Abstract
Gene-modified cell vaccines are the best way to achieve the immunotherapy for all types of acute leukemia. In this study, the recombinant eukaryotic expression vector (pDisplay-HSP70) of heat shock protein 70 (HSP70) of Bacille Calmette-Guérin (BCG) was constructed by amplifying the whole BCG HSP70 gene using polymerase chain reaction (PCR) and sub-cloning into the polyclone endonuclease sites in pDisplay. Then the HL-60 cell vaccine expressing the protein onto the cell surface was prepared by lipofectamine transfection and its anti-tumor effect and mechanism were further studied. Results showed that the fragment of BCG HSP70 was consistent with Mycobacterium tuberculosis HSP70 gene published in GeneBank. DNA sequencing showed that the recombinant vector was correctly constructed and named pDisplay-HSP70. After BCG HSP70 gene transfection, the yellow-green fluorescence on the HL-60 cells surface was observed under a fluorescence microscope. The immunogenicity of HSP70-transfected HL-60 cells exhibited upregulated proliferation of lymphocytes, increased cytokine secretion (IFN-γ) and enhanced killing activity. These results suggested that gene transfection of BCG HSP70 could significantly enhance the immunogenicity of HL-60 cells. It may be used as a suitable candidate gene-modified cell vaccine for cancer immunotherapy.
Introduction
Childhood leukemia is a curable disease, and combination chemotherapy can make long-term complete remission (CR) rate of 70–80%.Citation1 But there are still about 30% of the children died of leukemia relapse.Citation2 Minimal residual disease (MRD) of leukemia is the main cause of leukemia relapse and the major obstacle to cure leukemia. Its clearance, chemotherapy alone does not work, but need to rely on immune function.Citation3 Meanwhile, since the existence of individual differences, gene-modified cell vaccines are the best way to achieve the immunotherapy for all types of acute leukemia.
Our previous study has showed that Bacille Calmette-Guérin (BCG) and inactivated BCG could significantly improve the immune function of children with acute leukemia.Citation4 However, serious adverse reactions hinder the clinical application of the BCG; and due to strain variation in the process of passage and attenuation, the immune effects become unstable. BCG heat shock protein70 (BCG HSP70), which is the major antigenic component of BCG,Citation5 has a characteristic of immunodominant antigen and can be used as an adjuvant to stimulate a strong immune response against tumor antigens, without the need for additional adjuvant.Citation6 At present, the basic study on anti-tumor effect of BCG HSP70 mainly included the anti-tumor effect of dendritic cells induced by BCG HSP70 protein in vivo; and the major role on the solid tumors, such as melanoma,Citation7 liver cancer,Citation8 hepatoblastoma,Citation9 colorectal cancerCitation10 and glioblastoma etc.Citation11 There is no related research on leukemia. So, in this study, the recombinant eukaryotic expression vector of BCG HSP70 was constructed, and the HL-60 (standard acute promyelocytic cell line) cells vaccine expressing the protein onto the cell surface was prepared after gene transfection, so as to study its anti-tumor effect and mechanism.
Results
Vector construction
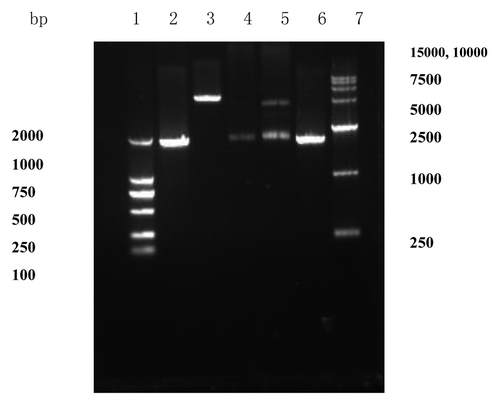
As demonstrated in , an approximate 1880bp fragment, obtained from genomic DNA of standard strains H37Rv by PCR amplification, and showed on the agarose gel electrophoresis, was consistent with Mycobacterium tuberculosis HSP70 gene released by GeneBank. Then the HSP70 was sub-cloned into the polyclone endonuclease sites in the expressing vector pDisplay. The two ways, restriction endonuclease digestion and PCR amplification, were used to identify that HSP70 gene was sub-cloned into the polyclone endonuclease sites in pDisplay. As demonstrated in , when the recombinant product was digested by restriction endonuclease BglII and SmaI respectively, the 1% agarose gel electrophoresis showed that two fragments (Lane 5), approximate 1880bp and 5300 bp, were consistent with the theoretical value of HSP70 (1878bp) and vector pDisplay (5300bp) (Lane 4 and Lane 3). The recombinant product being as a template, an approximate 1880bp fragment was obtained by PCR amplification (Lane 6). Then DNA sequencing showed that the recombinant vector was correctly constructed and named pDisplay-HSP70.
Figure 1. Agarose gel electrophoresis of PCR product. Lane 1: PCR product of HSP70; Lane 2: DNA marker (DL2000). The whole BCG HSP70 gene was amplified by using PCR and an approximate 1880bp fragment was consistent with Mycobacterium tuberculosis HSP70 gene released by GeneBank.
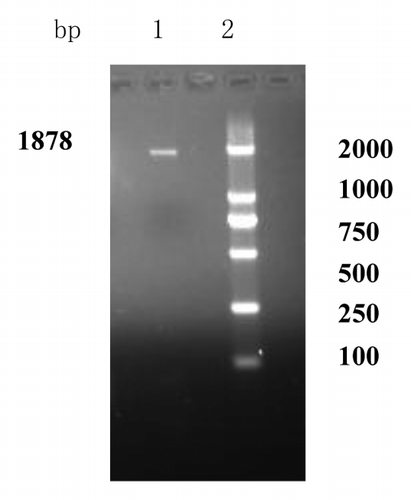
Figure 2. Identification of recombinant plasmid pDisplay-HSP70. Lane 1: DNA marker (DL2000); Lane 2: PCR product of HSP70; Lane 3: plasmid pDisplay digested with BglII and SmaI; Lane 4: HSP70 digested with BglII and SmaI; Lane 5: recombinant plasmid pDisplay-HSP70 digested with BglII and SmaI; Lane 6: PCR product from recombinant plasmid pDisplay-HSP70; Lane 7: DNA marker (DL15000). The two ways, restriction endonuclease digestion and PCR amplification, were used to identify that HSP70 gene was sub-cloned into the polyclone endonuclease sites in pDisplay.
Gene transfection
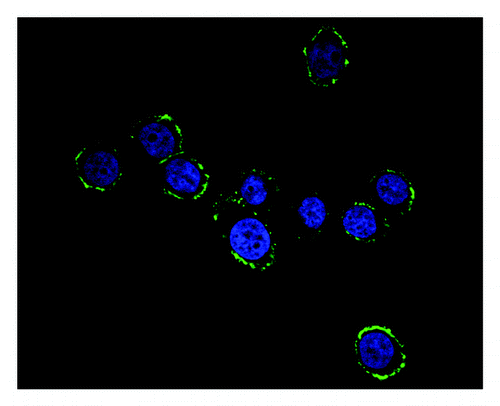
As demonstrated in , after being stained with DAPI and then labeled with heat shock protein 70 mAb and FITC conjugated goat anti-mouse IgG, the yellow-green fluorescence on the HL-60 cells surface was clearly observed on the basis of the nucleus stained blue under a fluorescence microscope with the appropriate band excitation. It showed that HSP70 was successfully transfected into HL-60 cells and expressed on the cells surface.
Figure 3. Fluorescene microscopy of HL60-HSP70 cells after staining with heat shock pritein 70 mAb and secondary Ab conjugated with FITC. The yellow-green fluorescence on the HL-60 cells surface was observed on the basis of the nucleus stained blue and it showed that HSP70 was successfully transfected into HL-60 cells surface.
Detection of the immunogenicity
Lymphocyte proliferation, cytokines secretion and cytotoxicity assay were measured to detect the immunogenicity of leukemia cells after gene transfection of BCG HSP70.
1. T-cell proliferation
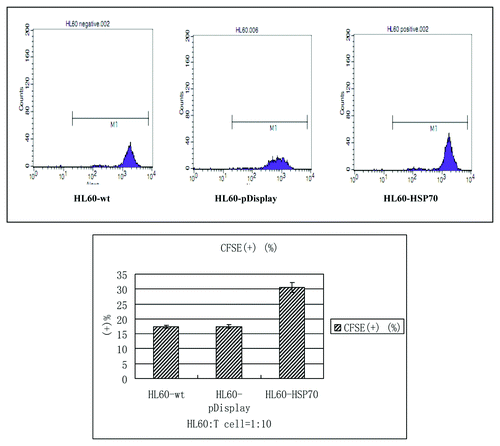
The HL-60 cells of different groups were cultured with the peripheral blood T cells at the radio of 1:10 for 72h. The proliferation indices of T cells were assayed by CFSE-staining method. The positive rates of the HL60-wt group, HL60-pDisplay group and HL60-HSP70 group were (17.42 ± 0.41)%, (17.49 ± 0.67)% and (30.58 ± 1.68)%, respectively. It showed that HSP70-transfected HL-60 cells stimulated the most significant T cell proliferation, compared with that of the HL60-wt group and HL60-pDisplay group (; ).
Table 1. Comparison of the positive rate of CFSE in different groups( ± S).
± S).
Figure 4. T cell proliferation. Purified T cells were co-cultured with the mitomycin C-treated different HL-60 cells at the radio of 10:1 for 72h. HSP70-transfected HL-60 cells stimulated the most significant T cell proliferation, compared with that of the HL60-wt group and HL60-pDisplay group. p < 0.05.
2. Cytokine level
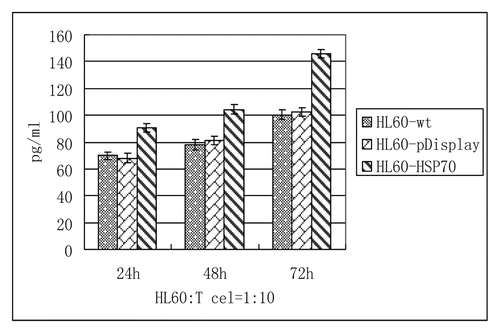
The culture supernatants were collected at 24h, 48h and 72h respectively, and the cytokine concentrations (IFN-γ) were detected by ELISA kits according to the instructions. As demonstrated in and , consistent with the T cells proliferation by CFSE-staining, the highest cytokine level (IFN-γ) was stimulated to secret in the group of HSP70-transfected HL-60 cells. And with the increase of time, the level of IFN-γ was rising.
Table 2. The contents of IFN-γ by ELISA ( ± S) (pg/ml)
± S) (pg/ml)
Figure 5. Regulation of T cells function (IFN-γ). Purified T cells were co-cultured with HL60-wt, HL60-pDisplay and HL60-HSP70 cells at the radio of 10:1. Cytokine production of IFN-γ from T cells was measured by ELISA. The highest cytokine level (IFN-γ) was stimulated to secret in the group of HSP70-transfected HL-60 cells. And with the increase of time, the level of IFN-γ was rising. p < 0.05.
3. Cytotoxicity assay
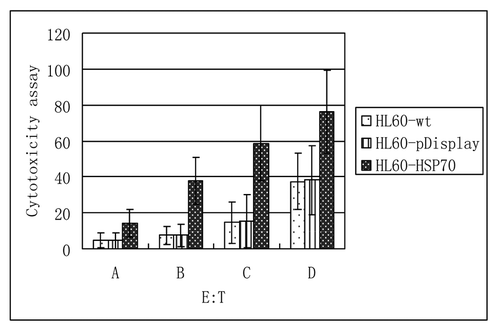
Cytotoxicity assay was measured by LDH release method according to the instruction. As shown in and , when the ratio of E: t = 10:1, the killing activity of the HL60-HSP70 group was (14.11 ± 7.48)%, which is higher than that of the HL60-wt group and HL60-pDisplay group; Similarly, at the different ratio of E:t = 20:1, 40:1, 80:1, the inhibiting activity of CTLs on HL-60 cells in the group of HSP70-transfected HL-60 cells was more significant, in comparison to that of wild-type HL-60 cells and pDisplay–transfected ones. And with the increase of the ratio from 10:1 to 80:1, the inhibiting activity of CTL in the HSP70-HL60 group was rising. It showed that gene transfection of BCG HSP70 could significantly enhance the killing activity.
Table 3. Cytotoxicity assay by LDH release method( ± S) (%)
± S) (%)
Figure 6. The inhibiting activity on HL60 cells by CTL cells (at the different ratio of E:t = .A (10:1), B (20:1), C (40:1), D (80:1)). The inhibiting activity of CTLs on HL-60 cells in the group of HSP70-transfected HL-60 cells was more significant, in comparison to that of wild-type HL-60 cells and pDisplay–transfected ones. And with the increase of the ratio from 10:1 to 80:1, the inhibiting activity of CTL in the HSP70-HL60 group was rising. p < 0.05.
From the aspects of the lymphocyte proliferation, cytokines secretion and cytotoxicity assay, it all indicated that gene transfection of BCG HSP70 significantly enhanced the immunogenicity of HL-60 cells.
Discussion
Immunotherapy is the most promising way to cure the children with acute leukemia, especially has an important in the clearance of MRD and relapse prevention. In addition, immunotherapy has the function of non-specific stimulating bone marrow, by promoting the proliferation of normal stem cells, so that the body can tolerate higher doses of chemotherapy and is less prone to bone marrow failure.Citation12 Because leukemia cells lack the necessary components of stimulating the immune response, and host T cells cannot effectively recognize and kill them, so immune escape occurs. Also, because of immune dysfunction, and reduced immune surveillance and immune clearance of leukemia cells, leukemia cells show the uncontrolled proliferation of malignant clones.Citation13,Citation14 Therefore, how to better stimulate the anti-tumor immunity and improve immune function becomes a research hotspot.
BCG HSP70, which is the major antigenic component of BCG, has a characteristic of immunodominant antigen and can induce and enhance the cellular immunity and humoral immunity.Citation15 Studies have shown that proteins of HSP70 family can enhance tumor cells to processing and presentation of tumor antigen, increase the levels of MHC-I molecules and activate directly tumor-specific T-cell response; Moreover, HSP70 itself has a strong immunogenicity and can be an adjuvant, without the need for additional adjuvant; Furthermore, HSP used in whole-cell vaccine has a unique advantage, that is, without identification and separation of tumor antigen, from the impact of tumor antigenic modulation, and breaking the tumor tolerance and avoiding the problem of tumor immune escape.Citation16-Citation18
From the classical immunological point, the target cells of immune destruction are tumor cells. Because of its possible tumor antigens, the use of tumor cells as a vaccine is the best choice.Citation19 Meanwhile, from the fundamental solution to the poor immunogenicity of leukemia cells themselves, which cannot produce anti-self-induced tumor-specific cellular immune response, and the existence of individual differences, gene-modified cell vaccines are the best way to achieve the immunotherapy for all types of acute leukemia. Therefore, in our study, the recombinant eukaryotic expression vector of BCG HSP70, which was named pDisplay-HSP70, was successfully constructed. And the HL-60 cells vaccine expressing the protein onto its surface was also successfully prepared.
Immunogenicity of leukemia cells is measured mainly by the lymphocyte proliferation, cytokines secretion and killing activity of cytotoxic T lymphocyte (CTL). At present, the lymphocyte proliferation after mixed lymphocyte culture has been detected by MTT assay and radioisotope method, but MTT assay is so rough that affect statistical results and there are hidden dangers in radioisotope method, so a more advanced CFSE (Carboxyfluorescein Succinimidyl ester) fluorescent dye method with flow cytometry was used in our study.Citation20,Citation21 CFSE showed that the BCG HSP70 gene transfected HL-60 cells significantly enhanced lymphocyte proliferation.
IFN-γ can promote Th0 cells to differentiate into Th1 cells, and promote the maturation and activity of CTL.Citation22 Our experimental results showed that the BCG HSP70 gene transfected HL-60 cells significantly increased the secretion of IFN-γ.
In addition, lactate dehydrogenase (LDH) release assay have been used to measure killing activity of NK cells/ CTLs.Citation23,Citation24 The results in our study, which showed that the killing rate of CTL in the group of HSP70-transfected HL-60 cells was significantly higher than that of the wild-type HL-60 cells and pDisplay–transfected ones, suggested that BCG HSP70 gene transfection could significantly enhance the killing activity of CTL on leukemia cells.
Based on those experiments in vitro, the eukaryotic expression vector of BCG HSP70 was successfully constructed and transfected into HL-60 cells surface. Moreover, gene transfection of BCG HSP70 significantly enhanced the immunogenicity of HL-60 cells. Thus, the immunological researches of BCG HSP70 gene-modified cells vaccine provide an experimental basis for the realization of individual and specific immunotherapy for all types of acute leukemia.
Materials and Methods
Reagents and cell cultures
Product of E.Z.N.A.® Endo-Free Plasmid Kit (Catalog#: D6926–01) was purchased from OMEGA. Restriction endonuclease BglII (Catalog#: D1021A) and SmaI (Catalog#: R0141) was purchased from New England Biolabs (NEB). LA tag enzyme (Catalog#: DRR02AG), T4 DNA ligase (Catalog#: D2011A) and E.coli Competent Cells DH5a (Catalog#: D9057) were purchased from TaKaRa. pDisplay vector (Catalog#: V660–20), Lipofectamine 2000 (Catalog#: 11668–019), and Carboxy-fluorescein in diacetate succinimidyl ester (CFSE) (Catalog#: C34544) were purchased from Invitrogen. Heat shock protein 70 mAb (mouse) (Catalog#: ABIN457460) and Fluorescein isothiocyanate (FITC) conjugated goat anti-mouse IgG (Catalog#:ABIN299978) were purchased from Cambridge bioscience (GmbH). The ELISA kit for detecting IFN-γ (Catalog#: BMS228TEN) was purchased from Jingmei Biotech. The LDH release kit (Catalog#: GMS50006) was purchased from Genmed Scientifics USA.
HL-60 cells were obtained from the American Type Culture Collection (ATCC) and cultured in IMDM medium with 10% fetal bovine serum (FBS) at 37°C in a fully humidified atmosphere with 5%CO2. Half of fresh medium were replaced every other day.
Construction of the recombinant eukaryotic expression vector of BCG HSP70
The whole gene encoding BCG HSP70 was obtained from genomic DNA of standard strains H37Rv (ATCC, NC_000962) by PCR amplification, using the forward primer 5′- GAAGATCTATGGCTCGTGCGGTCGGGAT -3′ (underline: BglII site) and the reverse primer 5′- TCCCCCGGGTGATTTCCGTCCGTCACTTG -3′ (underline: SmaI site). Then the HSP70, digested by restriction endonuclease BglII and SmaI respectively, was sub-cloned into the polyclone endonuclease sites in the expressing vector pDisplay by T4 DNA ligase. The recombinant product was transformed into DH5a competent cells from E.coli and the positive clones were picked out to amplify further. DNA was extracted and contaminated endotoxin preparation was removed by E.Z.N.A. ® Endo-Free Plasmid Mini Kit, of which endotoxin level was controlled less than 0.1EU/µg. The two ways, restriction endonuclease digestion and PCR amplification, were used to identify the recombinant product. Through the sequencing of genome further verified, and it’s named pDisplay-HSP70. Finally, the positive clones which were identified on the master plate were picked out to expand culture, and DNA was extracted by E.Z.N.A. ® Endo-Free Plasmid Maxi Kit.
Gene transfection
The recombinant eukaryotic expression vector pDisplay-HSP70 was transfected into HL-60 cells by using lipofectamine 2000 according to the instruction. 24h after transfection, the total cells were collected, added heat shock protein 70 mAb and incubated at 4°C overnight. The cells were washed twice by phosphate-buffered saline (PBS, 0.01M) and fixed in cold acetone at room temperature for 5–10min. Then the cells were washed twice again by PBS and FITC conjugated goat anti-mouse IgG were incubated at 4°C for 1h. Removal of the secondary antibody, the DAPI staining solution was added and incubated for 15min. The washed cells were detected by fluorescence microscope with .the appropriate band excitation.
Detection of the immunogenicity of HL-60 cells expressing HSP70
The test groups were divided into three subgroups, wild-type HL-60 cells (HL60-wt), pDisplay cells (HL60-pDisplay), and HSP70 cells (HL60-HSP70) respectively.
Peripheral blood mononuclear cells (PBMC) were collected from children with acute promyelocytic leukemia by density gradient separation in Ficoll-Hypaque and cultured with 10% FBS of RPMI 1640 at 37°C in a fully humidified atmosphere with 5%CO2. After culturing for 3h, suspension cells were collected and separated by nylon wool column to obtain T lymphocytes. Ten samples were obtained with written, informed consent from their parents, which were approved by the Ethics Committee of Human Experimentation in China.
1. T cell proliferation
The HL-60 cells of different groups were cultured with the peripheral blood T cells at the radio of 1:10 in the 96-well culture plates at a final volume of 200μl RPMI 1640 with 10% FBS at 37°Cand 5%CO2 for 72h. The proliferation indices of T cells were assayed by CFSE-staining method.Citation20,Citation21 According to the formula, the T cells were resuspended at a final concentration of 1 × 106/ml. Then the equal volume of CFSE (5uM) was added and incubated at 37°C for 10min. The staining was quenched by the addition of 5 volumes of ice-cold culture media to the cells and incubated 5min on ice. Then the cells were washed and resuspended in fresh media for a total of three times. The fluorescence intensity of CFSE was measured using flow cytometry with a 488nm excitation.
2. Cytokine production
The HL-60 cells of different groups were cultured with T cells at the radio of 1:10 at 37°Cand 5%CO2. The culture supernatants were collected at 24h, 48h and 72h, and then kept frozen at -80°C. The cytokine concentrations (IFN-γ) were detected by ELISA kits according to the instructions.
3. Cytotoxicity Assay
In the 96-well culture plates at a final volume of 200μl RPMI 1640 with 10% FBS, the T cells were co-cultured with the HL-60 cells of different groups at the radio of 10:1, added with IL-2 (20ng/ml), at 37°C and 5%CO2 for 6d to obtain cytotoxic T lymphocytes, which were used as effector cells, and continued to co-culture with the wild-type HL-60 cells, which was regarded as target cells, (at the different ratio of E: t = 10:1, 20:1, 40:1, 80:1), for another 12h. Cytotoxicity assay was measured by LDH release method according to the instruction. The optical density (OD) of 490nm was measured by microplate reader.
Killing activity (%) =[(experimental group OD-natural release OD) /(maximum release OD-natural release OD)] ×100%
Statistical analysis
The results were expressed as mean ± S. The different groups were compared by Analysis of Variance (ANOVA) and p < 0.05 is considered statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Pui CH, Robison LL, Look AT. Acute lymphoblastic leukaemia. Lancet 2008; 371:1030 - 43; http://dx.doi.org/10.1016/S0140-6736(08)60457-2; PMID: 18358930
- Pui CH, Carroll WL, Meshinchi S, Arceci RJ. Biology, risk stratification, and therapy of pediatric acute leukemias: an update. J Clin Oncol 2011; 29:551 - 65; http://dx.doi.org/10.1200/JCO.2010.30.7405; PMID: 21220611
- Stow P, Key L, Chen X, Pan Q, Neale GA, Coustan-Smith E, et al. Clinical significance of low levels of minimal residual disease at the end of remission induction therapy in childhood acute lymphoblastic leukemia. Blood 2010; 115:4657 - 63; http://dx.doi.org/10.1182/blood-2009-11-253435; PMID: 20304809
- Yang J, Sun LR, Pang XY, Lu Y, Li XR, Song AQ. [Effect of Bacillus Calmette-Guerin on the expansion of dendritic cells from peripheral blood of pediatric patients with leukemia in vitro]. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2010; 18:1240 - 3; PMID: 21129268
- Peng ML, Chen M, Ling N, Xu H, Qing Y, Ren H. Novel vaccines for the treatment of chronic HBV infection based on mycobacterial heat shock protein 70. Vaccine 2006; 24:887 - 96; http://dx.doi.org/10.1016/j.vaccine.2005.12.050; PMID: 16446013
- Wei MQ, Mengesha A, Good D, Anné J. Bacterial targeted tumour therapy-dawn of a new era. Cancer Lett 2008; 259:16 - 27; http://dx.doi.org/10.1016/j.canlet.2007.10.034; PMID: 18063294
- Ye J, Chen GS, Song HP, Li ZS, Huang YY, Qu P, et al. Heat shock protein 70 / MAGE-1 tumor vaccine can enhance the potency of MAGE-1-specific cellular immune responses in vivo. Cancer Immunol Immunother 2004; 53:825 - 34; http://dx.doi.org/10.1007/s00262-004-0536-6; PMID: 15127237
- Zhang Y, Li ZJ, Ma ZH, Chen X, Pan XY, Zhang FC. Enhancement of DNA vaccine potency by linkage of antigen gene to an HSP70 gene. Cancer Res 2000; 60:1025 - 42
- Onishi H, Kuroki H, Matsumoto K, Baba E, Sasaki N, Kuqa H. Monocyte-derived dendritic cells that capture dead tumor cells secret IL-12 and TNF-alpha through IL-12/TNF-alpha/NF-kappaB autocrine loop. J Cancer Immunol Immunother 2004; 53:1093 - 100; http://dx.doi.org/10.1007/s00262-004-0568-y
- Cheng WF, Hung CF, Chai CY, Hsu KF, He L, Rice CM, et al. Enhancement of Sindbis virus self-replicating RNA vaccine potency by linkage of Mycobacterium tuberculosis heat shock protein 70 gene to an antigen gene. J Immunol 2001; 166:6218 - 26; PMID: 11342644
- Hsu KF, Hung CF, Cheng WF, He L, Slater LA, Ling M, et al. Enhancement of suicidal DNA vaccine potency by linking Mycobacterium tuberculosis heat shock protein 70 to an antigen. Gene Ther 2001; 8:376 - 83; http://dx.doi.org/10.1038/sj.gt.3301408; PMID: 11313814
- June CH. Adoptive T cell therapy for cancer in the clinic. J Clin Invest 2007; 117:1466 - 76; http://dx.doi.org/10.1172/JCI32446; PMID: 17549249
- Ahmad M, Rees RC, Ali SA. Escape from immunotherapy: possible mechanisms that influence tumor regression/progression. Cancer Immunol Immunother 2004; 53:844 - 54; http://dx.doi.org/10.1007/s00262-004-0540-x; PMID: 15197495
- Rouas-Freiss N, Moreau P, Menier C, LeMaoult J, Carosella ED. Expression of tolerogenic HLA-G molecules in cancer prevents antitumor responses. Semin Cancer Biol 2007; 17:413 - 21; http://dx.doi.org/10.1016/j.semcancer.2007.07.003; PMID: 17881247
- Peng ML, Chen M, Ling N, Xu H, Qing Y, Ren H. Novel vaccines for the treatment of chronic HBV infection based on mycobacterial heat shock protein 70. Vaccine 2006; 24:887 - 96; http://dx.doi.org/10.1016/j.vaccine.2005.12.050; PMID: 16446013
- Mizukami S, Kajiwara C, Ishikawa H, Katayama I, Yui K, Udono H. Both CD4+ and CD8+ T cell epitopes fused to heat shock cognate protein 70 (hsc70) can function to eradicate tumors. Cancer Sci 2008; 99:1008 - 15; http://dx.doi.org/10.1111/j.1349-7006.2008.00788.x; PMID: 18341654
- Murshid A, Gong J, Calderwood SK. Heat-shock proteins in cancer vaccines: agents of antigen cross-presentation. Expert Rev Vaccines 2008; 7:1019 - 30; http://dx.doi.org/10.1586/14760584.7.7.1019; PMID: 18767951
- Yamaoka A, Guan X, Takemoto S, Nishikawa M, Takakura Y. Development of a novel Hsp70-based DNA vaccine as a multifunctional antigen delivery system. J Control Release 2010; 142:411 - 5; http://dx.doi.org/10.1016/j.jconrel.2009.11.005; PMID: 19913062
- Hockertz S. Present and future of cancer vaccines. Toxicology 2005; 214:151 - 61; http://dx.doi.org/10.1016/j.tox.2005.06.004; PMID: 16259078
- Venken K, Thewissen M, Hellings N, Somers V, Hensen K, Rummens JL, et al. A CFSE based assay for measuring CD4+CD25+ regulatory T cell mediated suppression of auto-antigen specific and polyclonal T cell responses. J Immunol Methods 2007; 322:1 - 11; http://dx.doi.org/10.1016/j.jim.2007.01.025; PMID: 17368474
- Tsuge I, Okumura A, Kondo Y, Itomi S, Kakami M, Kawamura M, et al. Allergen-specific T-cell response in patients with phenytoin hypersensitivity; simultaneous analysis of proliferation and cytokine production by carboxyfluorescein succinimidyl ester (CFSE) dilution assay. Allergol Int 2007; 56:149 - 55; http://dx.doi.org/10.2332/allergolint.O-06-457; PMID: 17460442
- Placek K, Coffre M, Maiella S, Bianchi E, Rogge L. Genetic and epigenetic networks controlling T helper 1 cell differentiation. Immunology 2009; 127:155 - 62; http://dx.doi.org/10.1111/j.1365-2567.2009.03059.x; PMID: 19476511
- Weidmann E, Brieger J, Jahn B, Hoelzer D, Bergmann L, Mitrou PS. Lactate dehydrogenase-release assay: a reliable, nonradioactive technique for analysis of cytotoxic lymphocyte-mediated lytic activity against blasts from acute myelocytic leukemia. Ann Hematol 1995; 70:153 - 8; http://dx.doi.org/10.1007/BF01682036; PMID: 7718644
- Piriou L, Chilmonczyk S, Genetet N, Albina E. Design of a flow cytometric assay for the determination of natural killer and cytotoxic T-lymphocyte activity in human and in different animal species. Cytometry 2000; 41:289 - 97; http://dx.doi.org/10.1002/1097-0320(20001201)41:4<289::AID-CYTO7>3.0.CO;2-5; PMID: 11084614