Abstract
Vaccines were urgently needed in 2009 against A/H1N1 pandemic influenza. Based on the H5N1 experience, it was originally thought that 2 doses of an adjuvanted vaccine were needed for adequate immunogenicity. We tested H1N1 vaccines with or without AF03, a squalene-based adjuvant, in children.
Two randomized, open-label, trials were conducted. Participants 3–17 y received two injections of 3.8 µg or 7.5 µg hemagglutinin (HA) with adjuvant or 15 µg HA without adjuvant. Participants aged 6–35 mo received two injections of 1.9 µg or 3.8 µg HA with full or half dose adjuvant or 7.5 µg HA without adjuvant.
All subjects 3 to 17 y reached seroprotection (hemagglutination inhibition (HI) titer ≥ 40) after the first dose of the adjuvanted vaccine, and 94% and 98% in the 3–8 and 9–17 y groups respectively with the non-adjuvanted vaccine. In children aged 6–35 mo responses were modest after one dose, but after two doses virtually all children were seroprotected regardless of HA or adjuvant dose. In this age group, antibody titers were 5 to 7 times higher after adjuvanted than non-adjuvanted vaccine. The higher responses with the adjuvanted vaccine were also reflected as better antibody persistence. There was no clustering of adverse events that would be suggestive of a safety signal.
While a single injection was sufficient in subjects from 3 y, in children aged 6–35 mo two injections of this A/H1N1 pandemic influenza vaccine were required. Formulation of this vaccine with adjuvant provided a significant advantage for immunogenicity in the latter age group.
Introduction
Within two months after the first confirmed cases of the novel influenza A (H1N1) 2009 virus outside of Mexico, the WHO declared on 11 June 2009 that the outbreak had become a pandemic.Citation1 It was soon realized that deaths and severe cases had occurred in disproportionally high numbers of previously healthy children and young adults, as well as in pregnant women.Citation2-Citation6 Therefore the risk groups were different from those for seasonal influenza and also the need for vaccination concerned much larger sections of the population than usual.Citation7 To meet the need for the unexpectedly high number of vaccine doses the strategy of “antigen sparing,” initially devised by vaccine manufacturers for H5N1 vaccine candidates, was applied. This meant reduction of the usual antigen dose of 15 µg hemagglutinin (HA) and addition of a squalene-based adjuvant to enhance the immunogenicity of the vaccine.
Squalene-based emulsion adjuvants had been used to enhance the immunogenicity of the poorly immunogenic H5N1 avian influenza virus vaccines in various age groups,Citation8-Citation10 as well as seasonal influenza vaccines for the elderly and young children who do not respond optimally to conventional influenza vaccine.Citation11-Citation13 Analogously, it was also planned to apply squalene-based adjuvants to enhance immunogenicity of vaccines against the H1N1 2009 virus, but it was not known whether adjuvants were needed at all or, if yes, for which target groups. The addition of adjuvant was driven by the assumed need for antigen sparing.
We report data from two clinical trials investigating the immunogenicity and safety of adjuvanted and non-adjuvanted pandemic influenza A H1N1 2009 vaccines in children and young infants. The studies were conducted in the fall of 2009, with a follow-up of antibody persistence until 8 or 13 mo later in a subset of the children.
Results
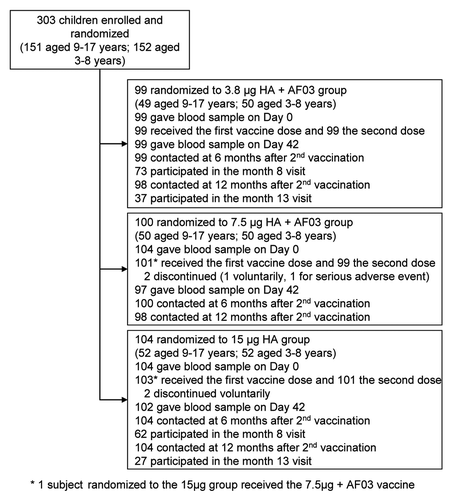
One of the vaccines was administered to 303 children and 401 infants/toddlers in the two studies, respectively ( and ). Fourteen children discontinued during the vaccination phase, two of them due to SAEs that were unrelated to vaccination (pneumonia responsive to antibiotic therapy and Duchenne muscular dystrophy). Four additional subjects were withdrawn due to non-compliance with the protocol. Subsets of 135 children from 3 y of age and 82 children less than 3 y participated in the assessment of antibody persistence at 8 mo after vaccination, and subsets of 64 and 28 children respectively in the visit organized at 13 mo after vaccination.
Vaccine groups were comparable for demographic characteristics at enrolment (). Except for 2 children less than 3 y, no participant was known to have been in contact with a case of pandemic H1N1 influenza before enrolment.
Table 1. Baseline characteristics of children, according to age and study group
Hemagglutination inhibiting antibody response to vaccination
The proportion of participants with pre-existing HI antibodies (≥ 10) against the H1N1 2009 strain was 21% and 0.7% in participants aged 9–17 and 3–8 y, respectively. Only one subject (0.25%) in the 6–35 mo age group was seropositive at baseline. The seroprotective titer of 40 was reached before vaccination by 7% of the children in the 9–17 y age groups, no child in the 3 to 8 y group and 1 child (0.25%) in the 6 to 35 mo group.
100% of children 3 y of age and above developed seroprotective HI titers after a single vaccination with either of the adjuvanted vaccine candidates, and even without adjuvant the seroprotection rate was more than 94%. Among the older children the HI GMTs were approximately 1.5-fold higher with adjuvant than without and in the 3–8 y-olds, they were 3- to 4-fold higher with adjuvanted vaccine than with non-adjuvanted vaccine ().
Table 2. Hemagglutination inhibition antibody response against H1N1 influenza 21 d after a single injection of H1N1 vaccine in children aged 3–17 y
For children less than 3 y of age, in the adjuvanted vaccine groups, antibodies against the A/California/07/2009 (H1N1) strain already increased significantly at 21 d after the first vaccination, with GMTRs exceeding 40 (). More than 96% of the subjects in these groups developed a seroprotective titer ≥ 40, regardless of their dosage of HA and AF03. After the second vaccination with the adjuvanted vaccines, 100% of the subjects seroconverted and had a seroprotective HI titer, with GMTs reaching at least 2500. In the recipients of non-adjuvanted vaccine, the GMT against the A/California/07/2009 (H1N1) strain 21 d after the first vaccination was 23. Seroprotective levels of HI antibody were reached by 33% of the subjects after the first dose and 98% after the second dose. After the second dose of non-adjuvanted vaccine, in these children aged less than 3 y, the GMT was more than 10-fold lower than following two doses of adjuvanted vaccine.
Table 3. Hemagglutination inhibition antibody response against H1N1 influenza in children aged 6 to 35 mo
Neutralizing antibody response to vaccination
The proportion of children with detectable NT antibodies (titer ≥ 10) before vaccination was 25% and 3%, respectively in the 9–17 and 3–8 y age groups. Only 1 child less than 3 y of age had detectable neutralizing antibodies at baseline.
All but 3 children had at least a 4-fold rise in neutralizing antibodies after one injection of adjuvanted vaccine, and all of them had at least a 4-fold increase after 2 injections. With the non-adjuvanted vaccine, in 9–17 and 3–8 y groups the rates of 4-fold titer rise after the first vaccination were 100% and 96%, respectively. In 6–35 mo old children who received a 7.5µg dose of the vaccine, the rate of 4-fold titer rise after the first vaccination was 81%. The GMTs were higher with the adjuvanted than the non-adjuvanted vaccine in all study groups: after the first dose, in the 9–17 y group, 3661 with 3.8 µg HA + AF03 vs. 2618 with the 15µg non-adjuvanted vaccine, in the 3–8 y group, 2094 with 3.8 µg HA + AF03 vs. 660 with the 15µg non-adjuvanted vaccine, and in the 6–35 mo group 952 with 1.9 µg HA + ½AF03 vs. 73 with the 7.5µg non-adjuvanted vaccine.
Persistence of HI antibody
Eight months after the first vaccination, a decrease in HI titers was observed compared with D42 in the subset of subjects evaluated, but HI titers were still higher than those observed 21 d after the first vaccination ().
Figure 3. Geometric mean hemagglutination inhibition antibody titer against influenza A (H1N1) 2009 before and after administration of adjuvanted or non-adjuvanted H1N1 vaccine in three age groups of children.
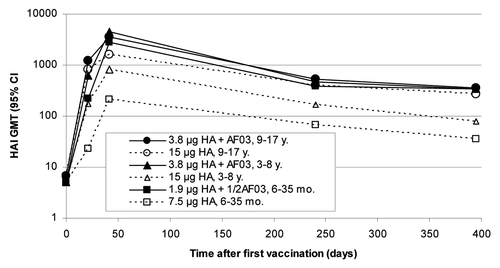
All children 3–17 y of age who received an adjuvanted vaccine remained seroprotected at the 8-mo time point. For those who received the non-adjuvanted vaccine, 97% of the children aged 9–17 y and 93% of those aged 3–8 y remained seroprotected. The persistence of the antibody response at seroprotective levels was confirmed in the subset of subjects who participated in the month 13 assessment: all subjects tested were still seroprotected in the 1.9 µg HA + ½ AF03 groups, all subjects in the 9–17 y non-adjuvanted group and 8/10 subjects in the 3–8 y non-adjuvanted group.
For children less than 3 y of age, all the subjects in the 1.9 µg HA + ½ AF03 group remained seroprotected, while 95.7% of subjects aged 12–35 mo and 66.7% of subjects aged 6–11 mo remained seroprotected in the non-adjuvanted group. Similar trends were seen in the small subset of subjects assessed at 13 mo after vaccination: 18/18 subjects were still seroprotected in the 1.9 µg HA + ½ AF03 group vs. 4/10 in the non-adjuvanted group.
Safety and reactogenicity
No deaths were reported. Six SAEs during the period D0-D42 and 25 additional SAEs during the 12-mo safety follow-up were reported across the 2 studies. None was considered by the investigator as related to the study vaccine. Among them, 5 episodes of febrile convulsions (adverse events of special interest) occurred in 4 subjects: one subject in the non-adjuvanted group, two subjects in the 1.9 µg HA + ½ AF03 group and one subject in the 3.8 µg HA + ½ AF03. All episodes of febrile convulsions occurred between 2 mo and 168 d after the vaccine injection.
Solicited adverse reactions reported within 7 d of each vaccination are summarized in . The incidence of solicited injection site reactions tended to be higher in the adjuvanted vaccine groups than in the non-adjuvanted vaccine group, and no major differences were detected in the incidence of solicited systemic reactions reported in the adjuvanted and non-adjuvanted vaccine groups. There was also no indication of a global increase in the incidence of solicited injection site and systemic reactions after the second vaccination as compared with the first.
Table 4. Safety data: % participants reporting at least one reaction after 1st or 2nd vaccination
Solicited adverse reactions reported in children less than 3 y of age in the 7-d period following each vaccination with the 1.9 µg HA + ½ AF03 vaccine and 7.5 µg HA vaccine are detailed in . Pain / tenderness and erythema were the most commonly reported injection site reactions. Regarding systemic reactions, there was a trend toward a higher incidence of fever with the adjuvanted vaccine than in the non-adjuvanted vaccine, and following the second vaccination compared with the first with the adjuvanted vaccine. Among children less than 3 y who received an adjuvanted vaccine, 2 and 9 subjects reported grade 3 fever (defined as rectal temperature > 39.5°C in children < 24 mo and axillary temperature ≥ 39.0°C in children ≥ 24 mo) respectively after 1st and 2nd vaccination, while none of the recipients of non-adjuvanted vaccine reported grade 3 fever.
Table 5. Reactogenicity of H1N1 vaccine in children less than 3 y: % participants reporting at least one solicited reaction between day 0 and 7 after 1st or 2nd vaccination
Laboratory analyses concluded in a similar or quite similar frequency of values out of range at 8 d after the first vaccination compared with the frequency observed at baseline. A few hematological values (leucocytes and neutrophils counts) that were under the lower limit of normality on day 8 were considered as clinically significant by the investigator in three 9–17 y-old subjects and one 3–8 y-old subject who all had received the non-adjuvanted vaccine. Some of the biochemistry and hematology values measured on day 8 in children less than 3 y of age were also considered as clinically significant by the investigators. For the biochemistry parameters, these consisted of isolated high creatinine values detected in 3 children who received an adjuvanted vaccine, and high liver enzymes values found in 3 children, of whom 2 had received an adjuvanted vaccine and 1 had received the non-adjuvanted vaccine. All six children presented with preexisting abnormal values already at baseline. Hematology abnormalities considered as clinically significant did not reveal any specific pattern, as they included high as well as low hemoglobin values, high platelets, leukocytes, neutrophils or lymphocytes counts as well as low leukocytes or neutrophils counts. These abnormal values were found both after administration of adjuvanted vaccines (8 children) and non-adjuvanted vaccines (4 children) and were most often preexisting at baseline or returned to normal at a later time-point.
Discussion
Trials of candidate pandemic vaccines against avian influenza A (H5N1) strains had shown that without adjuvant, doses of up to 90 µg hemagglutinin are required to elicit satisfactory immune responses, whereas with the use of adjuvants the dose of antigen required was much lower. To attain satisfactory antibody levels against H5N1, two-dose vaccination schedules were needed, irrespective of adjuvant content.Citation8-Citation10
The hemagglutinin of the influenza virus causing the 2009 pandemic was derived from the 1918 influenza A strain that entered swine around that time.Citation14 The H1N1 strains circulating in humans between 1918 and 1957, and again between 1977 to the present day, have drifted considerably so that seasonal influenza H1N1 strains and the H1N1 pandemic strain in 2009 had little antigenic similarity.Citation14,Citation15 Moreover, prior seasonal influenza vaccination induced little or no antibody cross-reactivity to the 2009 H1N1 pandemic strain.Citation16 It was originally hypothesized that for vaccines against the 2009 H1N1 pandemic virus, similarly to H5N1 and consistent with the two-dose vaccination schedule recommended for naïve young children receiving a seasonal influenza vaccine for the first time, a two-dose vaccination schedule would be needed. This hypothesis turned out to be incorrect, and a finding of our study was that a single vaccination induced robust antibody responses in children as young as three years. In the present study, 94% of children 3–8 y old and 100% of those aged 9–17 seroconverted (HI antibodies) after a single dose of non-adjuvanted vaccine. Other non-adjuvanted pandemic H1N1 2009 vaccines evaluated in children aged 3 y or above indicated similarCitation17 or lower immunogenicity.Citation18-Citation20 In the situations of lower immunogenicity, it is difficult to assess whether the observations reflect true differences in immunogenicity across vaccines from different manufacturers. Other factors, such as the dose of HA actually included in the vaccine or the laboratory and method used for assessing HI responses, may have also impacted the immunogenicity results. Nevertheless, in our study as well as in the others, in children less than 3 y of age, a second dose of the non-adjuvanted H1N1 vaccine was required, as is the case for seasonal vaccination.
Another key observation of this study was the differential adjuvant effect seen in the different age groups. Previous reports have indicated that in adult subjects, adjuvanted and non-adjuvanted vaccines display similar immunogenicity. In one study reported in 2010, 98% of adults 18 to 60 y seroconverted after a single dose of 5.25µg AS03 adjuvanted vaccine and 95% after a single 21 µg dose of a non-adjuvanted vaccine.Citation21 Another study conducted in young adults concluded that adjuvanted and non-adjuvanted vaccines showed similar immunogenicity. In this report, 21 d after a single injection of 7.5 µg HA without adjuvant or 7.5 µg HA with the MF59 adjuvant, respectively 72% and 73% of the subjects seroconverted.Citation22 In contrast, our data indicate that the effect of the adjuvant increases with decreasing age. This was illustrated by the comparison of HI and NT titers yielded by adjuvanted vs. non-adjuvanted vaccines, and by the fact that a single administration of the AF03 adjuvanted vaccine (but not the non-adjuvanted vaccine) induced seroconversion in most of the children less than 3 y of age. Interestingly, another study that compared adjuvanted and non-adjuvanted vaccines from different vaccine manufacturers in children aged 6 mo to 12 y reached a similar conclusion.Citation23 Therefore, it may be concluded that an adjuvant gave a significant advantage for the immune response in young children but that for children over the age of 3 y a non-adjuvanted pandemic H1N1 2009 vaccine would be sufficient.
Immunological priming by exposure to one or several influenza strains with common epitopes provides a potential, partial explanation for the observed response to vaccination that was more typical of the response to a seasonal vaccination than to a pandemic vaccination. We detected a clear age-dependent presence of pre-existing antibodies against the 2009 H1N1 strain, by both HI and NT methods. This was not directly related to recent seasonal vaccination, as approximately one third of 3–8 y olds had been vaccinated with the 2008/09 seasonal vaccine, yet only one child in this age group had detectable cross-reactive antibodies. While it is also possible that some of the individuals without a detectable antibody response at baseline had nevertheless been primed through past exposure, it appears more likely that the magnitude of the immune response to vaccination may also be explained by the inherent high immunogenicity of the H1N1 strain. The fact that high titers and seroprotection rates were reached after vaccination without adjuvant in children from 3 y of age supports the high intrinsic immunogenicity of the H1N1 vaccine strain. While the adjuvant did further increase antibody responses, particularly in young children, the principal advantage of the AF03 adjuvant with the pandemic H1N1 2009 vaccine strain was its ability to provide antigen dose-sparing. The level of dose-sparing achieved in this study with AF03 adjuvanted vaccine appeared similar to that of an AS03 adjuvanted vaccine used in pediatric populations, with an antigen dose as low as 3.75µg in children from 3 y of age and 1.9µg in children less than 3 y inducing seroconversion in more than 95% of the children after first vaccination.Citation24,Citation25 The immunogenicity data appeared somewhat higher than reported for an MF59 adjuvanted vaccine.Citation26 However, due to the lack of immunological assay standardization for influenza, it is difficult to compare the immunogenicity of vaccines in the absence of a head-to-head trial.
There were no safety concerns in either study, with no unexpected trends in adverse events. As expected, the adjuvanted vaccine was more reactogenic than the non-adjuvanted vaccine, and reaction rates were comparable with a previous report of an AF03-adjuvanted H5N1 vaccine candidate.Citation8 These safety data can also be considered as comparable to the results reported for other adjuvanted vaccines used in pediatric populations. In the trial of an AS03 adjuvanted vaccine conducted in children less than 3 y, somewhat higher rates of local and systemic reactions (including fever) were reported than in this study.Citation24 In another trial of an MF59 adjuvanted vaccine in children aged 3 to 8 y, local and systemic reactions appeared somewhat less frequent, even in the groups of subjects that received the full adult dose of adjuvant as in our study.Citation26 Erythema was reported as the most common local symptom after injection while in our study pain occurred more often. Such comparisons, however, have to be taken with caution due to differences across studies in the methods used for monitoring safety and reactogenicity, and because the trials were conducted in different populations, in different countries, which can affect the reporting of symptoms.
In summary, an inactivated monovalent vaccine against the influenza A (H1N1) 2009 strain elicited robust immune responses in children after a single injection, even without adjuvant. In a pandemic setting, a dose-sparing vaccine such as the AF03-adjuvanted 3.8 µg candidate is thought to have public health benefit over a conventional non-adjuvanted vaccine because more people could be immunised from each manufactured batch of vaccine. However, in the 2009 H1N1 pandemic this benefit was not required since the demand for vaccine was less than had been expected. A non-adjuvanted 15µg vaccine, produced and formulated according to the conventional process that has been used to produce seasonal influenza vaccines for several decades has the advantage of being a very well characterized product. Still, the availability of an adjuvanted vaccine provides an advantage for the rapid immunization of young children. However, the amount of adjuvant used in the vaccine should be adjusted to a level that is both effective and minimally reactogenic.
Patients and Methods
The immunogenicity and safety of various formulations of an H1N1 vaccine with or without squalene adjuvant were evaluated first in children aged 3–17 y, followed by infants/toddlers aged 6–35 mo in two randomized, open-label, multicenter phase 2 trials in Finland (study 1: 303 children; study 2: 401 children). Studies were conducted in accordance with the Declaration of Helsinki and Good Clinical Practice (as defined by the International Conference on Harmonization). The protocols were approved by the National Ethics Committee of Finland. The parents of all children gave their written informed consent before enrolment. Children aged 12 or older also gave written informed consent and children aged 6–11 y gave their assent before enrolment. These studies were registered with Clinicaltrials.gov (NCT00956046, NCT00956202).
Participants
Subjects were considered eligible to participate in the trial provided they had none of the following: systemic hypersensitivity to egg or chicken proteins or any of the vaccine constituents; history of a life-threatening reaction to the study vaccine or a vaccine containing the same constituents; acute febrile illness (oral temperature of ≥ 37.5°C); congenital or acquired immunodeficiency; treatment with immunosuppressive therapy within the previous 6 mo; long-term treatment with systemic corticosteroids; unstable chronic illness that could interfere with study conduct or completion; receipt of blood or blood-derived products in the previous 3 mo; any vaccination within the previous 4 weeks before the inclusion or planned up to the 3 weeks following the second vaccination, thrombocytopenia or a bleeding disorder contraindicating intramuscular vaccination, receipt of a vaccine with the pandemic A/H1N1 influenza strain, history of confirmed pandemic A/H1N1 infection, or receipt of any allergy medication in the previous 7 d or planned 7 d after vaccination. In addition, subjects aged 6–23 mo with history of seizures were not eligible.
Study procedures
We randomly assigned children to each of the study groups using randomization lists stratified by age group and trial center that were generated by the sponsor's biostatistics department using the block-permutation method. Participants received two injections 21 d apart in an unblinded manner. The vaccine was administered intramuscularly in the deltoid muscle or into the anterolateral aspect of the thigh (for subjects aged less than 1 y). Serum samples were collected before and 21 d after each vaccination for serology testing. A phone call was organized at 6 mo after the second vaccination to collect safety information. Subjects from 3 y of age who received the 3.8 µg + AF03 vaccine, subjects less than 3 y who received the 1.9 µg + ½ AF03 vaccine as well as all subjects who received a non-adjuvanted vaccine were then invited to participate in another study visit at 8 mo after primary vaccination for assessment of the persistence of their antibody response, as well as in two additional visits organized a few months later, in fall, for vaccination with trivalent inactivated seasonal influenza vaccine and analysis of the immune response to this vaccine. The outcome of this seasonal vaccination is not presented in this report, but the first of these 2 additional visits provided a supplementary evaluation of the persistence of the response to the H1N1 vaccine at 13 mo after vaccination. It is important to note that after completion of the primary series of immunization and collection of the post dose 2 blood specimen, many subjects received an additional H1N1 vaccination according to the Finnish national recommendation. Such children were not eligible for the antibody persistence assessment at 8- or 13 mo-follow up. A phone call was organized 12 mo after the second H1N1 vaccination to collect safety information from the subjects who did not participate in the seasonal influenza vaccination visits.
Vaccine
The influenza A/California/07/2009 (H1N1) New York Medical College X-179A vaccine seed strain was propagated in embryonated chicken eggs, inactivated and split according to the process used to produce a licensed seasonal influenza vaccine (Vaxigrip®, sanofi pasteur, Lyon, France). Vaccine was presented in multi-dose vials containing either 30 or 60 µg/ml HA and thiomersal as a preservative. The adjuvant (AF03, sanofi pasteur, Lyon, France), which was also presented in multi-dose vials, was a squalene-based, oil-in-water emulsion stabilized by two non-ionic surfactants and prepared to have a very fine droplet size (mean particle diameter: < 100 nm) and a narrow particle size distribution. Adjuvanted candidate vaccines were prepared extemporaneously. The vaccines were given intramuscularly. Each 0.5 ml dose contained either 15 µg HA and no adjuvant, 3.8 µg HA and adjuvant, or 7.5 µg HA and adjuvant. In children 3 to 17 y of age the same quantity of adjuvant was present in each adjuvanted vaccine (12.4 µg or 2.5% w/w squalene). In children 6 to 35 mo of age the amount of adjuvant was halved (6.2 µg or 1.25% w/w squalene) and given with antigen dose levels of 3.8 µg HA and 1.9 µg HA respectively.
Serology
Serum samples were tested for antibodies against the vaccine strain using hemagglutination inhibition (HI) and virus neutralization (NT) assays according to standard methods.Citation27,Citation28 HI testing was performed using turkey erythrocytes. The sample titer was the highest reciprocal serum dilution that inhibited hemagglutination completely. The neutralizing activity was measured by using a microneutralization assay format with enzyme-linked immunosorbent read-out. For both the HI assay and the NT assay, ten 2-fold dilutions from an initial dilution of 1:10 were tested. For calculation of geometric mean titers (GMTs), samples that were negative at the initial dilution of 1:10 were assigned the titer of 5. Each sample was tested twice (in two independent HI assay runs and in the same NT assay run), with the geometric mean of the two results recorded as the final titer, expressed as reciprocal dilution. Assays were performed at the sponsor's central clinical immunology laboratory (Swiftwater, PA, USA) under blinded conditions (group allocation was not indicated on the serum samples or on the accompanying listing). Both assays were validated according to ICH guidelines.
Safety and reactogenicity
Solicited and unsolicited injection site and systemic adverse events were recorded by the subjects’ parents on a daily basis in a diary card within 7 d of each vaccination. Solicited injection site reactions were pain, erythema, swelling, induration or ecchymosis. Solicited systemic reactions were fever, headache, malaise, myalgia and shivering for subjects aged at least 24 mo, and fever, vomiting, abnormal crying, drowsiness, lost appetite and irritability for subjects aged less than 24 mo. Unsolicited adverse events were recorded for 21 d after each vaccination and serious adverse events (SAEs) for the duration of the study. Occurrence of any of the following adverse events of special interest at any time during the study was also to be reported as SAE: anaphylaxis, Guillain-Barré syndrome, encephalitis, Bell’s palsy, neuritis, convulsions, vasculitis, demyelinating disorders, or vaccination failure (laboratory-confirmed influenza A H1N1 2009 infection). Besides, routine laboratory tests (hematology, AST, ALT, total bilirubin, GGT, and creatinine) were done before and 8 d following the first administration.
Statistical analysis
Immunogenicity data were expressed by group using GMT, geometric mean titer ratio (GMTR), HI seroprotection rate (% with titer ≥ 40), HI seroconversion rate (% with a pre-vaccination titer < 10 and a post-vaccination titer ≥ 40, or a pre-vaccination titer ≥ 10 and ≥ 4-fold increase after vaccination), NT seroconversion rate (% with ≥ 4-fold titer increase after vaccination).Citation29 Safety and reactogenicity data were summarized in terms of the number and proportion of participants per group reporting each type of reaction or adverse event, with unsolicited adverse events grouped by System Organ class and Preferred Term using the Medical Dictionary for Regulatory Activities. Injection site erythema, swelling, induration, and ecchymosis reported by children aged 12 or older were included in the analysis if they were at least 2.5 cm.Citation30 For younger children, all reactions were included. For each endpoint, 95% confidence intervals (CI) were calculated using the normal approximation for quantitative data and the exact binomial distribution (Clopper-Pearson method) for proportions. Immunogenicity data were analyzed according to the randomized group (full analysis set), safety data were analyzed according to the vaccine received.
Acknowledgments
The authors are grateful to the following for their contributions to the study: the clinical and project team members at sanofi pasteur, in particular to Anabelle Monnet, Yvan Couedel, Nathalie Rocher-Hélion, Fabienne Piras, Landry Bertaux, Jean-François Oudet, Pascale Chavand, Marie-Françoise Klucker, Christelle Leboucher, Levana Gionnet, Jérôme Richard, and Martin Dupuy. The contribution of Sanofi Pasteur’s central clinical immunology laboratory, and of Stephen W. Hildreth, Branda T. Hu, and Katherine Fries is particular, is also acknowledged. In Finland the study was conducted in the vaccine trial network of the Vaccine Research Center, University of Tampere. The work of Aino Karvonen, Head of the Study Clinics, and the following clinical investigators is gratefully acknowledged: Kristiina Kuismanen, Pia-Maria Lagerström-Tirri, Miia Virta, Johanna Immonen, Tiina Karppa, Tiina Korhonen, Niklas Lindblad, Ilkka Seppä, Simone Bianchi, Maarit Moisio and Tiina Haapaniemi. The final version of this manuscript was prepared with the assistance of Fred Vogel at Sanofi Pasteur.
Author contributions
All authors participated in the design and implementation of the trials. All authors were involved in the interpretation of data and the decision to submit for publication. TV and MD wrote the manuscript. All authors were involved at all steps of the preparation of the manuscript.
Funding statement
These 2 studies were sponsored by Sanofi Pasteur.
Disclosure of Potential Conflicts of Interest
TV declares consultancy fees from Merck, MedImmune, GlaxoSmithKline, Sanofi Pasteur / MSD and Novartis. He also received funding from Sanofi Pasteur for conducting the studies reported in this manuscript. SP, IK, AH and MD are employees of Sanofi Pasteur.
References
- World Health Organization. Pandemic (H1N1) 2009 – update 68. (Accessed October 9, 2009 at http://www.who.int/csr/don/2009_10_02/en/index.html)
- Chowell G, Bertozzi SM, Colchero MA, Lopez-Gatell H, Alpuche-Aranda C, Hernandez M, et al. Severe respiratory disease concurrent with the circulation of H1N1 influenza. N Engl J Med 2009; 361:674 - 9; http://dx.doi.org/10.1056/NEJMoa0904023; PMID: 19564633
- Centers for Disease Control and Prevention (CDC). Surveillance for pediatric deaths associated with 2009 pandemic influenza A (H1N1) virus infection - United States, April-August 2009. MMWR Morb Mortal Wkly Rep 2009; 58:941 - 7; PMID: 19730406
- Vaillant L, La Ruche G, Tarantola A, Barboza P, epidemic intelligence team at InVS. Epidemiology of fatal cases associated with pandemic H1N1 influenza 2009. Euro Surveill 2009; 14:19309; PMID: 19712643
- Shinde V, Bridges CB, Uyeki TM, Shu B, Balish A, Xu X, et al. Triple-reassortant swine influenza A (H1) in humans in the United States, 2005-2009. N Engl J Med 2009; 360:2616 - 25; http://dx.doi.org/10.1056/NEJMoa0903812; PMID: 19423871
- Jamieson DJ, Honein MA, Rasmussen SA, Williams JL, Swerdlow DL, Biggerstaff MS, et al, Novel Influenza A (H1N1) Pregnancy Working Group. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet 2009; 374:451 - 8; http://dx.doi.org/10.1016/S0140-6736(09)61304-0; PMID: 19643469
- Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, Hernandez M, Quiñones-Falconi F, Bautista E, et al, INER Working Group on Influenza. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med 2009; 361:680 - 9; http://dx.doi.org/10.1056/NEJMoa0904252; PMID: 19564631
- Levie K, Leroux-Roels I, Hoppenbrouwers K, Kervyn AD, Vandermeulen C, Forgus S, et al. An adjuvanted, low-dose, pandemic influenza A (H5N1) vaccine candidate is safe, immunogenic, and induces cross-reactive immune responses in healthy adults. J Infect Dis 2008; 198:642 - 9; http://dx.doi.org/10.1086/590913; PMID: 18576945
- Nolan T, Richmond PC, Formica NT, Höschler K, Skeljo MV, Stoney T, et al. Safety and immunogenicity of a prototype adjuvanted inactivated split-virus influenza A (H5N1) vaccine in infants and children. Vaccine 2008; 26:6383 - 91; http://dx.doi.org/10.1016/j.vaccine.2008.08.046; PMID: 18801398
- Banzhoff A, Gasparini R, Laghi-Pasini F, Staniscia T, Durando P, Montomoli E, et al. MF59-adjuvanted H5N1 vaccine induces immunologic memory and heterotypic antibody responses in non-elderly and elderly adults. PLoS One 2009; 4:e4384; http://dx.doi.org/10.1371/journal.pone.0004384; PMID: 19197383
- Vesikari T, Karvonen A, Tilman S, Borkowski A, Montomoli E, Banzhoff A, et al. Immunogenicity and safety of MF59-adjuvanted H5N1 influenza vaccine from infancy to adolescence. Pediatrics 2010; 126:e762 - 70; http://dx.doi.org/10.1542/peds.2009-2628; PMID: 20819892
- Frey S, Poland G, Percell S, Podda A. Comparison of the safety, tolerability, and immunogenicity of a MF59-adjuvanted influenza vaccine and a non-adjuvanted influenza vaccine in non-elderly adults. Vaccine 2003; 21:4234 - 7; http://dx.doi.org/10.1016/S0264-410X(03)00456-0; PMID: 14505903
- Vesikari T, Pellegrini M, Karvonen A, Groth N, Borkowski A, O’Hagan DT, et al. Enhanced immunogenicity of seasonal influenza vaccines in young children using MF59 adjuvant. Pediatr Infect Dis J 2009; 28:563 - 71; http://dx.doi.org/10.1097/INF.0b013e31819d6394; PMID: 19561422
- Garten RJ, Davis CT, Russell CA, Shu B, Lindstrom S, Balish A, et al. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science 2009; 325:197 - 201; http://dx.doi.org/10.1126/science.1176225; PMID: 19465683
- World Health Organization. Characteristics of the emergent influenza A (H1N1) viruses and recommendations for vaccine development. May 26 2009. (Accessed September 23, 2009, at http://www.who.int/csr/resources/publications/swineflu/H1N1Vaccinevirusrecommendation26May2009.pdf)
- Hancock K, Veguilla V, Lu X, Zhong W, Butler EN, Sun H, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med 2009; 361:1945 - 52; http://dx.doi.org/10.1056/NEJMoa0906453; PMID: 19745214
- Nolan T, McVernon J, Skeljo M, Richmond P, Wadia U, Lambert S, et al. Immunogenicity of a monovalent 2009 influenza A(H1N1) vaccine in infants and children: a randomized trial. JAMA 2010; 303:37 - 46; http://dx.doi.org/10.1001/jama.2009.1911; PMID: 20026597
- Arguedas A, Soley C, Abdelnour A, Sales V, Lindert K, Della Cioppa G, et al, Costa Rican H1N1 Vaccine Study Group. Assessment of the safety, tolerability and kinetics of the immune response to A/H1N1v vaccine formulations with and without adjuvant in healthy pediatric subjects from 3 through 17 years of age. Hum Vaccin 2011; 7:58 - 66; http://dx.doi.org/10.4161/hv.7.1.13411; PMID: 21285531
- Zhu F-C, Wang H, Fang H-H, Yang JG, Lin XJ, Liang XF, et al. A novel influenza A (H1N1) vaccine in various age groups. N Engl J Med 2009; 361:2414 - 23; http://dx.doi.org/10.1056/NEJMoa0908535; PMID: 19846844
- Plennevaux E, Blatter M, Cornish MJ, Go K, Kirby D, Wali M, et al. Influenza A (H1N1) 2009 two-dose immunization of US children: an observer-blinded, randomized, placebo-controlled trial. Vaccine 2011; 29:1569 - 75; http://dx.doi.org/10.1016/j.vaccine.2010.12.116; PMID: 21219979
- Roman F, Vaman T, Kafeja F, Hanon E, Van Damme P. AS03(A)-Adjuvanted influenza A (H1N1) 2009 vaccine for adults up to 85 years of age. Clin Infect Dis 2010; 51:668 - 77; http://dx.doi.org/10.1086/655830; PMID: 20687838
- Clark TW, Pareek M, Hoschler K, Dillon H, Nicholson KG, Groth N, et al. Trial of 2009 influenza A (H1N1) monovalent MF59-adjuvanted vaccine. N Engl J Med 2009; 361:2424 - 35; http://dx.doi.org/10.1056/NEJMoa0907650; PMID: 19745215
- Waddington CS, Walker WT, Oeser C, Reiner A, John T, Wilkins S, et al. Safety and immunogenicity of AS03B adjuvanted split virion versus non-adjuvanted whole virion H1N1 influenza vaccine in UK children aged 6 months-12 years: open label, randomised, parallel group, multicentre study. BMJ 2010; 340:c2649; http://dx.doi.org/10.1136/bmj.c2649; PMID: 20508026
- Carmona A, Omeñaca F, Tejedor JC, Merino JM, Vaman T, Dieussaert I, et al. Immunogenicity and safety of AS03-adjuvanted 2009 influenza A H1N1 vaccine in children 6-35 months. Vaccine 2010; 28:5837 - 44; http://dx.doi.org/10.1016/j.vaccine.2010.06.065; PMID: 20600478
- Garcia-Sicilia J, Gillard P, Carmona A, Tejedor JC, Aristegui J, Merino JM, et al. Immunogenicity and safety of AS03-adjuvanted H1N1 pandemic vaccines in children and adolescents. Vaccine 2011; 29:4353 - 61; http://dx.doi.org/10.1016/j.vaccine.2011.04.011; PMID: 21504774
- Nassim C, Christensen S, Henry D, Holmes S, Hohenboken M, Kanesa-Thasan N. Identification of antigen and adjuvant doses resulting in optimal immunogenicity and antibody persistence up to 1 year after immunization with a pandemic A/H1N1 influenza vaccine in children 3 to < 9 years of age. Pediatr Infect Dis J 2012; 31:e59 - 65; http://dx.doi.org/10.1097/INF.0b013e31824b9545; PMID: 22418661
- The World Health Organization (WHO). WHO Manual on Animal Influenza Diagnosis and Surveillance. 2002. Accessed October 21, 2009 at http://www.who.int/vaccine_research/diseases/influenza/WHO_manual_on_animal-diagnosis_and_surveillance_2002_5.pdf
- Kendal AP, Pereira MS, Skehel JJ, eds. Concepts and Procedures for Laboratory-Based Influenza Surveillance. Atlanta, GA: Centers for Disease Control and Prevention; 1982.
- Committee for Proprietary Medicinal Products (CPMP). Note for guidance on harmonisation of requirements for influenza vaccines. CPMP/BWP/214/96. The European Agency for the Evaluation of Medicinal Products (EMEA), March 1997. (Accessed September 25, 2009, at http://www.emea.europa.eu/pdfs/human/bwp/021496en.pdf)
- FDA Guidance for Industry. Toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. September 2007. Accessed October 15, 2009 at http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm091977.pdf