CDL antibodies.Citation3,Citation21,Citation22 Because NP and the M2 proteins are more conserved than the HA and NA among influenza A viruses,Citation23 some anti-influenza antibodies which can mediate the CDL are expected to be subtype cross-reactive.
The major limitation of this study was the heterogeneity of the study population in terms of history of natural infection and influenza vaccination. All but one of the subjects had received influenza vaccination previously; unvaccinated subjects were not included as a control group. Though it has been shown that the receipt of repeated vaccinations does not inhibit the development of influenza antibody responses,Citation24 many studies have shown that preexisting antibodies to a particular antigen limit the level of antibody production upon subsequent immunization.Citation4,Citation25 Therefore, it was not surprising that even though statistically significant increases in HAI and CDL responses were seen, fold increases post vaccination were low. This heterogeneity in terms of personal immunological history to influenza may have limited our ability to make proper comparisons between the results of these various antibody assays. Likewise, the seroconversion rate, defined as percentage of subjects with either a pre vaccination HI titer < 1:10 and a post vaccination titer > 1:40 or a minimum 4-fold rise in post vaccination titer was also low, seen in less than 20 percent of the subjects studied (data not shown). This means that in terms of criteria used by vaccine authorities to evaluate the immunogenicity of a vaccine, this vaccine underperformed. However, a recent study showed that in a partially seropositive population, using serological markers such as seroconversion can underestimate vaccination immunogenicity.Citation26 Finally, another limitation of this study was the variation in responses using different virus strains. Though the CDL assay has not undergone the same level of standardization testing as the HAI and MN assays, the use of the CDL assay in other viral systems has been described as highly reproducible.Citation11 We have done preliminary testing to qualify this assay using sera from several healthy subjects, testing twice a day on four separate days and found that that the variability in responses became more stable with specific lysis values > 20%. When looking at variability by serum dilution tested, variability was highest at the lowest dilution (1:8) and lower but similar at the higher dilutions (data not shown)
Understanding antibody responses following influenza vaccination is important due to the large number of subjects who are immunized each year in order to protect against influenza illness. In this study, we demonstrated statistically significant increases in CDL antibodies post vaccination with higher fold increases in these antibodies post vaccination compared with HAI or MN antibodies. Future studies will need to address the specificity (subtype specific or cross reactive) of these antibodies. The biological role that these CDL antibodies may play in protection against influenza illness needs to be studied in subjects followed prospectively with analyses of pre epidemic antibody levels and the observation of influenza illness in order to determine whether or not CDL, HAI and MN antibodies may correlate with protection in some individuals.
| Abbreviations: | ||
| MN | = | microneutralization |
| HAI | = | hemagglutination inhibition |
| CDL | = | complement dependent lytic |
| HA | = | hemagglutinin |
| GPC | = | guinea pig complement |
| NP | = | nucleoprotein |
| M2 | = | matrix protein 2 |
| SIL | = | specific immune lysis |
| MAB | = | monoclonal antibody |
Acknowledgments
We thank Dr. Sanjay Ram for useful advice and discussion in establishing the CDL assay. We thank Dr. Alexander Klimov of Centers for Disease Control and Prevention (CDC) for providing us with Influenza virus A/Solomon Islands/3/06 (H1N1). We thank Drs Robert Ryall and Michel De Wilde of Sanofi-Pastuer for the gifts of influenza A/New Caledonia/20/99 and A/Wisconsin/67/2005viruses. We thank Dr. George Reed, Dr. Qin Liu and Christine Foley for their input on the statistical methods used for analysis.
References
- de Jong JC, Palache AM, Beyer WE, Rimmelzwaan GF, Boon AC, Osterhaus AD. Haemagglutination-inhibiting antibody to influenza virus. Dev Biol (Basel) 2003; 115:63 - 73; PMID: 15088777
- Natsume A, Niwa R, Satoh M. Improving effector functions of antibodies for cancer treatment: Enhancing ADCC and CDC. Drug Des Devel Ther 2009; 3:7 - 16; PMID: 19920917
- Verbonitz MW, Ennis FA, Hicks JT, Albrecht P. Hemagglutinin-specific complement-dependent cytolytic antibody response to influenza infection. J Exp Med 1978; 147:265 - 70; http://dx.doi.org/10.1084/jem.147.1.265; PMID: 627837
- Co MD, Orphin L, Cruz J, Pazoles P, Rothman AL, Ennis FA, et al. Discordance between antibody and T cell responses in recipients of trivalent inactivated influenza vaccine. Vaccine 2008; 26:1990 - 8; http://dx.doi.org/10.1016/j.vaccine.2008.02.024; PMID: 18339461
- Szretter KJ, Balish AL, Katz JM. Influenza: propagation, quantification, and storage. Curr Protoc Microbiol 2006; Chapter 15:Unit 15G 1.
- Quinnan GV, Ennis FA, Tuazon CU, Wells MA, Butchko GM, Armstrong R, et al. Cytotoxic lymphocytes and antibody-dependent complement-mediated cytotoxicity induced by administration of influenza vaccine. Infect Immun 1980; 30:362 - 9; PMID: 7439982
- Reading PC, Hartley CA, Ezekowitz RA, Anders EM. A serum mannose-binding lectin mediates complement-dependent lysis of influenza virus-infected cells. Biochem Biophys Res Commun 1995; 217:1128 - 36; http://dx.doi.org/10.1006/bbrc.1995.2886; PMID: 8554567
- Papenburg J, Baz M, Hamelin MÈ, Rhéaume C, Carbonneau J, Ouakki M, et al. Evaluation of serological diagnostic methods for the 2009 pandemic influenza A (H1N1) virus. Clin Vaccine Immunol 2011; 18:520 - 2; http://dx.doi.org/10.1128/CVI.00449-10; PMID: 21228145
- Huber M, Trkola A. Humoral immunity to HIV-1: neutralization and beyond. J Intern Med 2007; 262:5 - 25; http://dx.doi.org/10.1111/j.1365-2796.2007.01819.x; PMID: 17598812
- Hicks JT, Ennis FA, Kim E, Verbonitz M. The importance of an intact complement pathway in recovery from a primary viral infection: influenza in decomplemented and in C5-deficient mice. J Immunol 1978; 121:1437 - 45; PMID: 701803
- Hicks JT, Klutch MJ, Albrecht P, Frank MM. Analysis of complement-dependent antibody-mediated lysis of target cells acutely infected with measles. J Immunol 1976; 117:208 - 15; PMID: 819582
- Qu Z, Liang X, Liu Y, Du J, Liu S, Sun W. Hepatitis B virus sensitizes hepatocytes to complement-dependent cytotoxicity through downregulating CD59. Mol Immunol 2009; 47:283 - 9; http://dx.doi.org/10.1016/j.molimm.2009.09.022; PMID: 19804910
- Sissons JG, Cooper NR, Oldstone MB. Alternative complement pathway-mediated lysis of measles virus infected cells: induction by IgG antibody bound to individual viral glycoproteins and comparative efficacy of F(ab’)2 and Fab’ fragments. J Immunol 1979; 123:2144 - 9; PMID: 489977
- Patrick Sissons JG, Schreiber RD, Perrin LH, Cooper NR, Müller-Eberhard HJ, Oldstone MB. Lysis of measles virus-infected cells by the purified cytolytic alternative complement pathway and antibody. J Exp Med 1979; 150:445 - 54; http://dx.doi.org/10.1084/jem.150.3.445; PMID: 479760
- Terajima M, Cruz J, Co MD, Lee JH, Kaur K, Wrammert J, et al. Complement-dependent lysis of influenza a virus-infected cells by broadly cross-reactive human monoclonal antibodies. J Virol 2011; 85:13463 - 7; http://dx.doi.org/10.1128/JVI.05193-11; PMID: 21994454
- Mozdzanowska K, Feng J, Eid M, Zharikova D, Gerhard W. Enhancement of neutralizing activity of influenza virus-specific antibodies by serum components. Virology 2006; 352:418 - 26; http://dx.doi.org/10.1016/j.virol.2006.05.008; PMID: 16777168
- Banzhoff A, Pellegrini M, Del Giudice G, Fragapane E, Groth N, Podda A. MF59-adjuvanted vaccines for seasonal and pandemic influenza prophylaxis. Influenza Other Respir Viruses 2008; 2:243 - 9; http://dx.doi.org/10.1111/j.1750-2659.2008.00059.x; PMID: 19453401
- Palese P. Influenza: old and new threats. Nat Med 2004; 10:Suppl S82 - 7; http://dx.doi.org/10.1038/nm1141; PMID: 15577936
- Wrammert J, Koutsonanos D, Li GM, Edupuganti S, Sui J, Morrissey M, et al. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J Exp Med 2011; 208:181 - 93; http://dx.doi.org/10.1084/jem.20101352; PMID: 21220454
- Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature 2008; 453:667 - 71; http://dx.doi.org/10.1038/nature06890; PMID: 18449194
- Yewdell JW, Bennink JR, Smith GL, Moss B. Influenza A virus nucleoprotein is a major target antigen for cross-reactive anti-influenza A virus cytotoxic T lymphocytes. Proc Natl Acad Sci USA 1985; 82:1785 - 9; http://dx.doi.org/10.1073/pnas.82.6.1785; PMID: 3872457
- Grandea AG 3rd, Olsen OA, Cox TC, Renshaw M, Hammond PW, Chan-Hui PY, et al. Human antibodies reveal a protective epitope that is highly conserved among human and nonhuman influenza A viruses. Proc Natl Acad Sci USA 2010; 107:12658 - 63; http://dx.doi.org/10.1073/pnas.0911806107; PMID: 20615945
- Palese P, Shaw M. Orthomyxoviridae: The Viruses and Their Replication. In: David M. Knipe PMH, ed. Fields' virology Philadelphia: Lippincott Williams & Wilkins 2007:1655,722-723.
- Gardner EM, Bernstein ED, Dran S, Munk G, Gross P, Abrutyn E, et al. Characterization of antibody responses to annual influenza vaccination over four years in a healthy elderly population. Vaccine 2001; 19:4610 - 7; http://dx.doi.org/10.1016/S0264-410X(01)00246-8; PMID: 11535308
- Hirota Y, Kaji M, Ide S, Goto S, Oka T. The hemagglutination inhibition antibody responses to an inactivated influenza vaccine among healthy adults: with special reference to the prevaccination antibody and its interaction with age. Vaccine 1996; 14:1597 - 602; http://dx.doi.org/10.1016/S0264-410X(96)00153-3; PMID: 9032887
- Beyer WE, Palache AM, Lüchters G, Nauta J, Osterhaus AD. Seroprotection rate, mean fold increase, seroconversion rate: which parameter adequately expresses seroresponse to influenza vaccination?. Virus Res 2004; 103:125 - 32; http://dx.doi.org/10.1016/j.virusres.2004.02.024; PMID: 15163500