Abstract
Paracoccidioidomycosis is a granulomatous pulmonary infection that is generally controlled by chemotherapy. The efficacy of treatment, however, is limited by the status of the host immune response. The inhibition of a Th-2 immunity or the stimulation of Th-1 cytokines generally increases the efficacy of antifungal drugs.Citation1 This has been achieved by immunization with an internal peptide of the major diagnostic antigen gp43 of Paracoccidioides brasiliensis. Peptide 10 (QTLIAIHTLAIRYAN) elicits an IFN-γ rich Th-1 immune response that protects against experimental intratracheal infection by this fungus. The combination of chemotherapy with P10 immunization showed additive protective effect even after 30 d of infection or in anergic mice, rendering in general, increased production of IL-12 and IFN-γ and reduction of IL-4 and IL-10. Immunotherapy with P10 even in the absence of simultaneous chemotherapy has been effective using various protocols, adjuvants, nanoparticles, P10-primed dendritic cells, and especially a combination of plasmids encoding the P10 minigene and IL-12. Gene therapy, in a long-term infection protocol succeeded in the virtual elimination of the fungus, preserving the lung structure, free from immunopathological side effects.
Introduction
Paracoccidioidomycosis (PCM) is the prevalent systemic fungal disease in Latin America. It is acquired by inhalation of conidia of Paracoccidioides brasiliensis. Initial foci of infection may regress or evolve with or without latency into clinical forms, acute or subacute, with lymphatic dissemination, and chronic, with localized lesions or spreading to different organs or systems. Characteristic of this mycosis is the granulomatous inflammatory response with formation of epithelioid tubercles, which represent an effective defense mechanism against invading yeast-forms of the fungus. Severe cases mainly in immune-compromised individuals may evolve to disseminated disease, with fatal cases representing half of the total deaths by systemic mycoses in Brazil.Citation2
Itraconazole is the choice treatment of PCM forms of low or moderate severity. The treatment may last from 6 to 18 mo. Trimethoprim together with sulfamethoxazole can also be used during 12 to 24 mo depending on the severity of the disease. Hospitalized patients are treated intravenously with amphothericin B.Citation3 Relapses are common events in PCM patients. After chemotherapy lung fibrosis is a frequent sequel. Stimulation of the immune response avoiding untoward effects of immunopathology and reduction of the fungal load are aims to be pursued for an effective treatment of severe cases of PCM.
Peptide 10 (P10) and protection against experimental PCM
The major diagnostic antigen of P. brasiliensis is the gp43 described in 1986.Citation4 A few other important antigenic molecules of this fungus have also been investigated,Citation5 but not systematically as a potential vaccine. The gp43 elicits an IFN-γ dependent Th-1 response that is protective against intratracheal infection by virulent yeast forms of Paracoccidioides. The T-CD4+ epitope was mapped to a 15-mer peptide (P10) that was presented by 3 murine haplotypes.Citation6 P10 was also promiscuously presented by HLA-DRs. The peptide and its analogous with N-terminal lysine and without the C-terminal asparagine bound to the 9 most prevalent HLA-DR molecules.Citation7 Using the Tepitope algorithm, the P10 and neighboring peptides were predicted to bind to 90% of the 25 Caucasian HLA-DR haplotypes.
Validation of P10 as a protective antigen was achieved using a series of protocols, adjuvants, delivery systems and treatment combinations on the intratracheal infection model in susceptible mice. Balb/c and B10.A mice were chosen for i.t. infection with virulent yeast forms of P. brasiliensis, isolate Pb18. The various modalities of protective P10-immunization testing are summarized on .
Table 1. Protective immunizations with P10: adjuvants, peptide delivery and gene therapy
Both the gp43 and P10 emulsified in complete Freund’s adjuvant protected mice against i.t. challenge with the virulent P. brasiliensis isolate. Disease progression is followed by counting tissue colony-forming units (CFUs) and histopathology. Protection depended on the production of IFN-γ which plays an important role in the organization of fungal containing granulomas. No protection by P10 immunization was observed in IFN-γ-R knockout mice.Citation8 In fact, upon i.t. challenge with virulent yeasts of P. brasiliensis all IFN-γ, IFN-γ-R or IRF-1 knockout mice died after 3–4 weeks in great contrast with infected wild-type mice.
The combination of chemotherapy and P10 immunization in complete Freund’s adjuvant (CFA) followed by incomplete FA, conferred additive protection in comparison with drug treatment or P10 alone, with significant reduction in the fungal load (measured as CFUs). Different protocols were used with chemotherapy starting after 48 h or after 30 d of i.t. infection. Mice received i.p. doses of itraconazole, fluconazole, ketoconazole, sulfamethoxazole, or trimethoprim-sulfamethoxazole every 24 h. P10 immunization was administered weekly, for 4 weeks.Citation9 Treatment with sulfamethoxazole rendered partial protection followed by relapse which was, however, controlled by P10 immunization. Chemotherapy which succeeded in reducing the fungal load stimulated a Th-2 response with increased IL-4 and IL-10. In contrast P10 administration induced an IFN-γ and IL-12-rich immune response. In a different protocol, drug treatment and P10 immunization were started after 60 and 120 d of infection. Also in this case 60–80% reduction of the fungal load (CFUs) was observed. More stringent conditions were tested using anergic mice by oral intake of dexamethasone-21 phosphate. After 30 d mice were infected and 15 d later they were treated with chemotherapeutics alone or combined with P10 immunization. The latter proved to be effective even in this case increasing the levels of IL-12 and IFN-γ while significantly reducing the infection.Citation10
Adjuvants and P10 delivery systems
In order to improve the efficacy of the immune response generated by P10 and aiming at human vaccination, different adjuvants and delivery systems were investigated. A multiple antigen peptide (MAP) construction with 4 equal LIAIHTLAIRYAN chains linked to branched lysine was as effective as P10 in stimulating sensitized lymph node cells proliferation and was protective in vivo without CFA adjuvancy.Citation11
In substitution for CFA, which is not allowed for human use other formulations were studied in combination with P10, e.g., aluminum hydroxide, flagellin and the cationic lipid, dioctadecyl-dimethylammonium bromide (DODAB). Balb/c mice infected with P. brasiliensis Pb18 were best treated by DODAB and P10 followed by Salmonella FliC flagellin and P10.Citation12,Citation13 Results were evaluated 52 d after i.t. infection and the effective treatment was translated as low numbers of viable yeast cells, little granuloma formation and fibrosis. Treated animals produced IFN-γ and TNF-α. Tissues from unimmunized infected control mice had abundant collagen I fibers (Masson’s staining) within cell infiltrates with large numbers of fungal cells. Immunization with FliC flagellin and P10 showed lungs with diffuse collagen but no yeast cells. Mice immunized with DODAB and P10 displayed preserved lung tissue with no increase in collagen fibers.Citation12 In the fungal infected lung a chronic damage leads to pulmonary fibrosis owing to persistent antigenic stimulation and active immune response. Granulomatous inflammatory response, with increased collagen I and III, is the basis of late fibrotic sequelsCitation14 therefore, the combination of DODAB and P10 while protective did not exacerbate the inflammatory response.
To improve the delivery of P10, the i.t. infection model was used and animals were treated daily with sulfamethoxazole/trimethoprim alone or combined with P10 entrapped in poly(lactic acid-glycolic acid) nanoparticles.Citation15 Mice with the combined therapy showed a marked reduced fungal load in the lungs in which P10 entrapped within the nanoparticles (1 μg/50μl) was more effective than free P10 emulsified in CFA (20 μg/50 μl) as an adjuvant to chemotherapyCitation14 remarkably lowering the peptide amount necessary to elicit a protective response.
Dentritic cells are 1,000-fold more efficient than CFA in activation of T cells. Therefore P10-primed DCs were tested for protective effects in the i.t. infection model in Balb/c mice. Prophylactic and therapeutic protocols were used, by subcutaneous vaccination of P10-primed DCs before infection, and by subcutaneous or intravenous vaccination weeks after infection.Citation16 Immunization with P10-primed DCs induced the production of IFN-γ and IL-12 with low IL-10 and IL-4, simultaneously with reduced fungal load and low tissue damage. Potentially, this kind of immunization may confer rapid protection against serious cases of PCM and successfully treat established disease.
Therapeutic DNA vaccine encoding P10
A vaccine against P. brasiliensis using plasmid DNA was originally tested in 2000Citation17 using a mammalian expression vector carrying the full gene of the gp43 under the control of CMV promoter with Freund's adjuvant. Immunization of Balb/c mice induced a mixed Th-1/Th-2 long-lasting cellular immune response, chiefly modulated by IFN-γ. Such immunization was protective in mice prior to i.t. challenge with virulent P. brasiliensis. A more directed immune response aiming at a Th-1 protective response was obtained with a plasmid expressing the minigene P10.
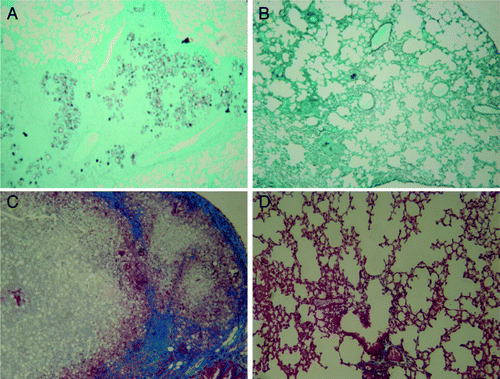
In previous protective experiments using P10, the medium susceptible mouse strain Balb/c was used in short-term experiments. In most cases definite but partial protection was achieved with reduced fungal load and various degrees of tissue damage, depending on the inflammatory response. A more stringent model with the highly susceptible B10.A mouse strain was then chosen to test a long-term protocol with a combination of plasmids encoding either the P10 minigene or the p70-IL-12 chain.Citation18 Regular immunoprophylactic and short-term therapeutic protocols were also run with Balb/c mice, resulting in remarkable protective results (). With the long-term (6 mo) protocol, involving intramuscular injection of plasmids expressing P10 and IL-12 given weekly for one month post-infection and as single injections every next month for 3 mo (last injection 1 mo before sacrifice) virtually eliminated detectable fungi while restoring the normal lung parenchyma. At least in preclinical experiments immunization with these plasmids proved to be a powerful procedure for treatment of installed PCM with no evidence of fibrotic sequels.
Figure 1. Histopathology of lungs from intratracheally infected BALB/c mice. Animals were infected with P. brasiliensis Pb18 for 30 d and treated with pcDNA3-P10 and pORF-mIL-12. (A and C) Untreated infected mice; (B and D) Infected mice immunized with plasmids pP10 and pIL-12 (A and B). Gomori staining showing yeast cells embedded in the lung infiltrate and clearing with plasmid treatment; (C and D) Masson’s trichrome staining showing blue collagen fibers, a granuloma and great number of yeast cells, and the effect of plasmid treatment. Original magnification, 100X.
Conclusions and Perspectives
The rare event of the complete identification of a diagnostic and protective antigen in a systemic mycosis makes it possible to examine a variety of immunization protocols and techniques that help to advance toward a human vaccine. Chemotherapy in fungal diseases needs to be complemented by an effective immune response for long-term control and eventual sterilization of the infection. Relapses should be prevented as well as sequels of hyper inflammation. Gp43 and its 15-mer internal peptide P10 have proved to be protective against experimental paracoccidioidomycosis using immune-prophylactic and immune-therapeutic protocols. The most encouraging result is that of DNA therapy encoding P10 and IL-12. Further adjuvancy is given by the plasmids themselves and, even if a primary response to the infection stimulated Th-1 effector cells producing pro-inflammatory cytokines, the long-term treatment may stabilize the immune response with memory cells and FoxP3+ T-regsCitation19 that control the infection while reducing the immune pathology.Citation20
Acknowledgments
The research work has been supported by Fundação de Amparo a Pesquisa do Estado de São Paulo, Brazil, grant 2010/51423–0, and the Brazilian National Research Council (CNPq).
Disclosure of Potential Conflicts of Interest
There is no conflict of interest. This work has been exclusively financed by a State Agency for support of Scientific Research (Fapesp) and the Brazilian National Research Council (CNPq)
References
- Romani L. Immunity to fungal infections. Nat Rev Immunol 2011; 11:275 - 88; http://dx.doi.org/10.1038/nri2939; PMID: 21394104
- Prado M, Silva MB, Laurenti R, Travassos LR, Taborda CP. Mortality due to systemic mycoses as a primary cause of death or in association with AIDS in Brazil: a review from 1996 to 2006. Mem Inst Oswaldo Cruz 2009; 104:513 - 21; http://dx.doi.org/10.1590/S0074-02762009000300019; PMID: 19547881
- Travassos LR, Taborda CP. Paracoccidioidocycosis: Advances in treatment incorporating modulators of the immune response. [Remedica Journals.] J Invasive Fungal Infect 2011; 5:1 - 6
- Puccia R, Schenkman S, Gorin PA, Travassos LR. Exocellular components of Paracoccidioides brasiliensis: identification of a specific antigen. Infect Immun 1986; 53:199 - 206; PMID: 2424841
- Travassos LR, Goldman G, Taborda CP, Puccia R. Insights in Paracoccidioides brasiliensis pathogenicity. In New Insights in Medical Mycology, Kevin Kavanagh, editor; Springer, Dordrechet, The Netherlands; 2007, pp. 241-265.
- Taborda CP, Juliano MA, Puccia R, Franco M, Travassos LR. Mapping of the T-cell epitope in the major 43-kilodalton glycoprotein of Paracoccidioides brasiliensis which induces a Th-1 response protective against fungal infection in BALB/c mice. Infect Immun 1998; 66:786 - 93; PMID: 9453642
- Iwai LK, Yoshida M, Sidney J, Shikanai-Yasuda MA, Goldberg AC, Juliano MA, et al. In silico prediction of peptides binding to multiple HLA-DR molecules accurately identifies immunodominant epitopes from gp43 of Paracoccidioides brasiliensis frequently recognized in primary peripheral blood mononuclear cell responses from sensitized individuals. Mol Med 2003; 9:209 - 19; PMID: 15208742
- Travassos LR, Taborda CP, Iwai LK, Cunha-Neto E, Puccia R. The gp43 from Paracoccidioides brasiliensis: a major diagnostic antigen and vaccine candidate. In The Mycota XII, Human Fungal Pathogens; JE Domer, GS Kobayashi, editors. Springer-Verlag, Berlin-Heildeberg; 2004, pp. 279-96.
- Marques AF, da Silva MB, Juliano MA, Travassos LR, Taborda CP. Peptide immunization as an adjuvant to chemotherapy in mice challenged intratracheally with virulent yeast cells of Paracoccidioides brasiliensis. Antimicrob Agents Chemother 2006; 50:2814 - 9; http://dx.doi.org/10.1128/AAC.00220-06; PMID: 16870776
- Marques AF, da Silva MB, Juliano MA, Munhõz JE, Travassos LR, Taborda CP. Additive effect of P10 immunization and chemotherapy in anergic mice challenged intratracheally with virulent yeasts of Paracoccidioides brasiliensis. Microbes Infect 2008; 10:1251 - 8; http://dx.doi.org/10.1016/j.micinf.2008.07.027; PMID: 18694844
- Taborda CP, Nakaie CR, Cilli EM, Rodrigues EG, Silva LS, Franco MF, et al. Synthesis and immunological activity of a branched peptide carrying the T-cell epitope of gp43, the major exocellular antigen of Paracoccidioides brasiliensis. Scand J Immunol 2004; 59:58 - 65; http://dx.doi.org/10.1111/j.0300-9475.2004.01359.x; PMID: 14723622
- Braga CJ, Rittner GM, Muñoz Henao JE, Teixeira AF, Massis LM, Sbrogio-Almeida ME, et al. Paracoccidioides brasiliensis vaccine formulations based on the gp43-derived P10 sequence and the Salmonella enterica FliC flagellin. Infect Immun 2009; 77:1700 - 7; http://dx.doi.org/10.1128/IAI.01470-08; PMID: 19204092
- Mayorga O, Muñoz JE, Lincopan N, Teixeira AF, Ferreira LC, Travassos LR, et al. The role of adjuvants in therapeutic protection against paracoccidioidomycosis after immunization with the P10 peptide. Front Microbiol 2012; 3:154; http://dx.doi.org/10.3389/fmicb.2012.00154; PMID: 22586420
- Naranjo TW, Lopera DE, Diaz-Granados LR, Duque JJ, Restrepo AM, Cano LE. Combined itraconazole-pentoxifylline treatment promptly reduces lung fibrosis induced by chronic pulmonary paracoccidioidomycosis in mice. Pulm Pharmacol Ther 2011; 24:81 - 91; http://dx.doi.org/10.1016/j.pupt.2010.09.005; PMID: 20851204
- Amaral AC, Marques AF, Muñoz JE, Bocca AL, Simioni AR, Tedesco AC, et al. Poly(lactic acid-glycolic acid) nanoparticles markedly improve immunological protection provided by peptide P10 against murine paracoccidioidomycosis. Br J Pharmacol 2010; 159:1126 - 32; http://dx.doi.org/10.1111/j.1476-5381.2009.00617.x; PMID: 20136827
- Magalhães A, Ferreira KS, Almeida SR, Nosanchuk JD, Travassos LR, Taborda CP. Prophylactic and therapeutic vaccination using dendritic cells primed with peptide 10 derived from the 43-kilodalton glycoprotein of Paracoccidioides brasiliensis. Clin Vaccine Immunol 2012; 19:23 - 9; http://dx.doi.org/10.1128/CVI.05414-11; PMID: 22089247
- Pinto AR, Puccia R, Diniz SN, Franco MF, Travassos LR. DNA-based vaccination against murine paracoccidioidomycosis using the gp43 gene from paracoccidioides brasiliensis. Vaccine 2000; 18:3050 - 8; http://dx.doi.org/10.1016/S0264-410X(00)00074-8; PMID: 10825609
- Rittner GM, Muñoz JE, Marques AF, Nosanchuk JD, Taborda CP, Travassos LR. Therapeutic DNA vaccine encoding peptide P10 against experimental paracoccidioidomycosis. PLoS Negl Trop Dis 2012; 6:e1519.
- Travassos LR, Taborda CP. New advances in the development of a vaccine against paracoccidioidomycosis. Front Microbiol 2012; 3:212; http://dx.doi.org/10.3389/fmicb.2012.00212; PMID: 22701452
- Carvalho A, Cunha C, Iannitti RG, Casagrande A, Bistoni F, Aversa F, et al. Host defense pathways against fungi: the basis for vaccines and immunotherapy. Front Microbiol 2012; 3:176; http://dx.doi.org/10.3389/fmicb.2012.00176; PMID: 22590466