Abstract
Objective: This study assessed the immunogenicity, long-term persistence of immune response and safety of a single dose of an A/California/07/2009 H1N1 pandemic influenza vaccine adjuvanted with AS03 (α-tocopherol and squalene based oil-in-water emulsion Adjuvant System) in subjects ≥ 65 y of age (NCT01114620).
Results: At Day 21, the HI immune response met all three European guidance criteria [seroconversion rate (SCR): 60.0%; seroprotection rate (SPR): 64.0%; geometric mean fold rise (GMFR): 10.2] and the US guidance criterion for SCR. At month 6, the HI immune response against the A/California/07/2009 H1N1 strain persisted but at levels lower than that observed at Day 21 (SCR: 38.8%; SPR: 42.9%; HI antibody geometric mean titer: 27.6); the European regulatory guidance criteria for SCR and GMFR were still met. Overall, the vaccine was well-tolerated.
Conclusion
A single dose of the 3.75µg HA AS03-adjuvanted H1N1 2009 pandemic vaccine induced immune responses against the vaccine strain that met the European regulatory guidance criteria at day 21 in the elderly Japanese population; the immune response persisted at lower levels at month 6. No safety concerns were identified. These results suggest that two vaccine doses might be useful for the elderly population to improve antibody induction and persistence.
Methods: In this open-label, single group study, 50 subjects received one dose of the 3.75 µg hemagglutinin (HA) AS03-adjuvanted H1N1 2009 vaccine. Immunogenicity assessments were made before vaccination, 21 days and six months after vaccination using hemagglutination inhibition (HI) and microneutralization assays. Immunogenicity end points were based on US and European regulatory criteria.
Introduction
The influenza pandemic of 2009 caused by the novel, swine-origin influenza A H1N1 2009 reaffirmed the unpredictable nature of influenza viruses.Citation1 This triple re-assortant influenza virus was characterized by a unique combination of genes from both North American and Eurasian swine lineages hitherto unidentified in human or swine populations.Citation1,Citation2 Due to its genetic divergence from the circulating seasonal H1N1 influenza viruses, the existing seasonal influenza vaccines were thought unlikely to confer protection against the influenza A H1N1 2009 virus.Citation1,Citation3,Citation4
The Center for Disease Control and Prevention (CDC) estimates indicated that contrary to the epidemiological pattern observed for seasonal influenza which is associated with high morbidity and mortality in the elderly, the number of influenza A H1N1 2009 cases and associated hospitalizations in the elderly population ≥ 65 y in the US were lower than that reported in adults and children; however, influenza A H1N1 2009 related complications led to a high mortality rate in those ≥ 65 y of age, which although lower than that in younger adults, was comparable to that in children.Citation5 The trends in the epidemiology of the H1N1 2009 pandemic in Japan were similar to these observations, where despite the infection rate in those ≥ 60 y of age being lower compared with that in the younger age groups, the case-fatality rate was relatively high among elderly adults, especially those with underlying medical conditions.Citation6,Citation7
Immunization is considered to be the most effective prophylactic approach to mitigate pandemic influenza associated illness and death.Citation8-Citation10 In order to effectively combat the H1N1 2009 influenza pandemic, the World Health Organization (WHO) endorsed the use of adjuvanted pandemic influenza vaccines in parallel with unadjuvanted vaccines to meet the vaccine dose requirements and to confer cross-reactive immunity.Citation11,Citation12 Consequently, a number of H1N1 2009 pandemic influenza vaccines at different doses with or without adjuvants were developed.Citation13 Two meta-analyses of trials evaluating these vaccines in various age groups have reported that the adjuvanted vaccines elicited higher immune responses than the non-adjuvanted vaccines and did not give rise to any safety concerns.Citation14,Citation15
Based on the experience of developing a pre-pandemic H5N1 influenza vaccine with an AS03 Adjuvant System (an Adjuvant System containing α-tocopherol and squalene in an oil-in-water emulsion) which was found to be highly immunogenic and well-tolerated in different populations,Citation16-Citation18 an AS03-adjuvanted H1N1 2009 pandemic influenza vaccine with 3.75µg hemagglutinin (HA) content was developed. This vaccine has been shown to be highly immunogenic (fulfilling the US and European regulatory guidance criteria for pandemic influenza vaccines) with a clinically acceptable safety and reactogenicity profile in adults.Citation19,Citation20 A previous study in Japan reported similar results following a single dose of the AS03-adjuvanted 3.75µg HA H1N1 2009 vaccine in adults 20−64 y of age.Citation21
As a post-licensure commitment to the Japanese health authorities [Ministry of Health, Labor and Welfare (MHLW)] for granting exceptional approval for the AS03-adjuvanted H1N1 2009 vaccine, this study was conducted to evaluate the immunogenicity and safety of the vaccine in older adults ≥ 65 y of age. The objective of the study was to demonstrate that a single dose of this H1N1 2009 vaccine could induce a humoral immune response in terms of H1N1 hemagglutination inhibition (HI) antibody titers meeting the US and European regulatory guidance criteria. In addition, the study also assessed the persistence of this immune response at Month 6 and the safety of the vaccine in this age group.
Results
Study population
The study was conducted between May 2010 and November 2010. All 50 subjects who were enrolled received one dose of the H1N1 2009 vaccine [Total vaccinated cohort (TVC)] and completed the study up to month 6; all subjects were included in the according-to-protocol (ATP) cohort for immunogenicity at day 21. One subject was excluded from the ATP cohort for immunogenicity at month 6 due to protocol violations. This 69 y-old male subject had received an investigational treatment for diabetes during the study; however, this protocol violation remained unreported until data validation for the month 6 time point, resulting in the inclusion of this subject in the ATP cohort for the day 21 analyses.
The mean age of subjects at the time of vaccination was 69.8 y (range: 65 to 80 y). The male to female ratio was 58.0%:42.0% and all subjects were of Japanese heritage.
Immunogenicity
HI immune response
Before vaccination, 24.0% of subjects were seropositive for HI antibodies against the vaccine homologous strain and the corresponding HI antibody geometric mean titer (GMT) was 6.6. Twenty-one days after vaccination, the seropositivity rate increased to 96.0% and the GMT to 67.2. All three Committee for Medicinal Products for Human Use (CHMP) criteria [seroprotection rate (SPR), seroconversion rate (SCR), geometric mean fold rise (GMFR)] for pandemic influenza vaccines in older adults were met (64.0%, 60.0% and 10.2, respectively). In addition, the Center for Biologics Evaluation and Research (CBER) criterion for SCR was met but not the criterion for the percentage of subjects with HI antibody titers ≥ 1:40 ().
Table 1. Immune response in terms of HI antibodies against the vaccine homologous A/California/7/2009 strain at all evaluation time points (ATP cohort for immunogenicity)
Six months from the first vaccine dose (month 6), the HI immune response against the H1N1 2009 strain persisted but at levels lower than that observed at day 21. Seropositivity rates persisted at 81.6%, with the corresponding HI antibody GMT of 27.6. The CHMP guidance criteria for SCR and GMFR were still met (38.8% and 4.2, respectively). The two CBER criteria were unmet at month 6. As the samples from month 6 and the samples from days 0 and 21 were tested by HI assay at different times, the potential variation of biologic assays over time must be taken into account.
Microneutralization assay
Before vaccination, 38.0% of subjects had seropositive levels of neutralizing antibodies against the A/Netherlands/602/09 strain. Twenty-one days after vaccination (day 21), the seropositivity rate rose to 82.0%, with a vaccine response rate (VRR) of 40.0%. Six months later (month 6), persistence of neutralizing antibody response against the A/Netherlands/602/09 strain was evident—seropositivity rate of 83.7% and VRR of 30.6%. The neutralizing antibody GMTs are presented in .
Table 2. Immune response in terms of neutralizing antibodies against the A/Netherlands/602/09 strain [antigenically homologous to the vaccine strain] at all evaluation time points (ATP cohort for immunogenicity)
Safety and reactogenicity
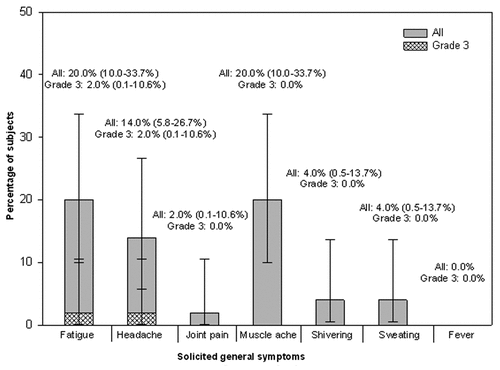
During the 7-day period following vaccination, at least one solicited or unsolicited adverse event assessed by the investigator to be vaccination-related was reported for 74.0% of the subjects (local: 70.0%; general: 38.0%). Pain at the injection site (reported for 66.0% of subjects) and fatigue and muscle ache (both reported for 20.0% of subjects) were the most frequently reported solicited local and general adverse events, respectively during the 7-day post-vaccination follow-up period. None of the subjects reported any solicited local adverse events of Grade 3 intensity. Fatigue and headache of Grade 3 intensity occurring on day 4 following vaccination was reported in 2.0% of subjects (one subject for each symptom) ( and ).
Figure 1. Percentage of subjects reporting solicited local adverse events during the 7-d post-vaccination follow-up period (Total vaccinated cohort).
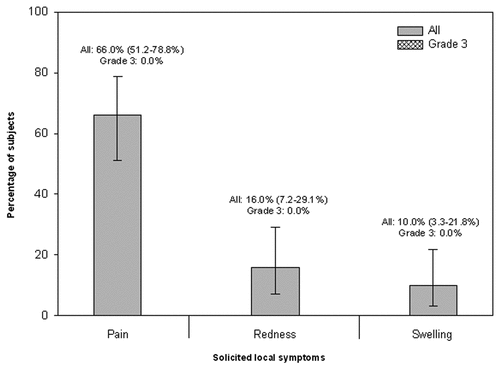
Figure 2. Percentage of subjects reporting solicited general adverse events during the 7-d post-vaccination follow-up period (Total vaccinated cohort).
Ten subjects (20.0%) reported at least one unsolicited adverse event during the 42-day post-vaccination follow-up period. One unsolicited adverse event, upper respiratory tract inflammation of Grade 2 intensity was considered by the investigator to be causally related to vaccination. A total of seven subjects (14.0%) experienced at least one medically attended adverse event (MAE) during the 42-day post-vaccination follow-up period.
Overall, serious adverse events (SAEs) were reported for four subjects (8.0%). A male subject 77 years of age reported relapsed liver cancer 27 days after vaccination (original diagnosis was in 2005, but stage and other details are not known) and another male subject 80 years of age was diagnosed with multiple myeloma 33 days after vaccination. Both subjects required hospitalization and the events remained unresolved at study conclusion. Two other subjects required hospitalization for the treatment of cataract and vertigo (onset 118 and 84 days after vaccination, respectively); both events resolved before study conclusion. None of these serious adverse events were considered by the investigator to be related to vaccination. No potential immune-mediated diseases (pIMDs) were identified.
Discussion
The number of doses of H1N1 2009 pandemic influenza vaccine required to induce an optimal immune response in the elderly adult population (a population at lower risk for H1N1 disease)Citation5 continues to be a topic of discussion. In this assessment of an AS03-adjuvanted H1N1 2009 pandemic influenza vaccine, a single dose induced strong HI immune responses 21 days later that met all three CHMP criteria for pandemic influenza vaccines in elderly adults; the CBER criterion for SCR was also met at day 21 but not the criterion for the percentage of subjects with HI antibody titers ≥ 1:40. In a study conducted in the UK, where 71 elderly subjects ≥ 65 years of age were enrolled in a larger study, a two dose regimen with an AS03-adjuvanted 3.75 µg HA formulated as split virion vaccine (n = 37) or unadjuvanted 7.5 µg HA formulated as whole-virion vaccine (n = 34) was recommended for elderly adults as European regulatory criteria were not met in this age stratum following one dose of 3.75 μg HA adjuvanted with AS03.Citation22 A study in the US in which 257 subjects ≥ 65 years of age received the AS03-adjuvanted 3.75 µg HA vaccine, 21 days following a single vaccine dose, European and US regulatory criteria were met.Citation23 In another study in the ≥ 65 years population, a single dose of unadjuvanted 7.5 µg HA vaccine also induced potentially protective immune responses.Citation24
The HI antibody response in the elderly study population in the present study persisted to six months after vaccination though at lower levels than at day 21, as evident from the high seropositivity rates (81.6%). The CHMP criteria for SCR and GMFR were still met at month 6. This is in agreement with observations made in two separate studies in the US and UK, which reported that in subjects ≥ 65 years of age two doses of the AS03-adjuvanted 3.75 µg HA H1N1) 2009 vaccine were necessary to induce long-term persistence of HI antibody.Citation22,Citation23
The evidence for an effective immune response induced by this AS03-adjuvanted H1N1 2009 vaccine in the elderly population is not well-established in Japan. However, data for Japanese adults under age 65 years is available from studies conducted just before the mass vaccination programs with adjuvanted and non-adjuvanted formulations of H1N1 2009 vaccine.Citation21,Citation25 These studies reported that the immune response after two doses of AS03-adjuvanted 3.75 µg HA H1N1 2009 vaccine in Japanese adults was much higher than that observed after two doses of non-adjuvanted 15 µg HA H1N1 2009 vaccine, although the immune response after a single dose of either the adjuvanted or the non-adjuvanted formulations was comparable. The above studies suggested that two doses of AS03-adjuvanted vaccine may improve the persistence of immune response in the elderly Japanese population.
The vaccine induced a strong neutralizing antibody response in the elderly Japanese population in the present study, as evident from the VRRs at day 21. The neutralizing antibody response decreased at month 6, similar to HI antibody persistence, as the VRR at month 6 was lower than that at day 21, as was the observation for the SPR and SCR for HI antibody response at month 6.
Overall, no safety concerns related to vaccination were identified in this study population ≥ 65 years of age. The majority of the solicited symptoms reported were of mild nature (< 2.0% were of Grade 3 intensity). A comparable observation was made in a previous study in Japanese adults 20−64 years of age using a similar vaccine.Citation21 The incidence of solicited adverse events, especially fever in the present study population of elderly adults was lower than that observed in previous studies in younger adults and children.Citation21,Citation28,Citation29 In a previous study in children 6 months to 10 years of age, following two doses of an AS03-adjuvanted H1N1 pandemic influenza vaccine, the HI antibody titers were found to be the highest in children with fever ≥ 38.0₫C, which indicated a possible correlation between the immunogenicity and reactogenicity profiles of the vaccine.Citation30 In contrast, post hoc exploratory analyses in this study indicated that there was no apparent correlation between GMT (for HI or neutralizing antibodies) and injection site pain (data not shown).
This study had certain drawbacks. First, it was a single-center study and the sample size was modest, which could be a plausible reason for not meeting the CBER regulatory guidance criteria following a single dose of the study vaccine. However, this relatively small sample size was proposed in the absence of a suitable reference study in the elderly Japanese population and taking into consideration the commitment to provide timely clinical evidence on the H1N1 2009 vaccine to the regulatory agencies at the time of the ensuing pandemic. And second, in the absence of a non-adjuvanted control group, no direct comparisons of immunogenicity or reactogenicity may be made between adjuvanted and non-adjuvanted vaccine formulations.
Since 2001, mass vaccination programs in Japan focused on two target groups – the ≥ 65 year-old population and those 60−64 years of age with chronic disorders of heart, kidney and lung.Citation31 In this context, long-term persistence data in the elderly population from this study will complement existing literature on immune response immediately following primary vaccination.
Conclusion
The data from this study showed that a single dose of the AS03-adjuvanted 3.75 µg HA H1N1 2009 pandemic influenza vaccine induced HI antibody response against the vaccine homologous A/California/7/2009 strain in subjects ≥ 65 yars of age, that met the European regulatory guidance criteria for pandemic influenza vaccines. The HI immune response persisted at lower levels compared with day 21, six months after vaccination. Similar trends were observed for the neutralizing antibody response against the vaccine strain at both time points. Overall, the vaccine did not give rise to any safety concerns in this study population.
Materials and methods
Study design and subjects
This phase IV, open-label, single center study (NCT01114620) trial took place in the context of fulfilling a post-licensure commitment to Japanese health authorities. Older adults ≥ 65 years of age residing in Japan and without a history of previous receipt of a pandemic H1N1 vaccine or any investigational or non-registered medicinal product within 30 days of study start or diagnosed with cancer or under treatment for cancer for three years preceding study start were enrolled to receive a single dose of a monovalent AS03-adjuvanted 3.75 µg HA A/California/7/2009 pandemic influenza vaccine. Serum samples were collected before vaccination and seven days after vaccination for hematological and biochemical assessments. For immunological assessments, serum samples were those collected before vaccination, 21 days (day 21) and six months after vaccination (month 6). Telephone contact was made with vaccinated subjects 42 days and 84 days after vaccination to collect information on the safety profile of the vaccine.
Written informed consent was obtained from all subjects prior to conducting any study-related procedures. The study was conducted in accordance with the Good Clinical Practice guidelines, the Declaration of Helsinki and local regulations. All study-related documents were approved by an Institutional Review Board.
Study vaccine
The H1N1 2009 pandemic influenza vaccine was a monovalent, inactivated, split-virion antigen adjuvanted with AS03A (Arepanrix™, a trademark of GlaxoSmithKline group of companies). The H1N1 viral seed for the vaccine was prepared from the reassortant virus NYMC X-179A (New York Medical College) generated from the A/California/07/2009 strain, as recommended by the WHO.Citation11 AS03A is an Adjuvant System containing α-tocopherol and squalene in an oil-in-water emulsion (squalene 10.69 mg, DL- α-tocopherol 11.86 mg and polysorbate 80 4.86 mg). The antigen and Adjuvant System were made available in multi-dose vials, which were mixed before vaccination. The vaccine was administered into the deltoid muscle.
Immunogenicity assessments
Serum samples collected before vaccination, 21 days after the vaccine dose (day 21) and six months later (month 6) were tested at GSK Biologicals Central Laboratory using a validated in-house hemagglutination inhibition (HI) assay [cut-off: ≥ 1:10] that used chicken erythrocytes as described previously.Citation32
The serum samples collected at all time points were tested using a viral microneutralization assay as previously described,Citation4,Citation33 and using a strain that is antigenically similar to the vaccine strain (A/Netherlands/602/09) at Viroclinics Biosciences (Rotterdam).Citation34 The neutralizing antibody titers were expressed as the reciprocal of the highest dilution achieving at least 50% neutralization of viral growth [neutralizing dose 50 (ND50)] by applying the Reed and Muench method,Citation35 which gave the cut-off value of 1:8.
The primary immunological assessments were based on the geometric mean titers (GMTs), seroprotection rate (SPR; percentage of subjects with HI antibody titers ≥ 1:40), seroconversion rate (SCR; percentage of subjects with either a pre-vaccination HI antibody titers ≤ 1:10 and post-vaccination titers ≥ 1:40 or a pre-vaccination HI antibody titers ≥ 1:10 and at least a 4-fold increase in post-vaccination HI antibody titers) and geometric mean fold rise (GMFR; fold increase in post-vaccination HI antibody GMTs compared with pre-vaccination) in terms of HI antibodies and on the vaccine response rates (VRRs; percentage of subjects with at least a 4-fold increase in post-vaccination neutralizing antibody titers compared with pre-vaccination) in terms of neutralizing antibodies against the vaccine homologous strain.
The immunological outcomes were assessed in terms of the Center for Biologics Evaluation and Research [CBER; lower limit of the 95% confidence interval (CI) for HI antibody SCR: ≥ 40% and SPR: ≥ 70%] and Committee for Medicinal Products for Human Use (CHMP; point estimates for HI antibody SCR: > 40%, SPR: > 70% and GMFR: > 2.5) guidance criteria for pandemic influenza vaccines.Citation36,Citation37
Safety and reactogenicity assessments
Diary cards were used to record the solicited local and general adverse events occurring within 7 dayd following vaccination, the unsolicited adverse events and medically-attended adverse events (MAEs) occurring within 42 days following vaccination; potential immune-mediated diseases (pIMD) and serious adverse events (SAEs) were recorded during the entire study period up to month 6. The intensity of all solicited adverse events except fever was graded on a standard scale of (0–3), Grade 1 being those that did not interfere with normal activities and Grade 3 being those that prevented normal activities (Grade 3 redness and swelling: diameter > 100 mm); fever was graded on a 0−4 scale, Grade 3 being axillary temperatures ≥ 39.0- ≤ 40.0°C and Grade 4 being axillary temperatures > 40.0°C.
Statistical analyses
A sample size of 50 subjects accounting for a 10.0% drop-out rate (45 evaluable subjects) gave 99.9% power to meet the primary objective to fulfil the CBER and CHMP criteria. The reference values for power calculation were chosen based on the results of the most recent study [NCT00985088] using a similar AS03-adjuvanted 3.75µg HA H1N1 2009 vaccine (SCR = 70.0%, SPR = 88.0% and GMFR = 8.0).
The analyses of immunogenicity were performed on the according-to-protocol (ATP) cohort for immunogenicity which included subjects who received vaccination as per protocol, complied with all protocol-define procedures and for whom the immunological results (both HI and neutralizing antibody) were available at the given time points (day 21 and month 6); the analyses of safety were performed on the total vaccinated cohort (TVC) which included all subjects with documented vaccination.
[Author: please cite ref# Citation26, Citation27 in text]
| Abbreviations: | ||
| ATP | = | according-to-protocol |
| CBER | = | Center for Biologics Evaluation & Research |
| CDC | = | Centers for Disease Control and Prevention |
| CHMP | = | Committee for Medicinal Products for Human Use |
| CI | = | confidence interval |
| GMFR | = | geometric mean fold rise |
| GMT | = | geometric mean titre |
| HA | = | hemagglutinin |
| HI | = | hemagglutination inhibition |
| MAE | = | medically-attended adverse event |
| MHLW | = | Ministry of Health, Labour and Welfare |
| pIMD | = | potential immune-mediated disease |
| SAE | = | serious adverse event |
| SCR | = | seroconversion rate |
| SPR | = | seroprotection rate |
| TVC | = | total vaccinated cohort |
| VRR | = | vaccine response rate |
| WHO | = | World Health Organization |
Financial Disclosure Statement
GlaxoSmithKline Biologicals was the funding source and was involved in all stages of the study conduct and analysis (ClinicalTrials.gov Identifier: NCT01114620). GlaxoSmithKline Biologicals also took in charge all costs associated with the development and the publishing of the present manuscript.
Trademark Statement
Arepanrix is a trademark of the GlaxoSmithKline group of companies.
Acknowledgments
All authors participated in the implementation of the study including substantial contributions to conception and design, the gathering of the data, or analysis and interpretation of the data. All authors were involved in the drafting of the article or revising it critically for important intellectual content, and final approval of the manuscript. We are grateful to the New York Medical College, New York for providing the vaccine virus reassortant and to the National Institute for Biological Standards and Control (NIBSC, UK) and Therapeutic Goods Administration (TGA) from the Australian Government for providing the reference standards. The authors are indebted to the participating study volunteers, clinicians, nurses and laboratory technicians at the study sites as well as to the sponsor’s project staff for their support and contributions throughout the study. In particular, we thank Yasunobu Kawakami. We are grateful to all teams of GSK Biologicals for their contribution to this study, especially Hiroshi Tamura, Shinobu Tamura, Yoko Nakagawa and Kenji Ishizuka for clinical study management and site monitoring, Veronique Grosjean and Ophélie Gascard for database management, Roger Bernhard and Urban Lundberg from the clinical and serological laboratory teams, Dorothy Slavin (Clinical Safety Representative) and Kimberly Cerenze for project management. Finally the authors thank Dr. Karl Walravens for critical review of the manuscript, Avishek Pal (GSK Biologicals) for providing medical writing services and Dr. Geraldine Drevon (GSK Biologicals) for editorial assistance and manuscript coordination. All authors had full access to the data and the corresponding author had final responsibility to submit for publication.
Disclosure of Potential Conflicts of Interest
Dr H.I. was the principal investigator. All participating institutions received compensation for study involvement. Drs K.T., A.M., D.V. and P.L. are employees of GlaxoSmithKline Biologicals.
References
- Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, et al, Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. [Erratum in: N Engl J Med 2009; 361:102] N Engl J Med 2009; 360:2605 - 15; http://dx.doi.org/10.1056/NEJMoa0903810; PMID: 19423869
- Garten RJ, Davis CT, Russell CA, Shu B, Lindstrom S, Balish A, et al. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science 2009; 325:197 - 201; http://dx.doi.org/10.1126/science.1176225; PMID: 19465683
- Katz J, Hancock K, Veguilla V, Zhong W, Lu XH, Sun H, et al, Centers for Disease Control and Prevention (CDC). Serum cross-reactive antibody response to a novel influenza A (H1N1) virus after vaccination with seasonal influenza vaccine. MMWR Morb Mortal Wkly Rep 2009; 58:521 - 4; PMID: 19478718
- Hancock K, Veguilla V, Lu X, Zhong W, Butler EN, Sun H, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med 2009; 361:1945 - 52; http://dx.doi.org/10.1056/NEJMoa0906453; PMID: 19745214
- Centers for Disease Control and Prevention (CDC). Updated estimates of 2009 H1N1 influenza cases, hospitalizations and deaths in the United States, April 2009–April 10, 2010. Available at: http://www.cdc.gov/h1n1flu/estimates_2009_h1n1.htm. Accessed on February 16, 2012.
- Wada K, Nishiura H, Kawana A. An epidemiological analysis of severe cases of the influenza A (H1N1) 2009 virus infection in Japan. Influenza Other Respi Viruses 2010; 4:179 - 86; http://dx.doi.org/10.1111/j.1750-2659.2010.00143.x; PMID: 20836793
- Ministry of Health. Labour and Welfare (MHLW); Press Release (August 11, 2010). Available at: http://www.mhlw.go.jp/kinkyu/kenkou/influenza/houdou/2010/08/dl/infuh0811-01.pdf. Accessed on February 16, 2012. Japanese.
- Clark TW, Pareek M, Hoschler K, Dillon H, Nicholson KG, Groth N, et al. Trial of 2009 influenza A (H1N1) monovalent MF59-adjuvanted vaccine. N Engl J Med 2009; 361:2424 - 35; http://dx.doi.org/10.1056/NEJMoa0907650; PMID: 19745215
- Liang XF, Wang HQ, Wang JZ, Fang HH, Wu J, Zhu FC, et al. Safety and immunogenicity of 2009 pandemic influenza A H1N1 vaccines in China: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet 2010; 375:56 - 66; http://dx.doi.org/10.1016/S0140-6736(09)62003-1; PMID: 20018364
- Vajo Z, Tamas F, Sinka L, Jankovics I. Safety and immunogenicity of a 2009 pandemic influenza A H1N1 vaccine when administered alone or simultaneously with the seasonal influenza vaccine for the 2009-10 influenza season: a multicentre, randomised controlled trial. Lancet 2010; 375:49 - 55; http://dx.doi.org/10.1016/S0140-6736(09)62039-0; PMID: 20018367
- World Health Organization (WHO). Global Alert and Response (GAR). Pandemic influenza A (H1N1) 2009 virus vaccine – conclusions and recommendations from the October 2009 meeting of the immunization Strategic Advisory Group of Experts, December 04, 2009. Available at: http://www.who.int/csr/disease/swineflu/meetings/sage_oct_2009/en/. Accessed on February 16, 2012.
- World Health Organization (WHO). WHO recommendations on pandemic (H1N1) 2009 vaccines. Pandemic (H1N1) 2009: briefing note 2. Available at: http://www.who.int/csr/disease/swineflu/notes/h1n1_vaccine_20090713/en/index.html. Accessed on February 16, 2012.
- World Health Organization (WHO). Global Advisory Committee on Vaccine Safety, 16-17 June 2010. Wkly Epidemiol Rec 2010; 85:285 - 92
- Manzoli L, De Vito C, Salanti G, D’Addario M, Villari P, Ioannidis JPA. Meta-analysis of the immunogenicity and tolerability of pandemic influenza A 2009 (H1N1) vaccines. PLoS One 2011; 6:e24384; http://dx.doi.org/10.1371/journal.pone.0024384; PMID: 21915319
- Yin JK, Khandaker G, Rashid H, Heron L, Ridda I, Booy R. Immunogenicity and safety of pandemic influenza A (H1N1) 2009 vaccine: systematic review and meta-analysis. Influenza Other Respi Viruses 2011; 5:299 - 305; http://dx.doi.org/10.1111/j.1750-2659.2011.00229.x; PMID: 21668694
- Chu DWS, Hwang SJ, Lim FS, Oh HML, Thongcharoen P, Yang PC, et al, H5N1 Flu Study Group for Hong Kong, Singapore, Taiwan and Thailand. Immunogenicity and tolerability of an AS03(A)-adjuvanted prepandemic influenza vaccine: a phase III study in a large population of Asian adults. Vaccine 2009; 27:7428 - 35; http://dx.doi.org/10.1016/j.vaccine.2009.07.102; PMID: 19683087
- Nagai H, Ikematsu H, Tenjinbaru K, Maeda A, Dramé M, Roman FP. A phase II, open-label, multicentre study to evaluate the immunogenicity and safety of an adjuvanted prepandemic (H5N1) influenza vaccine in healthy Japanese adults. BMC Infect Dis 2010; 10:338; http://dx.doi.org/10.1186/1471-2334-10-338; PMID: 21108818
- Leroux-Roels I, Bernhard R, Gérard P, Dramé M, Hanon E, Leroux-Roels G. Broad Clade 2 cross-reactive immunity induced by an adjuvanted clade 1 rH5N1 pandemic influenza vaccine. PLoS One 2008; 3:e1665; http://dx.doi.org/10.1371/journal.pone.0001665; PMID: 18301743
- Roman F, Vaman T, Gerlach B, Markendorf A, Gillard P, Devaster JM. Immunogenicity and safety in adults of one dose of influenza A H1N1v 2009 vaccine formulated with and without AS03A-adjuvant: preliminary report of an observer-blind, randomised trial. Vaccine 2010; 28:1740 - 5; http://dx.doi.org/10.1016/j.vaccine.2009.12.014; PMID: 20034605
- Roman F, Clément F, Dewé W, Walravens K, Maes C, Willekens J, et al. Effect on cellular and humoral immune responses of the AS03 adjuvant system in an A/H1N1/2009 influenza virus vaccine administered to adults during two randomized controlled trials. Clin Vaccine Immunol 2011; 18:835 - 43; http://dx.doi.org/10.1128/CVI.00480-10; PMID: 21450978
- Ikematsu H, Nagai H, Kawashima M, Kawakami Y, Tenjinbaru K, Maeda A, et al. Immunogenicity and safety of a novel AS03(A)-adjuvanted H1N1 2009 pandemic influenza vaccine in adults in Japan. Hum Vaccin 2010; 6:888 - 93; http://dx.doi.org/10.4161/hv.6.11.12851; PMID: 20980795
- Nicholson KG, Abrams KR, Batham S, Clark TW, Hoschler K, Lim WS, et al. Immunogenicity and safety of a two-dose schedule of whole-virion and AS03A-adjuvanted 2009 influenza A (H1N1) vaccines: a randomised, multicentre, age-stratified, head-to-head trial. Lancet Infect Dis 2011; 11:91 - 101; http://dx.doi.org/10.1016/S1473-3099(10)70296-6; PMID: 21168369
- Ferguson M, Risi G, Davis M, Sheldon E, Baron M, Li P, et al. Safety and long-term humoral immune response in adults after vaccination with an H1N1 2009 pandemic influenza vaccine with or without AS03 adjuvant. J Infect Dis 2012; 205:733 - 44; http://dx.doi.org/10.1093/infdis/jir641; PMID: 22315336
- Talaat KR, Greenberg ME, Lai MH, Hartel GF, Wichems CH, Rockman S, et al. A single dose of unadjuvanted novel 2009 H1N1 vaccine is immunogenic and well tolerated in young and elderly adults. J Infect Dis 2010; 202:1327 - 37; http://dx.doi.org/10.1086/656601; PMID: 20874515
- Ihara T. Evaluation of alum-adjuvanted whole virus influenza vaccine and future aspects of influenza A (H1N1) 2009 vaccine. Uirusu 2010; 60:69 - 78; http://www.flutrackers.com/forum/showthread.php?t=152740 http://dx.doi.org/10.2222/jsv.60.69; PMID: 20848866
- Nolan T, McVernon J, Skeljo M, Richmond P, Wadia U, Lambert S, et al. Immunogenicity of a monovalent 2009 influenza A(H1N1) vaccine in infants and children: a randomized trial. JAMA 2010; 303:37 - 46; http://dx.doi.org/10.1001/jama.2009.1911; PMID: 20026597
- Nolan T, Richmond PC, Formica NT, Höschler K, Skeljo MV, Stoney T, et al. Safety and immunogenicity of a prototype adjuvanted inactivated split-virus influenza A (H5N1) vaccine in infants and children. Vaccine 2008; 26:6383 - 91; http://dx.doi.org/10.1016/j.vaccine.2008.08.046; PMID: 18801398
- Leroux-Roels G. Prepandemic H5N1 influenza vaccine adjuvanted with AS03: a review of the pre-clinical and clinical data. Expert Opin Biol Ther 2009; 9:1057 - 71; http://dx.doi.org/10.1517/14712590903066695; PMID: 19555313
- Saitoh A, Tamura S, Nagai A, Tsuchida N, Sako M, Maekawa T, et al. Clinical evaluation of an AS03-adjuvanted pandemic influenza H1N1 2009 vaccine in children (Preliminary report). [Japanese.] J Jap Pediatr Soc. 2011; 115:578 - 84
- Andrews NJ, Walker WT, Finn A, Heath PT, Pollard AJ, Snape MD, et al. Predictor of immune response and reactogenicity to AS03B- adjyuvanted split virion and non-adjuvanted whole virion H1N1. Vaccine 2011; 29:7913 - 9; http://dx.doi.org/10.1016/j.vaccine.2011.08.076; PMID: 21875635
- Hirota Y, Kaji M. History of influenza vaccination programs in Japan. Vaccine 2008; 26:6451 - 4; http://dx.doi.org/10.1016/j.vaccine.2008.06.042; PMID: 18573292
- Hehme NW, Künzel W, Petschke F, Gisela T, Carmen R, Christian Van H, et al. Ten years of experience with the trivalent split-influenza vaccine, Fluarix™. Clin Drug Investig 2002; 22:751 - 69; http://dx.doi.org/10.2165/00044011-200222110-00004
- Leroux-Roels I, Borkowski A, Vanwolleghem T, Dramé M, Clement F, Hons E, et al. Antigen sparing and cross-reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine: a randomised controlled trial. Lancet 2007; 370:580 - 9; http://dx.doi.org/10.1016/S0140-6736(07)61297-5; PMID: 17707753
- Baras B, de Waal L, Stittelaar KJ, Jacob V, Giannini S, Kroeze EJ, et al. Pandemic H1N1 vaccine requires the use of an adjuvant to protect against challenge in naïve ferrets. Vaccine 2011; 29:2120 - 6; http://dx.doi.org/10.1016/j.vaccine.2010.12.125; PMID: 21238573
- Reed LT, Muench H. A simple method of calculating fifty percent end point. Am J Hyg 1938; 27:493 - 8
- European Committee for Proprietary Medicinal Products (CHMP). Guideline on influenza vaccine prepared from viruses with the potential to cause a pandemic and intended for use outside of the core dossier context (EMEA/CHMP/VWP/263499/2006). European Agency for the Evaluation of Medicinal Products, January 24, 2007. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003872.pdf
- US Food and Drug Administration. (FDA) Guidance for Industry. Clinical data needed to support the licensure of pandemic influenza vaccines. US Food and Drug Administration May 2007. Available at: http://www.fda.gov/cber/gdlns/panfluvac.htm