Abstract
Intramuscular injection of macaques with an IL-12 expression plasmid (0.1 or 0.4 mg DNA/animal) optimized for high level of expression and delivered using in vivo electroporation, resulted in the detection of systemic IL-12 cytokine in the plasma. Peak levels obtained by day 4–5 post injection were paralleled by a rapid increase of IFN-γ, indicating bioactivity of the IL-12 cytokine. Both plasma IL-12 and IFN-γ levels were reduced to basal levels by day 14, indicating a short presence of elevated levels of the bioactive IL-12. The effect of IL-12 as adjuvant together with an SIVmac239 DNA vaccine was further examined comparing two groups of rhesus macaques vaccinated in the presence or absence of IL-12 DNA. The IL-12 DNA-adjuvanted group developed significantly higher SIV-specific cellular immune responses, including IFN-γ+ Granzyme B+ T cells, demonstrating increased levels of vaccine-induced T cells with cytotoxic potential, and this difference persisted for 6 mo after the last vaccination. Coinjection of IL-12 DNA led to increases in Gag-specific CD4+ and CD4+CD8+ double-positive memory T cell subsets, whereas the Env-specific increases were mainly mediated by the CD8+ and CD4+CD8+ double-positive memory T cell subsets. The IL-12 DNA-adjuvanted vaccine group developed higher binding antibody titers to Gag and mac251 Env, and showed higher and more durable neutralizing antibodies to heterologous SIVsmE660. Therefore, co-delivery of IL-12 DNA with the SIV DNA vaccine enhanced the magnitude and breadth of immune responses in immunized rhesus macaques, and supports the inclusion of IL-12 DNA as vaccine adjuvant.
Introduction
The generation of potent, broad, and long-lasting immune responses able to prevent infection from a wide range of HIV variants is a key requirement for an effective HIV vaccine. DNA vaccination is one of the platforms being explored. The advantages of DNA as vaccine are safety, stability, cost, versatility, and repeated administration without immunity against the vector (reviewed in ref. Citation1). The immunogenicity using HIV/SIV DNA vaccines has been greatly improved over the last several years. To increase Gag and Env protein production, all steps regulating gene expression were optimized, including optimal transcription of the encoded gene, transport and stability of the mRNA, and translation by generating RNA/codon-optimized vectors.Citation2-Citation7 Additionally, the immunogenicity of some antigens was further improved by altering their intracellular trafficking.Citation8-Citation13 Another important improvement is the DNA vaccine delivery by in vivo electroporation (EP), which has been extensively used by several labs (reviewed in refs. Citation1,Citation14). In vivo EP as DNA delivery method significantly increased the expression of the DNA vectors and also the magnitude of cellular and humoral responses and was shown to induce potent SIV-specific immune responses in macaques.Citation8,Citation15-Citation21 DNA-only vaccination by in vivo EP was further able to induce immune responses able to control homologous SIVmac251 challengeCitation16 and heterologous SIVsmE660 challenge (our unpublished observation). Recently, human trials of HIV DNA vaccination by in vivo electroporation resulted in more efficient vaccine delivery and induced higher and longer-lasting immune responses than needle/syringe delivery.Citation22,Citation23 Yet, it should be mentioned that intramuscular (IM) SIV DNA delivery without electroporation in macaques also induced immune responses able to significantly dampen the high dose SIVmac251 challenge.Citation13,Citation16,Citation24-Citation26
The use of IL-12 plasmid DNA as adjuvant for SIV DNA vaccination was reported to induce higher and more consistent immune responses in mice and macaques using intramuscular (IM) injection with needle/syringe,Citation20,Citation27-Citation43 and in macaques with in vivo EPCitation18,Citation20 or together with an EP DNA prime/recombinant viral vector boost.Citation18,Citation21 The first in-man study using IM delivery of HIV gag DNA vaccine adjuvanted with IL-12 DNA followed by in vivo EP indicated no side effects and an increased number of responders.Citation44
In this report, we show that systemic levels of rhesus macaque (rm) IL-12 can be detected upon IM delivery followed by in vivo EP of 0.1 and 0.4 mg of optimized IL-12 plasmid DNA in macaques. The persistence of the produced IL-12 cytokine appeared short-lived in macaques, yet resulted in long-term modifications in immune responses. Testing the effect of the 0.1 mg of IL-12 DNA dose as vaccine adjuvant together with SIV DNA showed higher immune responses in the IL-12 DNA-adjuvanted macaques, with increased levels of SIV-specific cytotoxic T cells and higher and broader neutralizing antibodies.
Results
Detection of IL-12 and IFN-γ in the plasma of macaques upon intramuscular injection of IL-12 DNA
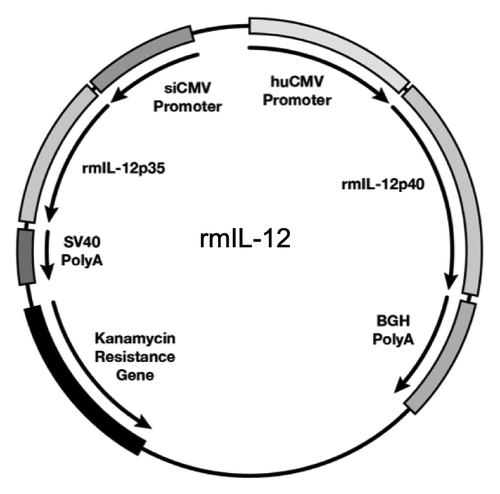
We generated an optimized rhesus macaque IL-12 DNA expression vector (plasmid AG157), which was injected intramuscularly into macaques followed by in vivo electroporation as DNA delivery method, to test whether systemic levels of the IL-12 cytokine can be detected in the plasma. The IL-12 plasmid produces the macaque IL-12p70 cytokine from a dual promoter plasmid (), optimized for high levels of expression by placing the IL-12p40 subunit under the control of the stronger human CMV promoter and the IL-12p35 subunit under the control of the weaker simian CMV promoter using RNA/codon optimized cDNAs, which is detailed elsewhere (Jalah et al., in preparation). This plasmid has recently been used in a vaccine study as adjuvant for the DNA prime/rAd5 boost regimen.Citation18
Figure 1. Rhesus macaque IL-12 expression vector. Map of the dual promoter plasmid (AG157) encoding the rmIL-12p70 cytokine. The rmIL-12p40 and rmIL-12 p35 genes are under the control of the human CMV and the simian CMV promoter, respectively. The polyadenylation signal from bovine growth hormone (BGH) and the simian virus 40 (SV40) are used for the p40 and the p35 subunit, respectively. The plasmid contains kanamycin resistance gene for selection in bacteria. The plasmid backbone is optimized for efficient growth in bacteria.
We measured systemic levels of the macaque IL-12 cytokine in the plasma of two cohorts of macaques (n = 4), injected IM with either 0.1 (; n = 4) or 0.4 mg (; n = 4) of the optimized rmIL-12 DNA followed by in vivo EP (upper panels). The plasma levels of the IL-12p40 subunit were measured at the indicated time points using a commercial macaque IL-12p40 ELISA. Of note, this ELISA does not distinguish between the solo IL-12p40 chain, the p40 homodimer or the IL-12p40 subunit associated with the IL-12p35 subunit forming the IL-12p70 or with the p19 subunit forming the IL-23, and this ELISA was selected because of its higher sensitivity than the commercially available macaque IL-12p70 ELISA.
Figure 2. In vivo bioactivity of optimized rmIL-12 DNA injected in rhesus macaques via intramuscular injection. (A, B) Plots from individual rhesus macaques depicting overlays of plasma macaque IL-12p40 (black solid line; left Y-axis) and IFN-γ (dotted line; right Y-axis). Macaques (n = 4) were injected intramuscularly followed by in vivo electroporation with either 0.1 mg (A, left upper panels) or 0.4 mg (B, right upper panels) of optimized rmIL-12 DNA or sham DNA (lower right and left panels; mean and SEM are shown). The animals in panel A were injected 3 times with intervals of 2 mo, respectively. The animals in panel B were injected twice with an interval of 9 weeks. Arrows mark the day of the injection. (C, D) The plots show the difference between basal levels (day 0; prior to injection) and at day 5 of plasma IL-12p40 (upper panels) and the peak levels of plasma rmIFN-γ (lower panels) after injection of 0.1 mg (C, left panels) or 0.4 mg (D, right panels) rmIL-12 DNA. Note for the 0.1 mg IL-12 DNA group at EP1 (left upper panel), the day 4 measurements were used since the day 5 samples were not collected. For rmIFN-γ, the peak values are shown. The basal levels of rmIFN-γ were below the threshold of detection in all macaques. The mean values are shown.
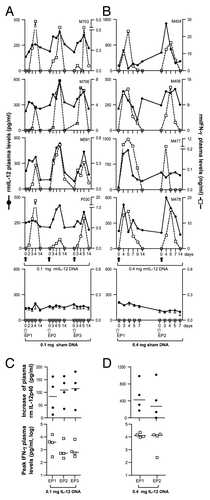
All animals showed detectable levels of endogenous IL-12p40 with a median of ~195 pg/ml (range of ~70–600 pg/ml) at the start of the study (). These IL-12p40 levels likely do not correspond to the IL-12p70 cytokine, because we failed to detect IFN-γ, an indicator of the presence of bioactive IL-12Citation45 in these plasma samples (see below). Upon intramuscular injection of 0.1 () or 0.4 mg () rmIL-12 DNA, the animals showed increased plasma IL-12p40 levels (solid line). Typically, the IL12p40 levels peaked by day 4 to 5 post injection and declined to basal levels by day 14. We noted that the duration of the increased IL-12p40 levels varied among animals. Upon injection of sham DNA, no changes of IL-12p40 were measured (, bottom panels). The animals were subjected to 2 additional cycles using the 0.1 mg IL-12 DNA dose (8 and 16 weeks later, respectively; ) or one additional cycle with the 0.4 mg IL-12 DNA dose (9 weeks later; ). The IL-12p40 levels increased with similar kinetics and to similar levels with each injection cycle, and no dampening was found upon subsequent IL-12p70 DNA injections (; 0.1 mg dose and D; 0.4 mg dose, upper panels). We measured a median increase of IL-12p40 to peak of ~120 pg/ml plasma in the 0.1 mg IL-12 DNA group and a higher increase of ~360 pg/ml plasma in the 0.4 mg IL-12 DNA group. These data indicated that no neutralizing antibodies against the rmIL-12 cytokine were induced, which likely would have dampened the detection of IL-12 upon repeated DNA injections. As controls, 4 macaques were injected in parallel with sham DNA (, lower panels). Changes between day 0 and days 4 to 5, when the IL-12 DNA groups reached peak levels, showed changes of less than 40 pg/ml, reflecting fluctuation of the measurements. Thus, these data confirmed that the IL-12p40 measured in the IL-12 DNA injected groups indeed reflected the de novo produced rmIL-12p70.
To further verify that the detected rmIL-12p40 indeed represented production of IL-12p70, we measured the plasma levels of IFN-γ, an indicator for the presence of bioactive IL-12p70.Citation45 Of note, macaque IFN-γ basal levels were below the threshold of detection (< 31 pg/ml; IFN-γ Duoset® ELISA Development System) in all rhesus macaques tested. (dotted lines) show rapid increases to ~0.2 to 25 ng rmIFN-γ/ml of plasma, followed by a sharp decrease to basal levels, concomitant with the increase and decrease of IL-12p40. Interestingly, despite the relative low increase in IL-12p40 in some animals (macaques M408, M478, P030) marked increases in IFN-γ levels were found. Repeated rmIL-12 DNA injections (, bottom panels) resulted in similar levels of IFN-γ. Animals receiving the higher dose of rmIL-12 DNA also produced higher levels of IFN-γ. These data further suggested the absence of negative feedback mechanisms (i.e., induction of IL-10) at these IL-12 levels, which would downregulate IL-12 and subsequently IFN-γ production.Citation46 Repeated IM injection of IL-12 DNA showed increases of IL-12p40 and IFN-γ after each injection. No increase in the IFN-γ levels was found in the sham DNA injected animals (, bottom panels), excluding the possibility that the IFN-γ increase was an effect of the DNA in vivo EP. These data further demonstrate that IFN-γ serves as a sensitive indicator for the detection of bioactive IL-12p70 heterodimer in the macaque plasma. Thus, effective IM injection of IL-12p70 plasmid DNA followed by in vivo EP resulted in detectable transient increase in IL-12p40 levels and in a more pronounced transient increase in IFN-γ levels. The levels of both cytokines returned to baseline levels by day 14 post injection. Thus, the effect of the DNA injected IL-12 cytokine was short-lived, which is desirable to avoid negative effects from prolonged IL-12 systemic levels.Citation47 Both doses, 0.4 mg and 0.1 mg of IL-12 DNA, were used in subsequent DNA vaccine studies (see also below).Citation18
IL-12 DNA as molecular adjuvant elicits higher levels of SIV-specific cytotoxic T cells in SIV DNA vaccinated macaques
Next, we examined the effect of the 0.1 mg IL-12 DNA dose as adjuvant of an SIV DNA vaccine in rhesus macaques. Two groups of animals (n = 8) were vaccinated with 2 mg of a mixture of DNAs expressing several SIVmac239 genes in the absence or presence of 0.1 mg of the optimized rmIL-12 plasmid DNA. The DNAs were delivered via intramuscular injection followed by in vivo EP. The animals received 4 vaccinations (EP1 to EP4) at 0, 8, 16 and 36 weeks () and were monitored for another 24 weeks after the last vaccination for the development of long-lasting humoral and cellular immune responses to Gag and Env.
Figure 3. The IL-12 DNA-adjuvanted vaccine group developed higher cytotoxic T cell responses. (A) The outline of the SIV vaccine study is shown. Two groups of macaques (n = 8 each) were subjected to intramuscular injection followed by in vivo EP using a mixture of SIV DNAs in the absence or presence of 0.1 mg of the optimized macaque IL-12 DNA plasmid (AG157) at 0, 8, 16, and 36 weeks. (B) Cellular immune responses to SIV Gag and SIV Env were measured from individual animals at 2 weeks post EP2 and 2 and 24 weeks post EP4 using polychromatic flow cytometry. Total (Env + Gag) SIV-specific IFN-γ+ T cells (upper panel) and the corresponding Granzyme B+ (GzmB+) cytotoxic T cell subpopulation (middle panel) and GzmB- T cell subpopulation (lower panel) are shown for the two groups of macaques. The bars represent the mean values and standard error of mean (SEM). Mann-Whitney non-parametric t-test was employed to compare the 2 groups using Prism software (GraphPad Software, Inc.). The significant p values are shown.
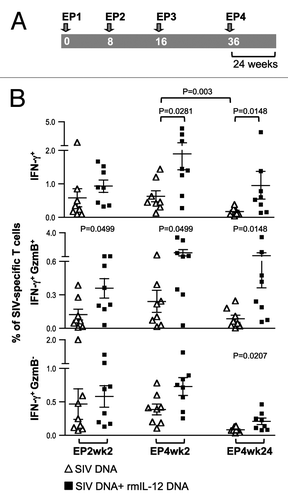
To address the development of SIV-specific cellular immune responses, PBMC collected at 2 weeks after EP2, and at 2 and 24 weeks after EP4 were examined by immunostaining and polychromatic flow cytometric analysis (, and ). DNA vaccination induced a median of 0.3% SIV-specific IFN-γ+ T cells at EP2wk2, and similar levels were detected at EP4wk2 (median 0.5%), indicating that 2 vaccinations were sufficient to reach maximal T cell responses (, upper panel). Consistent with other SIV DNA vaccination studies using in vivo EP in macaques,Citation18,Citation20 we found higher cellular immune responses (~3-fold higher) in the IL-12 DNA-adjuvanted cohort. The difference between the 2 groups reached significance after EP4, indicating that the IL-12-adjuvanted group benefited from additional vaccinations. We noted a significant decline (p = 0.003) in the SIV-specific T cell responses between week 2 and week 24 after EP4 in the absence of IL-12 (). In contrast, the T cell responses were sustained up to 24 weeks post EP4 in the IL-12 DNA-adjuvanted group, demonstrating preservation of the SIV-specific responses. Therefore, the inclusion of IL-12 DNA is important for the longevity of cellular vaccine-induced immune responses. These data demonstrate that IL-12 DNA as vaccine adjuvant provided a great benefit to the SIV-based DNA vaccine immunogenicity, even when using in vivo electroporation as the more efficient DNA delivery method.
Figure 4. Comparison of the Gag- and Env-specific CD4+, CD8+ and CD4+CD8+ double-positive (DP) T cell subsets. (A, B) Gag (A)- and Env (B)-specific IFN-γ + producing CD4+, CD8+ and CD4+CD8+ DP T cell populations were analyzed from individual animals by flow cytometry at EP2wk2, EP4wk2 and EP4wk24. The data are shown as % of total parent CD4+, CD8+ or CD4+CD8+ T cells. The bars represent mean values and SEM.
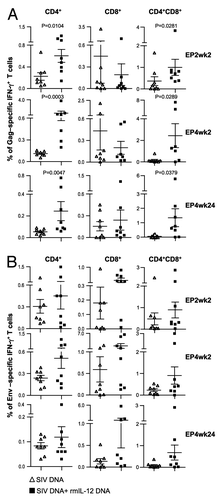
Figure 5. Comparison of the Gag- and Env-specific memory T cell subsets. (A, B) The frequency of (A) Gag-specific GzmB+ CD4+ and CD4+CD8+ DP transitional memory (TM) and effector memory (EM) T cells and (B) Env-specific GzmB+ CD8+ and CD4+CD8+ DP TM and EM T cells as analyzed by flow cytometry from individual animals at EP2wk2, EP4wk2 and EP4wk24 are shown. The SIV-specific IFN-γ producing GzmB+ cells are shown as % of total Gag- or Env-specific T cells. The mean and SEM values are shown. The significant p values are shown.
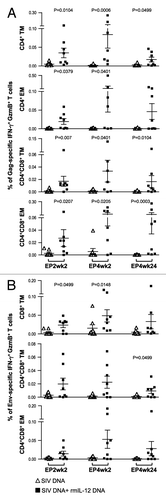
To further dissect the induced cellular responses, IFN-γ+ T cells were examined for their cytotoxic capability (, middle panel). The DNA-only group showed SIV-specific Granzyme B+ (GzmB+) T cell responses with a median of 0.1% after EP2 which increased to 0.2% after EP4, indicating that ~25–35% of the total SIV-specific IFN-γ+ cells have cytotoxic potential. In comparison, the IL-12 DNA-adjuvanted vaccine group showed significantly higher levels of SIV-specific GzmB+ T cells at all time points tested (~30–60% of the SIV-specific IFN-γ+ T cells). Co-injection of IL-12 DNA led to a significant difference in the Granzyme B negative SIV-specific T cell population at EP4wk24 (p = 0.0207) (, lower panel), a cell population with a yet unclear role. Together, the DNA vaccine including IL-12 DNA as adjuvant induced higher levels of SIV-specific cytotoxic T cells, an important T cell population armed to eliminate SIV infected cells.
Higher levels of SIV-specific memory T cells with cytotoxic potential induced in the IL-12 DNA-adjuvanted vaccine cohort
We further dissected the SIV-specific T cells for the presence of CD4+, CD8+ and CD4+CD8+ double-positive (DP) markers. Significantly more SIV Gag-specific IFN-γ producing CD4+ and CD4+CD8+ DP T cells were found in the IL-12 DNA-adjuvanted group (, left and right panels), and these responses were boosted after repeated vaccinations. IL-12 did not affect the levels of the Gag-specific IFN-γ+ CD8+ T cells (, middle panels). We noted a trend to higher levels of Env-specific CD8+ and CD4+CD8+ DP T cells populations in the presence of IL-12, which did not reach significance ().
The CD4+, CD8+ and CD4+CD8+ DP T cell subsets were further analyzed for their memory phenotypes and their ability to produce GzmB. Memory subsets were defined by polychromatic flow cytometry as previously described.Citation48,Citation49 Central memory T cells (CM) were defined as CD28+CD45RA-CD95+CCR7+; transitional Memory (TM) as CD28+CD45RA-CD95+CCR7- and effector memory (EM) as CD28-CD95+CCR7-. DNA vaccination induced SIV-specific IFN-γ+ CCR7- TM and EM T cells, and we did not detect de novo induction of IFN-γ+ CCR7+ CM T cells. Similarly, it was reported that no CM T cell populations were induced upon therapeutic DNA vaccination.Citation33 The IL-12 DNA-adjuvanted vaccine group showed significant increases in the Gag-specific IFN-γ producing GzmB+ CD4+ and CD4+CD8+ DP memory T cells (both TM and EM) at several time points measured (). This increase of Gag-specific memory T cells was observed for both IFN-γ producing GzmB+ () and GzmB- (data not shown) populations. In contrast, several of the macaques in the non-adjuvanted cohort had undetectable Gag-specific CD4+ EM and DP (both TM and EM) T cells at EP2wk2 and these levels were not boosted upon subsequent immunizations. The Env-specific responses were significantly increased in the IFN-γ producing GzmB+CD8+ TM T cells (), both at 2 weeks post EP2 and EP4, and in the DP memory T cells (TM and EM, ) at 24 weeks post EP4.
Together, these data showed that using IL-12 DNA as adjuvant induced distinct SIV Gag and Env-specific cellular responses. Significant increases were found in the Gag-specific GzmB+ T-cell population, mostly in the CD4+ and CD4+CD8+ DP TM and EM subsets. On the other hand, increased Env-specific GzmB+ cytolytic T cells were found mainly in the CD8+ and DP memory subsets, but not at all the time points analyzed.
Broader humoral immune responses in the IL-12 DNA adjuvanted vaccine group
We also measured the plasma levels of SIV-specific binding antibodies in the two vaccine groups (). Analysis of pooled plasma samples collected over time showed that the humoral responses to SIVmac251 Gag and Env developed with similar kinetics in the two vaccine groups, with the IL-12 DNA-adjuvanted group having slightly higher levels (, left panels). Analysis of individual animals at 2 weeks post EP4 confirmed the higher levels of Gag and SIVmac251 Env antibody titers in the IL-12 DNA adjuvanted group, but the significance in the difference was not maintained (, right panels). We found that the SIVmac239-based DNA vaccine induced high titers of Env binding antibody titers not only to the homologous SIVmac239 (, left panel), but also to the heterologous SIVsmE660 (, right panel), albeit with ~1 log lower titers. No significant differences were found in the bAb titers to SIVmac239 and SIVsmE660 Env comparing the 2 groups, thus, the difference was only noted for SIVmac251 Env. Our findings are consistent with other DNA vaccination studies in macaques, which reported increased humoral responses in the presence of IL-12 DNA at some time pointsCitation20 or not at all.Citation18
Figure 6. The IL-12 DNA-adjuvanted group developed broader humoral immune responses. (A) Endpoint titers of binding antibodies to SIVmac251 Gag (upper panel) and SIVmac251 Env (lower panel) were measured in pooled plasma samples (left panels). The right panels show the data from individual animals at 2 and 24 weeks post EP4. The mean and SEM values are shown. (B) Endpoint titers of binding antibodies to SIVmac239 (left panels) and SIVsmE660 (right panels) Env were measured in individual animal at 2 and 24 weeks post EP4. (C) Avidity index of the SIVmac239 Env bAb. The avidity of two of the 8 animals in the un-adjuvanted group could not be assessed at both time points. The antibody binding titers and avidity index were determined from parallel plates. (D) Neutralizing antibody titers from individual plasma samples from the vaccinated macaques against SIVmac251-TCLA (upper panel), and against the SIVsmE660 BR-CG7G (middle panel) and BR-CG7V (lower panel) Env proteins at 2, 8, 16 and 24 weeks post EP4 are shown. The bars represent mean and SEM values.
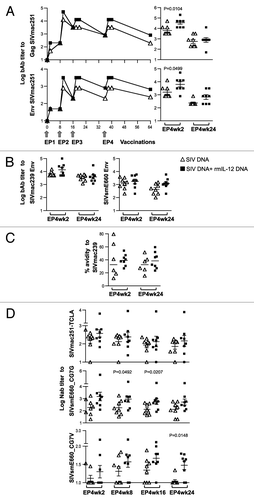
The bAb were further tested for their avidity. The avidity of the SIVmac239 bAb responses could be determined in 6 of the 8 animals from the unadjuvanted vaccine group, with a median avidity index of ~33%. The IL-12 DNA-adjuvanted group showed more consistent and higher avidity (median index of ~39%), but the difference between the groups did not reach significance. The avidity of sham DNA injected animals or pre-samples were below detection limit (assigned to 0.1%). The avidity of the SIVsmE660 bAb responses could not be determined due to the overall low bAb titers. Like the humoral responses, the avidity of SIVmac239 bAb responses did not change over time and persisted to week 24 post EP4.
We further examined the ability of the induced antibodies to neutralize a panel of SIV variants including the homologous TCLA-SIVmac251 and SIVmac239 and the heterologous SIVsmE660_CG7G and SIVsmE660_CG7V (). Plasma samples from different time points (week 2, 8, 16 and 24) after EP4 were compared. We found neutralizing antibodies (NAb) to TCLA-SIVmac251 with a median titer of 2.4 log at EP4wk2 in the DNA-only group (, upper panel). The NAb titers did not significantly change over time (compare week 2 to week 24), as noted for the binding antibody responses. No difference in the levels of TCLA-SIVmac251 NAb was found in the IL-12 DNA-adjuvanted vaccine group (median 2.5 log at EP4wk2) and a similar persistence over the 6 mo of follow-up is found. We also tested the plasma for the presence of NAb to the difficult-to-neutralize SIVmac239 and no responses were detected in either of the groups (data not shown). Thus, despite the optimization of DNA vaccine and vaccine delivery, no NAb to SIVmac239 were detected with this immunization method.
The antibodies were also examined for breadth of neutralization against the heterologous SIVsmE660 variants CG7G and CG7V. SIVsmE660 Env proteins and SIVmac239 share 83% identity, a diversity that is reminiscent of the heterogeneity found in HIV-1 clades. Interestingly, we found that vaccination with DNA expressing the SIVmac239 Env induced antibodies able to neutralize the ‘tier 1A-like’ SIVsmE660_CG7G (, middle panel). The IL-12 DNA adjuvanted group showed slightly higher NAb titers, which reached significance at week 16 post EP4 (p = 0.0207), but the difference was not maintained by week 24 post EP4. The plasma samples were also tested for their ability to neutralize the ‘tier-1B like’ SIVsmE660_CG7V (, bottom panel). We noted that the plasma samples from the DNA-only group neutralized the SIVsmE660_CG7V Env very poorly with 3 to 7 of the 8 animals showing titers below the 1:20 threshold (weeks 8 to 24). In contrast, the IL-12 DNA-adjuvanted vaccine group had more responders (6 to 7 of the 8 animals; weeks 8 to 24) and showed higher levels of NAb to SIVsmE660_CG7V, with a trend at weeks 8 and 16 post EP4 and reaching significance by week 24. These findings indicated that the Nab induced in the IL-12 DNA-adjuvanted group showed better longevity to the heterologous SIVsmE660 variants.
Discussion
In this report, we showed that intramuscular injection of 0.1 or 0.4 mg of an optimized rmIL-12 DNA plasmid delivered by in vivo electroporation resulted in detectable systemic levels of the IL-12 cytokine in the plasma of macaques. Using the less efficient needle and syringe injection as DNA delivery, it is not expected that IL-12 would have been detected in the plasma, since in vivo EP has been reported to yield 100–1000 fold higher antigen delivery.Citation14 Measurements of IL-12 and of IFN-γ, as the more sensitive read-out of the presence of bioactive IL-12, demonstrated that the biological effect of IL-12 DNA injection can only be detected for up to ~2 weeks after the DNA injection. Thus, this finding addressed one of the hurdles of using IL-12 DNA as vaccine adjuvant, namely the duration of the presence of the cytokine. Another major concern for the use of IL-12 DNA is potential side effects, because high systemic levels of IL-12 obtained upon injection of IL-12 protein were very toxic.Citation47 It has also been reported that high levels of IL-12 can suppress vaccine-induced responses.Citation50-Citation52 In agreement with these data, in DNA vaccinated mice using EP as delivery method, we also observed a negative effect of a high mouse IL-12 DNA dose (10 μg) on the vaccine induced responses, whereas a low dose (1 μg) provided positive adjuvant effects (our unpublished observation). Therefore, it is critical to define an optimal IL-12 DNA dose, which is safe and is biologically effective. Using the same optimized rhesus IL-12 DNA plasmid and in vivo EP as DNA delivery, a beneficial effect for DNA vaccination in macaques was found using doses of 0.1 mg, as reported in this work and other studies from our lab, and of 0.4 mg.Citation18 Together the work presented here showed that doses with a range of 0.1 to 0.4 mg of our optimized IL-12 DNA were bioactive, and well tolerated, and, thus this full-filled an important criterion.
Overall, our data are in agreement with other side-by-side comparative studies, which showed that both cellular and humoral immune responses were increased in the IL-12 DNA adjuvanted groups using in vivo electroporation as DNA delivery in macaques.Citation18,Citation20 Our work not only focused on the quantitative comparison, but also further expanded the qualitative analysis of the SIV-specific immune responses elicited using IL-12 DNA as adjuvant.
IL-12 was reported to enhance the CD8+ effector memory responses.Citation33,Citation53 In contrast to the DNA-only immunized cohort, we found higher levels of SIV-specific memory T-cells with cytotoxic potential (GzmB+) present in the IL-12 DNA-adjuvanted cohort early on during vaccination and these subsets were further boosted upon subsequent immunizations. We found more profound increases in the Gag-specific CD4+ and DP TM and EM subsets and in the Env-specific CD8+ and DP TM memory subsets. Such memory cells were shown to correlate with the control of SIV/HIV upon infection.Citation54,Citation55 Interestingly, the IL-12 DNA-adjuvanted vaccine induced higher levels of Gag-specific CD4+CD8+ DP T cells. This T cell subset has been described as differentiated effector memory T cells with antiviral role in HIVCitation56-Citation58 and other viral infections.Citation59,Citation60 During acute HIV infection, these DP T cells represent a significant portion of the anti-HIV cellular responses and are highly proliferative and multifunctional in HIV controllers. While our work focused solely on IFN-γ production upon stimulation with SIV-specific peptides, others also interrogated the production of cytokines like TNF-α and IL-2Citation18,Citation20 and reported that IL-12 DNA contributed to the generation of multifunctional SIV-specific T cells. Together, these studies support the inclusion of IL-12 DNA as an adjuvant for an effective HIV/SIV vaccine.
Another important goal of an HIV/SIV vaccine is to elicit high and broad humoral immune responses. We found that the IL-12 DNA-adjuvanted vaccine induced higher humoral immune responses, as observed by others.Citation18,Citation20 Testing of different SIVsmE660 variants which differ by ~20% from the vaccine SIVmac239, provided an excellent tool to test the breadth of the SIVmac239 Env induced responses. We demonstrated an important contribution of the IL-12 cytokine in the induction of increased NAb breadth with better longevity. Together, the presence of IL-12 DNA adjuvant increased humoral responses and led to the development of NAb with increased breadth, positively affecting the quality of the humoral immune responses.
It is also desirable that a vaccine is able to induce long-lasting immune responses. Here, we report the induction of long-lasting (24 weeks post EP4) cellular and humoral immune responses to Gag and Env by the SIV DNA vaccine. We report that IL-12 DNA as vaccine adjuvant positively affected the duration of the cellular SIV-specific immune responses. This finding is an extension of our previous observation,Citation15 where a Gag DNA vaccine adjuvanted with IL-12 DNA and delivered by in vivo EP showed efficient induction of long-lasting (> 2 y) cellular and humoral immune responses. In conclusion, the use of IL-12 DNA as vaccine adjuvant is an important component of a DNA vaccine protocol that uses in vivo EP as delivery method. Inclusion of IL-12 DNA vaccine adjuvant induces better humoral and cellular immune responses and should provide for a more efficacious HIV/SIV vaccine.
Materials and Methods
Animals
Indian rhesus macaques (Macaca mulatta) were housed and handled in accordance with the standards of the Association for the Assessment and Accreditation of Laboratory Animal Care International, at the Advanced BioScience Laboratories, Inc.
Rhesus macaque IL-12 DNA injection of macaques
Rhesus macaques were injected with of 0.1 mg or 0.4 mg (n = 4 each) of optimized rhesus macaque (rm) IL-12p70 expression vector (plasmid AG157; Jalah et al., in preparation) or only sham DNA (0.25 ml DNA/site, 2 injection sites in the left and right inner thighs). All DNA injections were performed intramuscularly followed by in vivo electroporation with the ELGEN® adaptive constant-current electroporation device (Inovio Pharmaceuticals, Inc.) using endotoxin-free preparations of DNA (Qiagen). The animals in the 0.1 mg group were research-naïve. The animals in the 0.4 mg group had been infected with SIVmac251 for one year and viremia was controlled by antiretroviral therapy (ART)Citation61 for the duration of this study. The levels of rmIL-12p40 in the plasma were measured using the rmIL-12p40 ELISA (Cat #CKM007; Cell Sciences, Inc.) having a threshold of detection of 5 pg/ml. The macaque IFN-γ plasma levels were measured using the primate IFN-γ Duoset® ELISA Development System (Cat #DY961; R&D Systems) having a threshold of detection of 31 pg/ml.
SIV DNA vaccination
Rhesus macaques (8 animals/group) received 4 vaccinations (0, 8, 16 and 36 weeks) using 0.5 ml of a plasmid mixture in water, consisting of 0.5 mg each of SIVmac239 gp160 Env (plasmid 99S), Gag [p57Gag (plasmid 206S) and the MCP3 p39 gag (plasmid 209S)], Pol [MCP3-pol (plasmid 216S), and LAMP-pol (plasmid 103S)], LAMP-Nef-Tat-Vif (plasmid 147S) described elsewhereCitation8,Citation13 in the absence or presence of 0.1 mg of the optimized rmIL-12 plasmid (plasmid AG157). All DNA injections were performed intramuscularly followed by in vivo electroporation as described above.
Cellular immune responses
The cellular immune responses were measured using isolated cryopreserved PBMCs stimulated with SIVmac239-specific peptide pools (15-mers with 11 aa overlap; Infinity Inc. Biotech Research and Resource) as described.Citation15 The cells were cultured for 12 h with monensin (Golgi Stop, BD PharMingen) to inhibit cytokine secretion. Immunostaining and flow cytometric analysis was performed as described.Citation15-Citation17 Briefly, cell surface staining was first performed using the following antibody cocktail: CD3 APCCy7 (BD PharMingen, #557757), CD4 AmCyan (BD, custom research), CD8 AF405 (Invitrogen; #MHCD0826), CD95 FITC (BD, #556640), CD28 PerCPCy5.5 (Biolegend, #302922), CD45RA AF700 (AbD serotec, #MCA88A700) and CCR7 APC (R&D Systems, #FAB197A). After permeabilization of the cells, intracellular staining was performed using IFN-γ PECy7 (BD, #557643) and Granzyme-PE (Invitrogen, #MHGB04). The data were acquired on an LSR II flow cytometer (BD Biosciences) and analyzed using the FlowJo software (Tree Star, Inc.).
Humoral immune responses
Binding antibody to SIV Gag and SIVmac251 Env were measured by standard ELISA (Advanced BioScience Laboratories) and the endpoint titers were determined. Cut-off values were defined as 2-fold the mean values obtained from control plasma. Antibody titers to SIVmac239 and SIVsmE660 (accession FJ579014.1) and antibody avidity comparisons upon treatment with 1.5 M sodium thiocyanate (NaSCN; Sigma-Aldrich) were measured as described.Citation62-Citation64 End-point binding Ab titers for SIVmac239 and SIVsmE660 were determined using values higher than the mean absorbance plus 3 times standard deviation of the negative control. The avidity index (%) was calculated by taking the ratio of the NaSCN-treated plasma dilution giving an absorbance of 0.5 to the TBS treated plasma dilution giving an OD of 0.5 and multiplying by 100. Neutralizing antibody titers were determined using the M7-luc assayCitation65 for the TCLA-SIVmac251/H9 and the TZM-bl assayCitation65 for SIVmac239CS.23, SIVsmE660/BR-CG7G.IR1 (SIVsmE660_CG7G) and SIVsmE660/BR-CG7V.IR (SIVsmE660_CG7V). The assay stock of TCLA-SIVmac251 was produced by infection in H9 cells. The assay stock of SIVmac239CS.23 was produced by co-transfection of the corresponding gp160-containing plasmid and a backbone plasmid (pSG3ΔEnv) in 293T cells. Assay stocks of SIVsmE660 CG7G and CG7VCitation66 were produced in transfection of full-length infectious molecular clone DNA in 293T cells.
| Abbreviations: | ||
| EP | = | electroporation |
| rm | = | rhesus macaque |
| GzmB | = | GranzymeB |
| DP | = | CD4+CD8+ double-positive |
| CM | = | central memory |
| TM | = | transitional memory |
| EM | = | Effector memory |
Acknowledgments
We are grateful to D. Weiss, J. Treece, I. Kalisz, V. Kalyanaraman, P. Markham and staff at Advanced BioScience Laboratories, Inc., Rockville, for their expert help. We thank A. Valentin for discussions, J. Bear and B. Chowdhury for technical assistance, and T. Jones for editorial assistance. This work was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health (NCI/NIH) and by NIH HHSN 27201100016C.
B.K.F., G.N.P.: designed, coordinated the study, analyzed the data, and wrote the paper
R.J., M.R., V.P., V.K., C.A., B.G., A.vG.: performed experiments and analyzed the data
C.L., D.C.M.: performed and analyzed NAb assays
W.H., Y.G.: performed binding Ab and avidity assays
K.E.B., N.Y.S.: contributed electroporation delivery methods and devices
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Hutnick NA, Myles DJ, Bian CB, Muthumani K, Weiner DB. Selected approaches for increasing HIV DNA vaccine immunogenicity in vivo. Curr Opin Virol 2011; 1:233 - 40; http://dx.doi.org/10.1016/j.coviro.2011.08.003; PMID: 22440782
- Schneider R, Campbell M, Nasioulas G, Felber BK, Pavlakis GN. Inactivation of the human immunodeficiency virus type 1 inhibitory elements allows Rev-independent expression of Gag and Gag/protease and particle formation. J Virol 1997; 71:4892 - 903; PMID: 9188551
- Schwartz S, Campbell M, Nasioulas G, Harrison J, Felber BK, Pavlakis GN. Mutational inactivation of an inhibitory sequence in human immunodeficiency virus type 1 results in Rev-independent gag expression. J Virol 1992; 66:7176 - 82; PMID: 1433510
- Schwartz S, Felber BK, Pavlakis GN. Distinct RNA sequences in the gag region of human immunodeficiency virus type 1 decrease RNA stability and inhibit expression in the absence of Rev protein. J Virol 1992; 66:150 - 9; PMID: 1727477
- Graf M, Deml L, Wagner R. Codon-optimized genes that enable increased heterologous expression in mammalian cells and elicit efficient immune responses in mice after vaccination of naked DNA. Methods Mol Med 2004; 94:197 - 210; PMID: 14959831
- Deml L, Bojak A, Steck S, Graf M, Wild J, Schirmbeck R, et al. Multiple effects of codon usage optimization on expression and immunogenicity of DNA candidate vaccines encoding the human immunodeficiency virus type 1 Gag protein. J Virol 2001; 75:10991 - 1001; http://dx.doi.org/10.1128/JVI.75.22.10991-11001.2001; PMID: 11602739
- Nasioulas G, Zolotukhin AS, Tabernero C, Solomin L, Cunningham CP, Pavlakis GN, et al. Elements distinct from human immunodeficiency virus type 1 splice sites are responsible for the Rev dependence of env mRNA. J Virol 1994; 68:2986 - 93; PMID: 8151769
- Kulkarni V, Jalah R, Ganneru B, Bergamaschi C, Alicea C, von Gegerfelt A, et al. Comparison of immune responses generated by optimized DNA vaccination against SIV antigens in mice and macaques. Vaccine 2011; 29:6742 - 54; http://dx.doi.org/10.1016/j.vaccine.2010.12.056; PMID: 21195080
- de Arruda LB, Chikhlikar PR, August JT, Marques ET. DNA vaccine encoding human immunodeficiency virus-1 Gag, targeted to the major histocompatibility complex II compartment by lysosomal-associated membrane protein, elicits enhanced long-term memory response. Immunology 2004; 112:126 - 33; http://dx.doi.org/10.1111/j.1365-2567.2004.01823.x; PMID: 15129672
- Marques ET Jr., Chikhlikar P, de Arruda LB, Leao IC, Lu Y, Wong J, et al. HIV-1 p55Gag encoded in the lysosome-associated membrane protein-1 as a DNA plasmid vaccine chimera is highly expressed, traffics to the major histocompatibility class II compartment, and elicits enhanced immune responses. J Biol Chem 2003; 278:37926 - 36; http://dx.doi.org/10.1074/jbc.M303336200; PMID: 12824194
- Valentin A, Chikhlikar P, Patel V, Rosati M, Maciel M, Chang KH, et al. Comparison of DNA vaccines producing HIV-1 Gag and LAMP/Gag chimera in rhesus macaques reveals antigen-specific T-cell responses with distinct phenotypes. Vaccine 2009; 27:4840 - 9; http://dx.doi.org/10.1016/j.vaccine.2009.05.093; PMID: 19539586
- Biragyn A, Belyakov IM, Chow YH, Dimitrov DS, Berzofsky JA, Kwak LW. DNA vaccines encoding human immunodeficiency virus-1 glycoprotein 120 fusions with proinflammatory chemoattractants induce systemic and mucosal immune responses. Blood 2002; 100:1153 - 9; http://dx.doi.org/10.1182/blood-2002-01-0086; PMID: 12149191
- Rosati M, von Gegerfelt A, Roth P, Alicea C, Valentin A, Robert-Guroff M, et al. DNA vaccines expressing different forms of simian immunodeficiency virus antigens decrease viremia upon SIVmac251 challenge. J Virol 2005; 79:8480 - 92; http://dx.doi.org/10.1128/JVI.79.13.8480-8492.2005; PMID: 15956591
- Sardesai NY, Weiner DB. Electroporation delivery of DNA vaccines: prospects for success. Curr Opin Immunol 2011; 23:421 - 9; http://dx.doi.org/10.1016/j.coi.2011.03.008; PMID: 21530212
- Patel V, Valentin A, Kulkarni V, Rosati M, Bergamaschi C, Jalah R, et al. Long-lasting humoral and cellular immune responses and mucosal dissemination after intramuscular DNA immunization. Vaccine 2010; 28:4827 - 36; http://dx.doi.org/10.1016/j.vaccine.2010.04.064; PMID: 20451642
- Rosati M, Bergamaschi C, Valentin A, Kulkarni V, Jalah R, Alicea C, et al. DNA vaccination in rhesus macaques induces potent immune responses and decreases acute and chronic viremia after SIVmac251 challenge. Proc Natl Acad Sci U S A 2009; 106:15831 - 6; http://dx.doi.org/10.1073/pnas.0902628106; PMID: 19717425
- Rosati M, Valentin A, Jalah R, Patel V, von Gegerfelt A, Bergamaschi C, et al. Increased immune responses in rhesus macaques by DNA vaccination combined with electroporation. Vaccine 2008; 26:5223 - 9; http://dx.doi.org/10.1016/j.vaccine.2008.03.090; PMID: 18468743
- Winstone N, Wilson AJ, Morrow G, Boggiano C, Chiuchiolo MJ, Lopez M, et al. Enhanced control of pathogenic SIVmac239 replication in macaques immunized with a plasmid IL12 and a DNA prime, viral vector boost vaccine regimen. J Virol 2011; 85:9578 - 87; http://dx.doi.org/10.1128/JVI.05060-11; PMID: 21734035
- Luckay A, Sidhu MK, Kjeken R, Megati S, Chong SY, Roopchand V, et al. Effect of plasmid DNA vaccine design and in vivo electroporation on the resulting vaccine-specific immune responses in rhesus macaques. J Virol 2007; 81:5257 - 69; http://dx.doi.org/10.1128/JVI.00055-07; PMID: 17329330
- Hirao LA, Wu L, Khan AS, Hokey DA, Yan J, Dai A, et al. Combined effects of IL-12 and electroporation enhances the potency of DNA vaccination in macaques. Vaccine 2008; 26:3112 - 20; http://dx.doi.org/10.1016/j.vaccine.2008.02.036; PMID: 18430495
- Vojnov L, Bean AT, Peterson EJ, Chiuchiolo MJ, Sacha JB, Denes FS, et al. DNA/Ad5 vaccination with SIV epitopes induced epitope-specific CD4⁺ T cells, but few subdominant epitope-specific CD8⁺ T cells. Vaccine 2011; 29:7483 - 90; http://dx.doi.org/10.1016/j.vaccine.2011.07.048; PMID: 21839132
- Vasan S, Hurley A, Schlesinger SJ, Hannaman D, Gardiner DF, Dugin DP, et al. In vivo electroporation enhances the immunogenicity of an HIV-1 DNA vaccine candidate in healthy volunteers. PLoS One 2011; 6:e19252; http://dx.doi.org/10.1371/journal.pone.0019252; PMID: 21603651
- Vasan S, Schlesinger SJ, Huang Y, Hurley A, Lombardo A, Chen Z, et al. Phase 1 safety and immunogenicity evaluation of ADVAX, a multigenic, DNA-based clade C/B’ HIV-1 candidate vaccine. PLoS One 2010; 5:e8617; http://dx.doi.org/10.1371/journal.pone.0008617; PMID: 20111582
- Boyer JD, Robinson TM, Kutzler MA, Vansant G, Hokey DA, Kumar S, et al. Protection against simian/human immunodeficiency virus (SHIV) 89.6P in macaques after coimmunization with SHIV antigen and IL-15 plasmid. Proc Natl Acad Sci U S A 2007; 104:18648 - 53; http://dx.doi.org/10.1073/pnas.0709198104; PMID: 18000037
- Muthumani K, Bagarazzi M, Conway D, Hwang DS, Manson K, Ciccarelli R, et al. A Gag-Pol/Env-Rev SIV239 DNA vaccine improves CD4 counts, and reduce viral loads after pathogenic intrarectal SIV(mac)251 challenge in rhesus Macaques. Vaccine 2003; 21:629 - 37; http://dx.doi.org/10.1016/S0264-410X(02)00571-6; PMID: 12531331
- Boyer JD, Maciag PC, Parkinson R, Wu L, Lewis MG, Weiner DB, et al. Rhesus macaques with high levels of vaccine induced IFN-gamma producing cells better control viral set-point following challenge with SIV239. Vaccine 2006; 24:4498 - 502; http://dx.doi.org/10.1016/j.vaccine.2005.08.016; PMID: 16185790
- Boyer JD, Cohen AD, Ugen KE, Edgeworth RL, Bennett M, Shah A, et al. Therapeutic immunization of HIV-infected chimpanzees using HIV-1 plasmid antigens and interleukin-12 expressing plasmids. AIDS 2000; 14:1515 - 22; http://dx.doi.org/10.1097/00002030-200007280-00007; PMID: 10983638
- Boyer JD, Robinson TM, Kutzler MA, Parkinson R, Calarota SA, Sidhu MK, et al. SIV DNA vaccine co-administered with IL-12 expression plasmid enhances CD8 SIV cellular immune responses in cynomolgus macaques. J Med Primatol 2005; 34:262 - 70; http://dx.doi.org/10.1111/j.1600-0684.2005.00124.x; PMID: 16128921
- Chattergoon MA, Saulino V, Shames JP, Stein J, Montaner LJ, Weiner DB. Co-immunization with plasmid IL-12 generates a strong T-cell memory response in mice. Vaccine 2004; 22:1744 - 50; http://dx.doi.org/10.1016/j.vaccine.2004.01.036; PMID: 15068858
- Chong SY, Egan MA, Kutzler MA, Megati S, Masood A, Roopchard V, et al. Comparative ability of plasmid IL-12 and IL-15 to enhance cellular and humoral immune responses elicited by a SIVgag plasmid DNA vaccine and alter disease progression following SHIV(89.6P) challenge in rhesus macaques. Vaccine 2007; 25:4967 - 82; http://dx.doi.org/10.1016/j.vaccine.2006.11.070; PMID: 17335943
- Egan MA, Chong SY, Megati S, Montefiori DC, Rose NF, Boyer JD, et al. Priming with plasmid DNAs expressing interleukin-12 and simian immunodeficiency virus gag enhances the immunogenicity and efficacy of an experimental AIDS vaccine based on recombinant vesicular stomatitis virus. AIDS Res Hum Retroviruses 2005; 21:629 - 43; http://dx.doi.org/10.1089/aid.2005.21.629; PMID: 16060834
- Egan MA, Megati S, Roopchand V, Garcia-Hand D, Luckay A, Chong SY, et al. Rational design of a plasmid DNA vaccine capable of eliciting cell-mediated immune responses to multiple HIV antigens in mice. Vaccine 2006; 24:4510 - 23; http://dx.doi.org/10.1016/j.vaccine.2005.08.024; PMID: 16140439
- Halwani R, Boyer JD, Yassine-Diab B, Haddad EK, Robinson TM, Kumar S, et al. Therapeutic vaccination with simian immunodeficiency virus (SIV)-DNA + IL-12 or IL-15 induces distinct CD8 memory subsets in SIV-infected macaques. J Immunol 2008; 180:7969 - 79; PMID: 18523260
- Kim JJ, Ayyavoo V, Bagarazzi ML, Chattergoon MA, Dang K, Wang B, et al. In vivo engineering of a cellular immune response by coadministration of IL-12 expression vector with a DNA immunogen. J Immunol 1997; 158:816 - 26; PMID: 8992999
- Kim JJ, Nottingham LK, Tsai A, Lee DJ, Maguire HC, Oh J, et al. Antigen-specific humoral and cellular immune responses can be modulated in rhesus macaques through the use of IFN-gamma, IL-12, or IL-18 gene adjuvants. J Med Primatol 1999; 28:214 - 23; http://dx.doi.org/10.1111/j.1600-0684.1999.tb00272.x; PMID: 10593488
- Kim JJ, Simbiri KA, Sin JI, Dang K, Oh J, Dentchev T, et al. Cytokine molecular adjuvants modulate immune responses induced by DNA vaccine constructs for HIV-1 and SIV. J Interferon Cytokine Res 1999; 19:77 - 84; http://dx.doi.org/10.1089/107999099314441; PMID: 10048771
- Kim JJ, Trivedi NN, Nottingham LK, Morrison L, Tsai A, Hu Y, et al. Modulation of amplitude and direction of in vivo immune responses by co-administration of cytokine gene expression cassettes with DNA immunogens. Eur J Immunol 1998; 28:1089 - 103; http://dx.doi.org/10.1002/(SICI)1521-4141(199803)28:03<1089::AID-IMMU1089>3.0.CO;2-L; PMID: 9541605
- Morrow MP, Yan J, Pankhong P, Ferraro B, Lewis MG, Khan AS, et al. Unique Th1/Th2 phenotypes induced during priming and memory phases by use of interleukin-12 (IL-12) or IL-28B vaccine adjuvants in rhesus macaques. Clin Vaccine Immunol 2010; 17:1493 - 9; http://dx.doi.org/10.1128/CVI.00181-10; PMID: 20685940
- Robinson TM, Sidhu MK, Pavlakis GN, Felber BK, Silvera P, Lewis MG, et al. Macaques co-immunized with SIVgag/pol-HIVenv and IL-12 plasmid have increased cellular responses. J Med Primatol 2007; 36:276 - 84; http://dx.doi.org/10.1111/j.1600-0684.2007.00245.x; PMID: 17669216
- Schadeck EB, Sidhu M, Egan MA, Chong S-Y, Piacente P, Masood A, et al. Plasmid encoded IL-12 functions as a DNA vaccine adjuvant and augments SIVgag-specific cell-mediated and humoral immune responses in Rhesus macaques. Vaccine 2006; 24:4677 - 87; http://dx.doi.org/10.1016/j.vaccine.2005.10.035; PMID: 16288822
- Schadeck EB, Sidhu M, Egan MA, Chong SY, Piacente P, Masood A, et al. A dose sparing effect by plasmid encoded IL-12 adjuvant on a SIVgag-plasmid DNA vaccine in rhesus macaques. Vaccine 2006; 24:4677 - 87; http://dx.doi.org/10.1016/j.vaccine.2005.10.035; PMID: 16288822
- Xu R, Megati S, Roopchand V, Luckay A, Masood A, Garcia-Hand D, et al. Comparative ability of various plasmid-based cytokines and chemokines to adjuvant the activity of HIV plasmid DNA vaccines. Vaccine 2008; 26:4819 - 29; http://dx.doi.org/10.1016/j.vaccine.2008.06.103; PMID: 18657584
- O’Neill E, Bostik V, Montefiori DC, Kraiselburd E, Villinger F. IL-12/GM-CSF coadministration in an SIV DNA prime/protein boost protocol enhances Gag-specific T cells but not virus-specific neutralizing antibodies in rhesus macaques. AIDS Res Hum Retroviruses 2003; 19:883 - 90; http://dx.doi.org/10.1089/088922203322493058; PMID: 14585220
- Kalams SA, Parker S, Jin X, Elizaga M, Metch B, Wang M, et al, NIAID HIV Vaccine Trials Network. Safety and immunogenicity of an HIV-1 gag DNA vaccine with or without IL-12 and/or IL-15 plasmid cytokine adjuvant in healthy, HIV-1 uninfected adults. PLoS One 2012; 7:e29231; http://dx.doi.org/10.1371/journal.pone.0029231; PMID: 22242162
- Manetti R, Gerosa F, Giudizi MG, Biagiotti R, Parronchi P, Piccinni MP, et al. Interleukin 12 induces stable priming for interferon gamma (IFN-gamma) production during differentiation of human T helper (Th) cells and transient IFN-gamma production in established Th2 cell clones. J Exp Med 1994; 179:1273 - 83; http://dx.doi.org/10.1084/jem.179.4.1273; PMID: 7908322
- Meyaard L, Hovenkamp E, Otto SA, Miedema F. IL-12-induced IL-10 production by human T cells as a negative feedback for IL-12-induced immune responses. J Immunol 1996; 156:2776 - 82; PMID: 8609396
- Leonard JP, Sherman ML, Fisher GL, Buchanan LJ, Larsen G, Atkins MB, et al. Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-gamma production. Blood 1997; 90:2541 - 8; PMID: 9326219
- Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, et al. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med 2009; 15:293 - 9; http://dx.doi.org/10.1038/nm.1935; PMID: 19219024
- Pitcher CJ, Hagen SI, Walker JM, Lum R, Mitchell BL, Maino VC, et al. Development and homeostasis of T cell memory in rhesus macaque. J Immunol 2002; 168:29 - 43; PMID: 11751943
- Gherardi MM, Ramírez JC, Esteban M. Interleukin-12 (IL-12) enhancement of the cellular immune response against human immunodeficiency virus type 1 env antigen in a DNA prime/vaccinia virus boost vaccine regimen is time and dose dependent: suppressive effects of IL-12 boost are mediated by nitric oxide. J Virol 2000; 74:6278 - 86; http://dx.doi.org/10.1128/JVI.74.14.6278-6286.2000; PMID: 10864637
- Lasarte JJ, Corrales FJ, Casares N, López-Díaz de Cerio A, Qian C, Xie X, et al. Different doses of adenoviral vector expressing IL-12 enhance or depress the immune response to a coadministered antigen: the role of nitric oxide. J Immunol 1999; 162:5270 - 7; PMID: 10228002
- Lee K, Overwijk WW, O’Toole M, Swiniarski H, Restifo NP, Dorner AJ, et al. Dose-dependent and schedule-dependent effects of interleukin-12 on antigen-specific CD8 responses. J Interferon Cytokine Res 2000; 20:589 - 96; http://dx.doi.org/10.1089/10799900050044787; PMID: 10888115
- Chowdhury FZ, Ramos HJ, Davis LS, Forman J, Farrar JD. IL-12 selectively programs effector pathways that are stably expressed in human CD8+ effector memory T cells in vivo. Blood 2011; 118:3890 - 900; http://dx.doi.org/10.1182/blood-2011-05-357111; PMID: 21832277
- Killian MS, Johnson C, Teque F, Fujimura S, Levy JA. Natural suppression of human immunodeficiency virus type 1 replication is mediated by transitional memory CD8+ T cells. J Virol 2011; 85:1696 - 705; http://dx.doi.org/10.1128/JVI.01120-10; PMID: 21147929
- Picker LJ, Hansen SG, Lifson JD. New paradigms for HIV/AIDS vaccine development. Annu Rev Med 2012; 63:95 - 111; http://dx.doi.org/10.1146/annurev-med-042010-085643; PMID: 21942424
- Frahm MA, Picking RA, Kuruc JD, McGee KS, Gay CL, Eron JJ, et al. CD4+CD8+ T cells represent a significant portion of the anti-HIV T cell response to acute HIV infection. J Immunol 2012; 188:4289 - 96; http://dx.doi.org/10.4049/jimmunol.1103701; PMID: 22461689
- Holznagel E, Norley S, Holzammer S, Coulibaly C, Kurth R. Immunological changes in simian immunodeficiency virus (SIV(agm))-infected African green monkeys (AGM): expanded cytotoxic T lymphocyte, natural killer and B cell subsets in the natural host of SIV(agm). J Gen Virol 2002; 83:631 - 40; PMID: 11842258
- Howe R, Dillon S, Rogers L, Palmer B, MaWhinney S, Blyveis N, et al. Phenotypic and functional characterization of HIV-1-specific CD4+CD8+ double-positive T cells in early and chronic HIV-1 infection. J Acquir Immune Defic Syndr 2009; 50:444 - 56; http://dx.doi.org/10.1097/QAI.0b013e31819aa8c4; PMID: 19360930
- Nascimbeni M, Pol S, Saunier B. Distinct CD4+ CD8+ double-positive T cells in the blood and liver of patients during chronic hepatitis B and C. PLoS One 2011; 6:e20145; http://dx.doi.org/10.1371/journal.pone.0020145; PMID: 21647449
- Nascimbeni M, Shin EC, Chiriboga L, Kleiner DE, Rehermann B. Peripheral CD4(+)CD8(+) T cells are differentiated effector memory cells with antiviral functions. Blood 2004; 104:478 - 86; http://dx.doi.org/10.1182/blood-2003-12-4395; PMID: 15044252
- Valentin A, von Gegerfelt A, Rosati M, Miteloudis G, Alicea C, Bergamaschi C, et al. Repeated DNA therapeutic vaccination of chronically SIV-infected macaques provides additional virological benefit. Vaccine 2010; 28:1962 - 74; http://dx.doi.org/10.1016/j.vaccine.2009.10.099; PMID: 20188252
- Guan Y, Sajadi MM, Kamin-Lewis R, Fouts TR, Dimitrov A, Zhang Z, et al. Discordant memory B cell and circulating anti-Env antibody responses in HIV-1 infection. Proc Natl Acad Sci U S A 2009; 106:3952 - 7; http://dx.doi.org/10.1073/pnas.0813392106; PMID: 19225108
- Moore JP, Wallace LA, Follett EA, McKeating JA. An enzyme-linked immunosorbent assay for antibodies to the envelope glycoproteins of divergent strains of HIV-1. AIDS 1989; 3:155 - 63; http://dx.doi.org/10.1097/00002030-198903000-00006; PMID: 2540772
- Xiao P, Zhao J, Patterson LJ, Brocca-Cofano E, Venzon D, Kozlowski PA, et al. Multiple vaccine-elicited nonneutralizing antienvelope antibody activities contribute to protective efficacy by reducing both acute and chronic viremia following simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques. J Virol 2010; 84:7161 - 73; http://dx.doi.org/10.1128/JVI.00410-10; PMID: 20444898
- Montefiori DC. Evaluating neutralizing antibodies against HIV, SIV, and SHIV in luciferase reporter gene assays. Curr Protoc Immunol 2005; Chapter 12:Unit 12 1.
- Keele BF, Li H, Learn GH, Hraber P, Giorgi EE, Grayson T, et al. Low-dose rectal inoculation of rhesus macaques by SIVsmE660 or SIVmac251 recapitulates human mucosal infection by HIV-1. J Exp Med 2009; 206:1117 - 34; http://dx.doi.org/10.1084/jem.20082831; PMID: 19414559