Abstract
How to tap into dilutive and non-dilutive funding sources for immunotherapy projects.
Introduction
The Phacilitate Immunotherapy Leaders‘ Forum has been again bringing together companies and scientists active in developing novel immunotherapies, be it therapeutic antibodies or antibody-like proteins, next generation antibodies, e.g., with effector functions or bi-specific molecules, and of course from the “re-buoying” field of therapeutic cancer vaccines. While most topics of the sessions, presentations and workshops, circled around the scientific advances and advanced in product development in the immunotherapy field, this years’ meeting also included a session around how to secure funding for developing immunotherapeutics (in case one is not a large pharmaceutical company).
In this session, venture capital investors and biotech CEOs discussed funding needs and different sources from scientific grants, governmental programs and charities to angel investors, family offices, VCs, corporate VCs and public equity funding. Most of these funding sources have significant cyclical and/or regional aspects, and pros and cons that need to be considered. Partnering a certain product candidate or a portion of a platform technology with larger pharmaceutical companies is another source that can be tapped. The panelists also presented and discussed brief case examples of biotech companies that got funding – one or the other way – to develop immunotherapies. Questions that where discussed included: What do venture capital investors like to see as prerequisite to fund such projects or companies, the experience with non-dilutive funding and whether it should influence the selection of the geography where the company is set up, at which stage pharmaceutical companies are willing to partner, and whether there is a public equity market for such companies.
Main Content
The session was kicked off by an introductory presentation by the session’s chairman, Dr. Klaus Breiner, Managing Partner at BB Biotech Ventures and Executive Chairman at Vaximm AG. The presentation covered the cost and sources of funding for developing immunotherapies, as well as case studies from the BB Biotech Ventures’ portfolio.
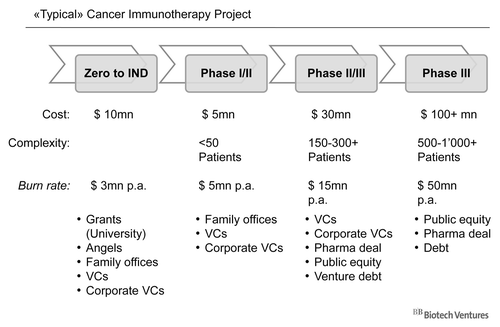
Cost of developing a typical (cancer) immunotherapy
summarizes approximate numbers with regard to the cost associated with the different stages of development of an immunotherapy. This approximation may vary with regard to the indication (example used is one of the larger cancer indications), the geographic location, the nature of the active pharmaceutical ingredient (“API”), the cost structure for a peptide vaccine is different from that of a therapeutic antibody, and other circumstances, such as e.g., preexisting data, material and methods.
When such a project or a respective company is started, the initial costs from concept stage to get to the clinic are about 10 million dollars. The time span is around three years, and includes in case of a biological, the generation of cell banks, the development of a manufacturing process for the API, the production of the API and pharmacodynamic and toxicological studies. The burn rate slightly increases when clinical studies are initiated. However, cash needs as well as managerial complexity during this period still remains relatively modest. A drastic increase in burn rate and complexity occurs, when a project moves from clinical phase I/II to phase II/III, which may involve more than one study and in the hundreds of patients. During this phase, the burn rate may easily exceed 15 million dollars per year. This creates the need to raise substantial amounts of money at least every other year to fund the program and the company behind it. Phase III development is basically only financeable for companies with access to public equity markets to raise large amounts of money, for large pharmaceutical companies with substantial free cash flow, or through partnerships with the former. ()
The different amounts required at the different project stages are typically associated with different types and sources of funding. In the initial phases, grants, family and friends, business angels and smaller family offices can contribute significantly to the financing needs of such companies. Venture capital firms (VCs) and Corporate VCs (typically associated with a large pharmaceutical company) do invest over the entire development phase, as long as the company is still privately held. In later phases, public equity (going public and follow-on public offerings on a stock exchange), debt instruments and deals with large pharmaceutical companies become essential to cover the huge burn rate.
The different sources of funds an their particularities
For all the different sources of funds mentioned in there are certain aspects to consider. Grants and soft loans are typically regional or national in nature and as a consequence there is a significant difference in the different countries and even between regions. Later in the session, Dr. Joel Crouzet, CEO of the French biotech company InnaVirVax, presented the funding history of his company showing over a third of of the total funding amount from grants. Especially projects in the field of HIV/AIDS (InnaVirVax’ focus) and other third world diseases attract grant money and non-dilutive money from foundations. As far as government funds are concerned, the French research tax credit system, involving tax cuts for monies invested into companies with the “Young Innovative Start-up” label, significantly enhance the willingness of private investors to put money into this segment. Dr. Ekaterina Smirnyagina, Venture Partner at Alta Partners, a US-based VC firm, mentioned the example of Innate Pharma, a publicly listed French biotech company developing antibodies modulating innate immunity to treat cancer and inflammation. Since inception, Innate Pharma has received € 40 million from the French state in form of research tax credit, direct equity investment and R&D related loans. The Dutch program “Innovation Loans” was also mentioned. In this program, the Dutch government provides up to 50% of direct project cost as a loan, if the company finances the other half from private investors. Only in case the project is successful, those loans have to be paid back. It serves as a leverage tool to give the investors a larger reward, if projects go well. While there are countries that have fiscal instruments in place to promote drug development in smaller biotech companies, other geographies such as Switzerland lack them almost entirely. A recent development was mentioned in Russia, where the state-owned fund Rusnano has an allocation to invest into drug development, if part of the development is performed in Russia, or Russian product rights are transferred to a Rusnano subsidiary. The choice of the geographic location of a newly to be founded company, especially in times of dire funding perspectives, should certainly include the availability of governmental financial support.
Business Angels typically invest only in their own territory. They invest smaller amounts, and have almost never a meaningful expertise the field of immunotherapy. For their investment decision, they typically rely on advisors, or simply enjoy investing in private companies that have an exciting story to tell based on their gut feeling. Family offices are a more refined version of Business Angels. Family offices too have often a regional bias. But there are players in this category, who are quite educated and/or have a proper network of advisors. Family offices that have been quite influential investors in biotech in Germany, and immunotherapeutic development in particular, include the SAP founder Dietmar Hopp (Dievini), as well as the former owners of the German generics company Hexal, Dres. Andreas and Thomas Stüngmann (Athos). One of the advantages of family offices as a source of funding is that they follow their own cycle. They are less influenced by the macroeconomic environment and they have less of a problem with the longer timelines associated with drug development projects. During his brief introduction, Dr. Matthias Kromeyer, a member of the Executive Board of MIG Verwaltungs AG, a German VC firm, made the point that it is paramount to ensure the support from a strong syndicate of potent investors for such projects, already from the very beginning. His firm has good experience with co-investing with larger family offices, which have deep pockets and are being rather patient investors.
VCs are more broadly investing across regions, across Europe or the US. Some VCs are even transatlantic or consider transatlantic investments. They are typically investors specialized in healthcare/life sciences and try to add value to their portfolio companies by sharing their know-how and network. In turn VCs want to have much more influence in the companies they invest in, than other investors. However, access to VC money in Europe and in the US has become increasingly difficult. Since the onset of the financial crises in 2008, the IPO exit market for the portfolio companies of VCs has more or less shut and fund flows from VC to their investors have suffered. As a consequence, many VCs have difficulties raising new funds to invest into biotech companies. This gap is partially filled by corporate VCs, which have become more active in recent times. Corporate VCs that invest in immunotherapy projects are part of pharmaceutical companies. Some of them have a strategic mission and some are purely financial return driven. Corporate VCs often draw from the expertise or follow the strategic direction of the mother company.
Vaximm AG, a Swiss-German cancer vaccines company that develops an oral T-cell vaccine to target the tumor vasculature, was presented as a case example that managed to raise monies from VC and corporate VCs. In this case, the strategic interest of the corporate VC encouraged two VC firms to join the syndicate of new investors in the company’s second financing round. Involvement of a (strategic) corporate VC can serve as a certain validation for financial VCs. On the downside, Corporate VCs are sometimes subject to substantial shifts in strategy by their parent companies, often with repercussions on their portfolio companies, especially if another refinancing is required.
After an extended period of low IPO activity on the public equity markets, due to the ongoing financial crisis, which started four years ago, the markets are now showing first signs of reopening both in the US and in Europe. Public biotech stocks have been rallying over the past nine months, which has helped public investors’ sentiment a lot. Moreover, several take-overs of public biotech companies by large pharmaceutical players seem to keep the rally going. Acquisitions of biotech companies also flushes cash into biotech investors’ pockets that needs to be redeployed. Manish Singh, CEO of the publicly listed US biotech company ImmunoCellular Therapeutics, made the point that “back door” listings on public markets, like reverse mergers or cold IPOs (where no new money is raised), is a viable strategy to address another type of investors in times where VC money is getting too scarce and/or expensive. There have been recent examples of immunotherapy companies that have chosen that route, such as OncoSec Medical and ImmunoCellular Therapeutics, both in the US.
Another way to obtain funding is through partnering a project or a technology platform with large pharmaceutical companies. Such “pharma deals” are happening at a steady rate, uncorrelated with macroeconomic cycles. It was predicted that the interest especially for cancer vaccines will increase in the near term. After many failures of cancer vaccines in clinical development, the success of Dendreon’s Provenge has broken the ice for investors and pharmaceutical players. MerckSerono and GSK have advanced cancer vaccine programs in clinical Phase 3 development. Both are expected to report pivotal data in the first quarter next year. If these data are positive, one can expect more deals in this area.
Deals can range from option deals for limited rights - to complete take overs. Two case examples where discussed during the session where pharma partnering played a significant role. Dr. Breiner mentioned the company Molecular Partners, a Swiss biotech company that is developing therapeutics based on DARPins (designed ankyrin repeat proteins), small binding proteins that have antibody-like functions. Next to raising two rounds of VC funding, Molecular Partners did three larger partnership deals so far. In parallel to the first VC financing, the company entered a partnership with J&J Centocor (now J&J Janssen Biotech) to develop multiple Darpins against certain targets. This deal served as a first validation of the commercial potential of the platform. The company was also able to partner their lead product with Allergan for a significant upfront payment plus further payments upon achievement of development milestones and sales. Another multi-target partnership with Janssen brought additional monies and potential future payments to the company. A company with an attractive platform technology can through intelligent deal-making become self-sustainable quickly and generate the monies required for their own development programs. Dr. Smirnagyna mentioned Innate Pharma as partnering case example. In 2011, Innate Pharma partnered one of its lead compounds with Bristol Myers Squibb for a significant upfront payment plus further payments down the road. Those monies can now be used to advance Innate’s remaining pipeline of immunotherapeutics.
In summary, all types of funding have their pro’s and con’s. As companies are typically using different sources, one has to weigh them and try to avoid incompatibilities. Even though in scarce times as the one we are currently living through, prospective founders and companies should not lightly disregard money from any source. What today looks like “expensive” money, may well turn out to be a sugar sweet deal tomorrow.