Abstract
The efficacy and effectiveness of influenza vaccines depend primarily on the vaccine recipient and the virus similarity to the endemic virus. Regulatory T cells (Tregs) and cytokines are known to restrict immune responses against viral infections. We conducted this study to explore the role of Tregs, cytokines, and antibody production after influenza vaccination. The whole blood was collected from healthy subjects (n = 36) before and two weeks after influenza vaccine immunization for two or three consecutive years. The cell surface markers, intracellular staining of Foxp3+ Tregs, and Th1/Th2 cytokines were determined. The antibody titer was detected using the hemagglutination inhibition test. The CD3+, CD127+, CD4+CD25+ and CD4+Foxp3+cells were increased significantly post vaccination. The plasma level of the transforming growth factor (TGF-β), but not interleukin (IL)-2, IL-4, IL-5, IL-10, IFN-γ, TNF-α, was also found to increase significantly after vaccination. We further correlated the cytokine fold-increases with the anti-influenza antibody titer for individual post vaccination. It was found that the IL-10 level after vaccination correlated with the fold-increases of anti-H1N1, anti-H3N2, anti-B/Yamagata, and anti-B/Victoria antibodies. But, a negative relationship occurs between the TGF-β level and fold-increases of anti-H1N1, anti-H3N2, anti-B/Yamagata, and anti-B/Victoria antibodies post vaccination. Treg cells and TGF-β seem to participate in the downregulation of the anti-influenza antibody response post influenza vaccination. Alteration of Treg activity might enhance influenza vaccine antibody responses and efficacy.
Introduction
Influenza viruses belong to the Orthomyxoviridae family and are the major cause of respiratory disease in humans. Three influenza types/subtypes circulate in the population, A/H3N2, A/H1N1 and B.Citation1 Influenza infections in the elderly and young children can lead to secondary bacterial infections that result in severe symptoms and occasional death.Citation2 A highly pathogenic avian influenza virus, the H5N1 strain, has caused outbreaks of disease in domestic poultry in Asian countries.Citation3 Furthermore, a novel influenza virus, 2009 A/H1N1 pandemic virus, emerged from the animal reservoir of influenza viruses and became transmissible among humans.Citation4
Anti-influenza viral immunity is a complex process involving both of innate and adaptive immunity. The degree of immunity within an influenza virus subtype is mainly dependent on previous exposure to natural infection, the individual’s immune status, and the immunity developed to the annual influenza vaccination.Citation5 Vaccination represents the most cost-effective and efficient defense against virus-induced diseases. Trivalent inactivated vaccines (TIV) contain strains of influenza viruses that are antigenically equivalent to the annually recommended strains: one influenza A (H3N2) virus, one influenza A (H1N1) virus, and one influenza B virus. Current immunization strategy relies heavily on the induction of strain-specific serologic immunity by TIV that must be redesigned and produced annually to reflect circulating strains.Citation6,Citation7 Studies of the immune response to influenza vaccination and infection are often limited to measures of antibody titers.
Regulatory T cells (Treg) play key roles in the maintenance of lymphoid homeostasis in a number of immune circumstances. The so-called ‘natural’ CD4+CD25+ Tregs arise as a distinct lineage from the thymus in mice and humans.Citation8 Regulatory function can also be acquired by uncommitted, CD4+ T cells under particular conditions of antigenic stimulation. These so-called ‘induced’ Tregs are likewise heterogeneous. A firm molecular definition for these cells came about with the discovery that they express the forkhead-winged helix transcription factor Foxp3. In humans, regulatory activity is mostly confined to the CD4+CD25high subset.Citation8,Citation9 Interleukine (IL)-7 plays an essential role in the development and maintenance of T lymphocytes. The biological effects of IL-7 are mediated via the hematopoietic IL-7 receptor (IL-7R) complex, a heterodimer of an IL-7 receptor α (CD127) chain.Citation10 CD127 expression has proven crucial during thymocyte maturation and has been suggested to be a crucial step for effector or memory differentiation. It is commonly shared by many cytokines including IL-2, IL-4, IL-9, IL-15 and IL-21.Citation11
Cytokines are key regulators of the immune system. They are essential in shaping the innate and adaptive immune responses, as well as for the establishment and maintenance of immunological memory. Vaccines aimed at establishing long lasting immunity should manipulate the cytokine milieu to induce the appropriate immune effector mechanisms for each particular pathogen, and to establish a large pool of long-lived memory cells.Citation11 The incorporation of cytokines as molecular adjuvants in vaccines has been attempted to strengthen vaccine-induced immune responses, and as a rational approach to modulate cytokine milieu in vivo and tailor host immunity for specific situations.Citation12
Various cytokines might exert different effects on Treg suppression, thereby contributing to tuning the magnitude of suppression.Citation13 Adaptive Treg cells include Foxp3+ cells that develop extrathymically and share most phenotypic and functional features of natural Treg cells, as well as Foxp3- cells that seem to exert their regulatory activity mainly by a cell contact-independent,Citation14 cytokine-dependent mechanism that involves both IL-10 and the transforming growth factor (TGF)-β.Citation11 TGF-β participates in the development and/or maintenance of all Treg subsets, whether they originate from Foxp3+ or Foxp3- thymic precursor cells, in addition to its involvement in the development of the IL-17-producing T helper cell effector lineage.Citation15
Little is known about Treg responses induced post influenza vaccination. The approach taken in this study was to address the issue of Treg Foxp3 and cytokine expression, and the relationship between these immunologic indicators and antibody production in healthy subjects who received seasonal influenza vaccination.
Results
Demographic data
The study enrolled 36 (male 18, female 18) healthy subjects. The mean age was 35.6 ± 12.5 y. All the subjects received influenza vaccinations for at least two consecutive years. No underlying disease was reported in the subjects.
Immunophenotype expressions
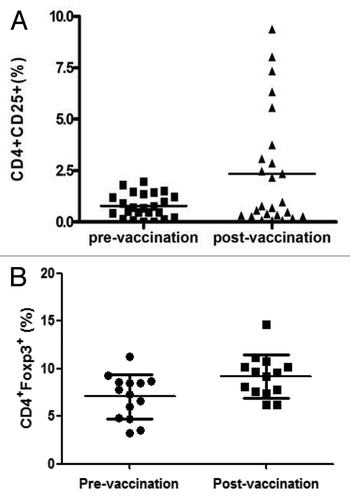
The expression frequency of CD3+ (p = 0.025) increased significantly after vaccination. No significant differences in expression frequency for CD4+ (p = 0.761), CD8+ (p = 0.716), CD20+ (p = 0.409), or CD14+ (p = 0.548) were found after vaccination (). The expression frequency of CD4+CD25+ and CD4+Foxp3+ was studied in 24 and 14 subjects, respectively. The expression frequency of CD4+CD25+ (0.8 ± 0.6% vs. 2.5 ± 2.9%, p = 0.011) increased significantly after vaccination. In addition, the expression frequency of CD4+Foxp3+ (7.1 ± 2.4% vs. 9.2 ± 2.2%, p = 0.039) () and the expression frequency of CD127+ (p < 0.01) increased significantly after vaccination.
Table 1. The changes of expression frequency or ratio of immunophenotypes in subjects before and after influenza vaccination
Changes of cytokines expression after vaccination
The changes of cytokine profile after vaccination were summarized in . In comparison with the cytokine level before vaccination, the plasma TGF-β level (p < 0.05) of the subjects elevated significantly after vaccination. Other cytokines such as IL-2, IL-4, IL-5, IL-10, IFN-γ, and TNF-α did not show a marked change after vaccination.
Table 2. The changes of cytokines expression in subjects before and after influenza vaccination
Antibody titer
The antibody titers to the influenza A H1N1, influenza A H3N2, influenza B/Yamagata, and influenza B/Victoria below 1:40 were found in 29–65%, 20–33%, 4–40% and 17–44% of the subjects before vaccination, respectively. After vaccination, titers above 1:40 for the subjects to influenza A H1N1 were 96–100%, to influenza A H3N2, 85–100%, to influenza B/Yamagata, 78–100%, and to influenza B/Victoria, 85–100%, indicating the influenza vaccine indeed induce anti-influenza antibody.
Correlation of cytokines and antibody titers
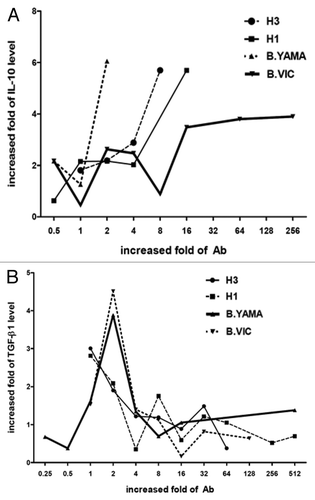
Since the duration of antibody titer is short that are usually decreased after one year post immunization, we attempt to investigate whether this related to the regulatory mechanism of the immune system. Through the serum cytokine level and the anti-influenza antibody titer for individual immunized persons (n = 19), the fold-increases of the cytokine and fold-increases of the antibody titer was plotted and correlated. Since the antibody titer was determined by hemagglutination inhibition test, the 4-fold increase was considered to be significant. There is positive correlation between the fold-increases of IL-10 () and anti-H1N1, anti-H3N2, anti-B/Yamagata, and anti-B/Victoria antibodies for individuals post immunization. On the contrary, there was a negative relationship between the fold-increases of the TGF-β level and the fold-increases of the anti-H1N1, anti-H3N2, anti-B/Yamagata, and anti-B/Victoria antibody titer (). Whereas, fold-increases of IL-2, IL-4, IL-5, IFN-γ, or TNF-α level showed different correlation to fold-increases of each anti-influenza antibody titer.
Discussion
Immune correlates of protection against influenza infection after vaccination include serum hemagglutination inhibition antibody and neutralizing antibody. Increased levels of antibody induced by vaccination decrease the risk for illness caused by endemic strains that are similar antigenically to those strains included in the vaccine.Citation16-Citation18
Tregs are increasingly important in vaccine strategies. Tregs can influence the magnitude of the vaccination response in several ways. Short-range mechanisms, such as regulatory molecule secretion of IL-10, adenosine, IL-35, galectins, and carbon monoxide will suppress the local effector cells during adaptive immune responses. In the longer run, Tregs create a regulatory environment by producing TGF-β and/or change DC functions to stimulate additional Treg production.Citation19 Cytokines (IL-2, IL-10, IFN-γ, TGF-β) could influence “natural” Tregs that are present without an apparent immune response. Additionally, some cytokines could amplify the number or activity of Tregs. The influence of cytokines on Tregs is factored into the activation/conversion or effector function. Most cytokines have a positive stimulating or supportive effect although some downregulate the regulatory cell. Whether the downregulation effect is on the regulatory cell or the cytokine renders the effector cell “target” resistant to suppression should be considered.Citation20
Influenza A/PR/8/34 (PR8) is an H1N1 human strain adapted to efficiently replicate in and kill mice. Surls and coworkers found that immunization of BALB/c mice with a prototype of UV-inactivated PR8 virus vaccine expanded the CD4+Foxp3+ Treg pool and fostered the development of virus-specific CD4+Foxp3+ Treg cells. Increasing the size of the Foxp3+ Treg pool did not alter the primary PR8-specific B-cell response, but it did suppress the primary and memory PR8-specific T helper responses induced by vaccination.Citation21 Since the CD4 T cells play an important role in anti-influenza viral protection through a quick recall of the adaptive B memory cell response, and Tregs are expanded as consequence of vaccination. These findings may support the notion of the increasing expression frequency of CD4+Foxp3+ after influenza vaccination in our study.
Peripheral Tregs are heterogeneous, and may include some cells with transient Foxp3 expression. CD4+Foxp3+ Treg pool is a critical modulatory component of the adaptive CD4+ T cell primary and memory responses induced by vaccination. The CD4+Foxp3+ is not limited to CD4+CD25+ T cells. Human CD4+CD25− T cells can also transiently express Foxp3 after TcR stimulation without the requirement of other extrinsic signals. Foxp3-expressing T cell encompassed in the CD25− cell population convert to CD25+ after homeostatic expansion.Citation22 In vitro human studies also indicate that stable and high Foxp3 expression in CD4+ CD25− T cells is consistent with acquisition of Treg phenotype and function.Citation23 One of the contributions of Tregs was to provide rapid helper activity during the initial early window after vaccine administration, at a time when conventional, antigen specific CD4+ helper cells had not started to activate and clonally expand.Citation24
Differential CD127 expression on Treg depends on their localization and their activation status. Simonetta et al. showed that Treg exhibit high CD127 expression when activated, in contrast to their low expression in non-immunized settings.Citation25 They also suggested in contrast to conventional T-cell activation, CD127 expression may be more prominent during Treg activation. Low CD127 expression is not an intrinsic characteristic of Treg and that differential CD127 expression on Treg depends on their localization and their activation status. CD127hi effector cells express more survival factors and undergo apoptosis to a lesser extent in vivo than do CD127lo effector cells, and strongly support the model in which CD127hi effector cells are selected to survive and develop into long-lived memory cells.Citation26 Huang et al. showed that prime-immunization with SARS-CoV S DNA vaccine can induce both CD4+ and CD8+ T cell responses. The SARS-CoV S-specific CD4+ and CD8+ T cells were 30 to 50% of the cells expressing CD127, which can generate antigen-specific humoral and cellular immune responses that may contribute to long-term protection.Citation27 These studies may provide some explanation for the increased expression of CD4+Foxp3+ and CD127 simultaneously after influenza vaccination.
Antibodies that are present in the serum and on mucosal surfaces are good correlates of immunity to influenza. McElhaney et al.Citation28 found that cytokine production and the proliferation of T cells in the presence of influenza antigens correlated with protection in elderly adults. They reported different types of helper T-cell responses to each of the vaccine strains of the virus. IL-10, which is produced in a Th2 response, was higher in PBMC stimulated with A/Texas/36/91 (H1N1) than in A/Johannesburg/33/94 (H3N2). The strain of influenza virus contained in the vaccine appeared to be an important determinant of the T-cell response to vaccination.Citation29 Influenza-specific T cells play a crucial role in the control of viral influenza infection and are capable of producing cytokines and killing infected cells. A comparative study of a single vs. booster dose of influenza vaccination in elderly subjects revealed much different immune responses.Citation30 The single immunization led to an expected increase in IFN-γ and Granzyme B which positively correlated with increased antibody levels and decreased IL-10. In contrast, boosting 16 weeks after the initial dose lead to decreased IFN-γ and Granzyme B as compared with the single boost group.
CD4 T cell reactivity to hemagglutinin (HA) is particularly important to quantify because of the recent evidence that CD4 help for B cell responses might occur optimally if the epitopes are derived from the same antigen.Citation31 The exposure of CD4+ T cells to TGF-β may convert these cells to CD4+Foxp3+ Treg cells. This conversion could occur in any immune privileged site that contains TGF-β. T cell sensitivity to TGF-β is required for the effector cells to be suppressed by CD8+ Treg cells themselves induced by CD4+Foxp3+ Treg cells. Further, Casares and coworkers also identified peptide P60 is able to inhibit the immunosuppressive activity of murine and human-derived Tregs and enhances the effector T cell stimulation in vitro, and most importantly, they show that P60 can improve the immunogenicity of viral vaccines.Citation32 Therefore, TGF-β and CD4+Foxp3+ Treg cells may act as checkpoints between the antibodies production and vaccination. These findings may also provide the explanation, at least in part, of the lower antibody titer of anti-B/Yamagata and anti-B/Victoria post vaccination.
Limitations of this study, the use of CD4+CD25+ is limited given the more sophisticated nature of Treg identification. Other report utilized CD45RO+RA-CD25+CD127- as a definitive marker of Tregs.Citation33 Further, the possibility that Foxp3 could be also expressed by activated human T cells.Citation34 Instead of analyzing the frequencies of CD4+CD25+ and CD4+Foxp3+ T cells, might consider analyzing the frequency of Tregs using tetramer technology. Because of relatively small number of subjects, the analysis of the correlations between cytokine and antibody responses is limited, though the trend exists. We did not evaluate the frequency of B cells taken from the human cohort. Correlation of the increased B cell frequencies, antibodies and cytokines, together with the functional analysis of Foxp3+Treg cells would attach more credence to this study.
In summary, we suggest that Treg cells may be activated post influenza vaccination. The altered expression of Treg cells may be used as a surrogate measurement of regulatory function that influenced the production of anti-influenza antibody. It can also be the targets to enhance the efficacy of influenza vaccine.
Material and Methods
Selection of subjects
Healthy volunteers (n = 36), at the time of blood collection, donated whole blood via venous puncture before an influenza vaccination and two weeks after the immunization in 2006–2009. The Clinical Research Ethics Committee of the National Cheng Kung University and Hospital approved the study protocol. Informed consent was obtained from all the enrolled subjects.
Vaccine
Single lots of licensed 2006–2007 (A/New Caledonia/20/99 (H1N1)-like virus; an A/Wisconsin/67/2005 (H3N2)-like virus; a B/Malaysia/2506/2004-like virus), 2007–2008 (A/Solomon Islands/3/2006 (H1N1)-like (new for this season), A/Wisconsin/67/2005 (H3N2)-like, and B/Malaysia/2506/2004-like viruses), and/or 2008–2009 (A/Brisbane/59/2007 (H1N1)-like, A/Brisbane/10/2007 (H3N2)-like, and B/Florida/4/2006-like antigens) trivalent inactivated preservative-free split-virion inactivated influenza vaccine (Sanofi Pasteur, Fluzone® or GlaxoSmithKline, Fluarix®) were used throughout the trial.
Sample preparation
Peripheral blood mononuclear cells (PBMCs) were isolated from ethylenediamine tetraacetic acid (EDTA) whole blood using Ficoll separation (Ficoll-Paque plus; Amersham Biosciences). After two washes in PBS, PBMCs were subjected to further analyses. The plasma was stored at −80°C for further use.
Peripheral blood mononuclear cell (PBMC) preparation and flow cytometry analysis
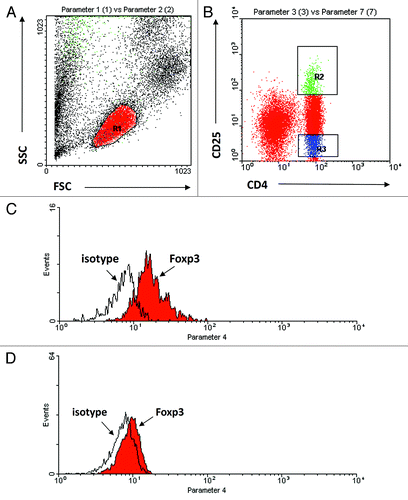
PBMCs were extracted from peripheral blood using a standard Ficoll density-gradient centrifugation and were immediately tested using fluorescence activated cell sorter (FACS) analysis. The cells then were washed with PBS and fixed with 0.5 mL of 0.1% glutaraldehyde solution in PBS. Stained lymphocytes were analyzed using flow cytometry (Becton Dickinson Immunocytometry Systems). Data was acquired and analyzed by Cell Quest software (Becton Dickinson). The following fluorescent MAbs were used for staining and in sorting: peridinin chlorophyll protein–conjugated Leu 4 (CD3; pan T), phycoerythrin-conjugated Leu-3a (CD4 T cells), Leu-2a (CD8 T cells and NK cells), Leu-11c (CD16; NK lymphocytes), Leu-19 (CD56; NK lymphocytes and T lymphocyte subset), CD25 (M-A251), and CD127 according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN, USA). CD4+ Tregs showed Foxp3 expression as determined by FACS intra-cytoplasmic staining with anti-Foxp3Ab (eBioscience, San Diego, CA, USA). The gating strategy for each population is demonstrated in .
Figure 3. Representative plot illustrating gating strategy. (A) Lymphocytes were gated according to forward and side scatter (R1), (B) CD4+CD25+ lymphocytes (R2), CD4+CD25− lymphocytes (R3). From the gated lymphocyte population, the total Foxp3 expression and isotype control were analyzed (C) (R2) and (D) (R3).
Measurement of cytokines
Plasma for cytokine determination was harvested from the subjects within 30 min of venipuncture from EDTA-anticoagulated venous blood samples and was stored at -70°C until analyzed. The cytometric bead array assay (CBA) (BD PharMingen, CA, USA) consisted of six bead populations with distinct fluorescence intensities. The concentrations of IL-2, IL-4, IL-5, IL-10, interferon (IFN)-γ and tumor necrosis factor (TNF)-α were measured using the human Th1/Th2 cytokine CBA kits. An ELISA for quantitative determination of TGF-β (Quantikine; R&D Systems) was used. The limits of detection of these immunoassays were 2.6 pg/mL for IL-2, 2.6 pg/mL for IL-4, 2.4 pg/mL for IL-5, 2.8 pg/mL for IL-10, 2.8 pg/mL for TNF-α, and 7.1 pg/mL for IFN-γ.
Hemagglutination inhibition (HAI) assay
Serum samples were pre-treated with receptor-destroying enzyme (Denka Seiken, Tokyo, Japan) overnight at 37°C and then incubated for 1 h at 56°C to remove nonspecific inhibitors. The HAI test was conducted with 8 HA units of the appropriate virus and 0.7% (v ⁄ v) turkey erythrocytes, as described elsewhere. Serum HAI antibody titers of 40 were considered to be protective.
Statistical analysis
Data were expressed as mean ± SD. Statistical significance was determined by pair or nonpair nonparametric tests, using SPSS software (version 11.5, Chicago, IL, USA). A p < 0.05 was considered significant.
Acknowledgments
This study was supported by grants from Department of Health, Executive Yuan, Taiwan (DOH100-TD-B-111–102), and National Cheng Kung University Hospital (NCKUH-96–024, NCKUH-9701003), Taiwan.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest have been disclosed.
References
- Wright PF, Neuman G, Kawaoka Y. Orthomyxoviruses. In: Knipe DM, Howley PM, Griffin DE, Martin MA, Ramb RA, Roizman B, et al, editors. Fields virology 5th ed. Philadelphia Lippincott-Raven; 2007, 1691-740.
- McCullers JA, Rehg JE. Lethal synergism between influenza virus and Streptococcus pneumoniae: characterization of a mouse model and the role of platelet-activating factor receptor. J Infect Dis 2002; 186:341 - 50; http://dx.doi.org/10.1086/341462; PMID: 12134230
- de Jong MD, Bach VC, Phan TQ, Vo MH, Tran TT, Nguyen BH, et al. Fatal avian influenza A (H5N1) in a child presenting with diarrhea followed by coma. N Engl J Med 2005; 352:686 - 91; http://dx.doi.org/10.1056/NEJMoa044307; PMID: 15716562
- Trifonov V, Khiabanian H, Rabadan R. Geographic dependence, surveillance, and origins of the 2009 influenza A (H1N1) virus. N Engl J Med 2009; 361:115 - 9; http://dx.doi.org/10.1056/NEJMp0904572; PMID: 19474418
- Neuzil KM, Jackson LA, Nelson J, Klimov A, Cox N, Bridges CB, et al. Immunogenicity and reactogenicity of 1 versus 2 doses of trivalent inactivated influenza vaccine in vaccine-naive 5-8-year-old children. J Infect Dis 2006; 194:1032 - 9; http://dx.doi.org/10.1086/507309; PMID: 16991077
- Russell CA, Jones TC, Barr IG, Cox NJ, Garten RJ, Gregory V, et al. Influenza vaccine strain selection and recent studies on the global migration of seasonal influenza viruses. Vaccine 2008; 26:Suppl 4 D31 - 4; http://dx.doi.org/10.1016/j.vaccine.2008.07.078; PMID: 19230156
- Ohmit SE, Victor JC, Rotthoff JR, Teich ER, Truscon RK, Baum LL, et al. Prevention of antigenically drifted influenza by inactivated and live attenuated vaccines. N Engl J Med 2006; 355:2513 - 22; http://dx.doi.org/10.1056/NEJMoa061850; PMID: 17167134
- Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol 2004; 22:531 - 62; http://dx.doi.org/10.1146/annurev.immunol.21.120601.141122; PMID: 15032588
- Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat Immunol 2002; 3:756 - 63; PMID: 12089509
- Ziegler SE, Morella KK, Anderson D, Kumaki N, Leonard WJ, Cosman D, et al. Reconstitution of a functional interleukin (IL)-7 receptor demonstrates that the IL-2 receptor gamma chain is required for IL-7 signal transduction. Eur J Immunol 1995; 25:399 - 404; http://dx.doi.org/10.1002/eji.1830250214; PMID: 7875201
- Chabalgoity JA, Baz A, Rial A, Grille S. The relevance of cytokines for development of protective immunity and rational design of vaccines. Cytokine Growth Factor Rev 2007; 18:195 - 207; http://dx.doi.org/10.1016/j.cytogfr.2007.01.016; PMID: 17347024
- Yucesoy B, Johnson VJ, Fluharty K, Kashon ML, Slaven JE, Wilson NW, et al. Influence of cytokine gene variations on immunization to childhood vaccines. Vaccine 2009; 27:6991 - 7; http://dx.doi.org/10.1016/j.vaccine.2009.09.076; PMID: 19819209
- Wan YY, Flavell RA. ‘Yin-Yang’ functions of transforming growth factor-β and T regulatory cells in immune regulation. Immunol Rev 2007; 220:199 - 213; http://dx.doi.org/10.1111/j.1600-065X.2007.00565.x; PMID: 17979848
- Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J Exp Med 2003; 198:1875 - 86; http://dx.doi.org/10.1084/jem.20030152; PMID: 14676299
- Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity 2006; 24:677 - 88; http://dx.doi.org/10.1016/j.immuni.2006.06.002; PMID: 16782025
- Potter CW, Oxford JS. Determinants of immunity to influenza infection in man. Br Med Bull 1979; 35:69 - 75; PMID: 367490
- Hirota Y, Kaji M, Ide S, Kajiwara J, Kataoka K, Goto S, et al. Antibody efficacy as a keen index to evaluate influenza vaccine effectiveness. Vaccine 1997; 15:962 - 7; http://dx.doi.org/10.1016/S0264-410X(96)00302-7; PMID: 9261942
- Fiore AE, Uyeki TM, Broder K, Finelli L, Euler GL, Singleton JA, et al, Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep 2010; 59:RR-8 1 - 62; PMID: 20689501
- van’t Land B, Schijf M, van Esch BCAM, van Bergenhenegouwen J, Bastiaans J, Schouten B, et al. Regulatory T-cells have a prominent role in the immune modulated vaccine response by specific oligosaccharides. Vaccine 2010; 28:5711 - 7; http://dx.doi.org/10.1016/j.vaccine.2010.06.046; PMID: 20600499
- Cone RE, Bhowmick S. Cytokines and sympathy: the control of regulatory T cells. Int J Interferon Cytokine Mediator Res 2010; 2:41 - 7; http://dx.doi.org/10.2147/IJICMR.S6758
- Surls J, Nazarov-Stoica C, Kehl M, Casares S, Brumeanu T-D. Differential effect of CD4+Foxp3+ T-regulatory cells on the B and T helper cell responses to influenza virus vaccination. Vaccine 2010; 28:7319 - 30; http://dx.doi.org/10.1016/j.vaccine.2010.08.074; PMID: 20832492
- Zelenay S, Lopes-Carvalho T, Caramalho I, Moraes-Fontes MF, Rebelo M, Demengeot J. Foxp3+ CD25- CD4 T cells constitute a reservoir of committed regulatory cells that regain CD25 expression upon homeostatic expansion. Proc Natl Acad Sci USA 2005; 102:4091 - 6; http://dx.doi.org/10.1073/pnas.0408679102; PMID: 15753306
- Wang J, Ioan-Facsinay A, van der Voort EIH, Huizinga TWJ, Toes REM. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol 2007; 37:129 - 38; http://dx.doi.org/10.1002/eji.200636435; PMID: 17154262
- Sharma MD, Hou DY, Baban B, Koni PA, He Y, Chandler PR, et al. Reprogrammed foxp3(+) regulatory T cells provide essential help to support cross-presentation and CD8(+) T cell priming in naive mice. Immunity 2010; 33:942 - 54; http://dx.doi.org/10.1016/j.immuni.2010.11.022; PMID: 21145762
- Simonetta F, Chiali A, Cordier C, Urrutia A, Girault I, Bloquet S, et al. Increased CD127 expression on activated FOXP3+CD4+ regulatory T cells. Eur J Immunol 2010; 40:2528 - 38; http://dx.doi.org/10.1002/eji.201040531; PMID: 20690182
- Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol 2003; 4:1191 - 8; http://dx.doi.org/10.1038/ni1009; PMID: 14625547
- Huang J, Ma R, Wu CY. Immunization with SARS-CoV S DNA vaccine generates memory CD4+ and CD8+ T cell immune responses. Vaccine 2006; 24:4905 - 13; http://dx.doi.org/10.1016/j.vaccine.2006.03.058; PMID: 16621188
- McElhaney JE, Xie D, Hager WD, Barry MB, Wang Y, Kleppinger A, et al. T cell responses are better correlates of vaccine protection in the elderly. J Immunol 2006; 176:6333 - 9; PMID: 16670345
- McElhaney JE, Upshaw CM, Hooton JW, Lechelt KE, Meneilly GS. Responses to influenza vaccination in different T-cell subsets: a comparison of healthy young and older adults. Vaccine 1998; 16:1742 - 7; http://dx.doi.org/10.1016/S0264-410X(98)00133-9; PMID: 9778750
- McElhaney JE, Hooton JW, Hooton N, Bleackley RC. Comparison of single versus booster dose of influenza vaccination on humoral and cellular immune responses in older adults. Vaccine 2005; 23:3294 - 300; http://dx.doi.org/10.1016/j.vaccine.2005.01.080; PMID: 15837235
- Sette AM, Moutaftsi M, Moyron-Quiroz J, McCausland MM, Davies DH, Johnston RJ, et al. Selective CD4+ T cell help for antibody responses to a large viral pathogen: deterministic linkage of specificities. Immunity 2008; 28:847 - 58; http://dx.doi.org/10.1016/j.immuni.2008.04.018; PMID: 18549802
- Casares N, Rudilla F, Arribillaga L, Llopiz D, Riezu-Boj JI, Lozano T, et al. A peptide inhibitor of FOXP3 impairs regulatory T cell activity and improves vaccine efficacy in mice. J Immunol 2010; 185:5150 - 9; http://dx.doi.org/10.4049/jimmunol.1001114; PMID: 20870946
- Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med 2006; 203:1693 - 700; http://dx.doi.org/10.1084/jem.20060468; PMID: 16818676
- Gavin MA, Torgerson TR, Houston E, DeRoos P, Ho WY, Stray-Pedersen A, et al. Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc Natl Acad Sci USA 2006; 103:6659 - 64; http://dx.doi.org/10.1073/pnas.0509484103; PMID: 16617117