Abstract
Introduction: Invasive pneumococcal disease is associated with substantial morbidity, mortality and cost implications, which could be reduced by vaccination.
Aim: To assess the cost-effectiveness of a 23-valent pneumococcal vaccine in the elderly (65 and older) in Poland.
Methods: A Markov model with a 1-year cycle length was developed, allowing up to 10 cohorts to enter the model over the lifetime horizon (35 years). In the base case, costs and benefits were assessed using the public health care payer (NFZ) perspective. The analysis included routine vaccination of all elderly and high-risk (HR) elderly versus no vaccination. The analysis assumed that the government would reimburse 50% of the vaccine price. Costs and benefits were discounted 5%, with costs expressed in 2009 Polish Zloty (PLN). Extensive sensitivity analyses were carried out.
Results: PPV23 vaccination targeting all elderly and HR elderly in Poland would avoid 8,935 pneumococcal infections, 2,542 hospitalisations, 671 deaths and 5,886 infections, 1,673 hospitalisations and 441 deaths respectively. The incremental cost per QALY gained would be PLN 3,382 in all elderly and PLN2,148 in HR elderly.
Conclusion: Vaccinating adults 65 and older regardless of risk status with a 23-valent pneumococcal vaccine, is cost-effective, resulting in clinical and economic benefits including a non-negligible reduction of ambulatory doctor visits, hospitalizations and, deaths in Poland.
Cost-effectiveness of polysaccharide pneumococcal vaccination in people aged 65 and above in Poland
Introduction
Streptococcus pneumoniae is a bacterial pathogen that colonises the upper respiratory tract and is responsible for a range of infections. These include non-invasive forms, such as non-bacteraemic pneumococcal pneumonia (NBPP), otitis media and sinusitis, and more serious invasive pneumococcal disease (IPD), which includes bacteraemic pneumococcal pneumonia,(BPP) and pneumococcal meningitis (PM). Invasive pneumococcal diseases are often accompanied by severe complications that can lead to permanent sequelae and death.Citation1,Citation2 In industrialised countries, the overall case-fatality rate (CFR) for pneumococcal bacteraemia may reach 15–20% in adults and 30–40% in elderly patients.Citation3 Age-related reduction of immune function (immunosenescence) and co-morbidities predispose older adults to IPD, which will be more frequent and more deadly.Citation3,Citation4
Pneumococcal infections are primarily treated with antibiotics. However, treatment success has been compromised by a marked increase in antimicrobial resistance, including multi-drug resistance, caused by years of misuse and overprescribing of antibiotics. Treatment of IPD may thus incur higher costs due to an increased duration of hospitalisation and the need for more costly alternative antimicrobial therapies.Citation5
A pneumococcal polysaccharide vaccine covering 23 serotypes (PPV23) was licensed during the 1980s to prevent pneumococcal infections (PIs). PPV23 is indicated for persons aged two years and older. Since its licensure, many countries have recommended and financed its use in adults aged 65 years and older and in persons at high risk of developing pneumococcal infections such as the immunocompromised and the chronically ill.Citation6 When considering the countries surrounding Poland, PPV23 has already been recommended and publicly funded in Germany, Czech Republic, Hungary, Slovenia, Slovakia and Croatia for all adults over 59 or 64 years old.
Poland is the largest country in Central-Eastern Europe, with a total population of about 38.19 million in 2010.Citation7 In Poland, antimicrobial resistance of S. pneumoniae reached as high as 29.8% for penicillin, 16.1% for macrolides and 14.7% for dual penicillin and macrolide resistance,Citation8 showing the value of prevention of pneumococcal disease over its treatment with antibiotics.
As part of the health care financing system in Poland, universal coverage is provided through both public and private funding but currently PPV23 vaccination program is not part of the publicly funded benefit package. Consequently, PPV23 vaccine is paid for out-of-pocket. Therefore, despite current recommendations for its use, coverage rates for PPV23 vaccine have remained very low (less than 1%) meaning that little to no benefit of the vaccine has been seen in Poland and individuals remain largely unprotected.
In Poland, pneumonia is a leading cause of death and it is recognized that about 30–50% of all community-acquired pneumonia (CAP) are caused by S. pneumoniae.Citation9 Pneumonia can occur as a primary illness or can result as a common complication of influenza in the elderly. A recent burden of illness study in Poland collected data from three different sites (a general practice family clinic and two Warsaw hospitals) between 2007 and 2009. This study costed the resource items using a micro-costing approach for ambulatory costs and a Diagnosis-Related Group (DRG) approach was used for inpatient costs. This study showed that actual costs including hospitalization for CAP far exceed those borne by the national health fund (Narodowy Fundusz Zdrowia; NFZ). This study also showed that pneumococcal infection results in substantial costs, and that measures should be taken to protect the frail and reduce the cost burden.Citation10
In this context, the implementation of a universal programme of pneumococcal vaccination has great potential to prevent pneumococcal infections, particularly IPD. Therefore, vaccination is expected to result in significant health gains.
Several cost-effectiveness analyses have been conducted to assess the benefit of routine pneumococcal vaccination, although to date none have been conducted for Poland. In general, these studies have shown that vaccination policies range from very cost-effective to even cost-saving in the high risk and elderly population.Citation11-Citation13
The objectives of the present study were first to evaluate the current medical and economic burden of pneumococcal infections in Poland in persons aged 65 years or older (taking into consideration the results of the recent Polish study mentioned above), and second to analyze the cost-effectiveness of implementing a public vaccination programme in this population based on an assumed 50% reimbursement of PPV23 vaccine from the NFZ.
Results
Analysis on all elderly
Based on the estimations of our model, if the status quo of no pneumococcal vaccination for the elderly were to be maintained in Poland during the next years, 857,990 pneumococcal infections would be expected to occur in the population aged 65 years or older, of which 54,451 would be IPD, with the remainder being NBPP infections. These infections would result in a total of 168,896 hospitalizations (31% being IPD-related) and 44,225 deaths (31% due to IPD). Furthermore, our analysis indicates that if a PPV23 vaccination policy was not implemented in Poland within the following years, 232,926 life-years and 210,040 quality-adjusted life years (QALYs) would be lost due to pneumococcal infections in individuals aged 65 years and older (see ). Based on the level of reimbursement paid to health care providers by the NFZ (base case scenario), the cost of managing these infections would be PLN464.3 million, of which PLN405.9 million would be due to inpatient care, PLN51 million due to outpatient care, and PLN7.5 million related to long-term sequelae of meningitis. Since the actual hospitalisation costs incurred by health care providers are not fully covered by the NFZ,Citation10 an additional analysis was conducted based on the actual hospitalization costs incurred in the management of pneumococcal infections (Health Care System: HCS scenario). From this scenario, pneumococcal infections would result in a total cost of PLN936 million, 94% of which (i.e., PLN877.7 million) would be spent on hospitalisations, 5% (i.e., PLN 50.7 million) on treating infections in the outpatient setting, and 1% (PLN8.5 million) in dealing with post-meningitis long-term sequelae.
Table 1. Burden of disease for the time period considered at analysis in the absence of a pneumococcal vaccination program
If a PPV23 vaccination program was implemented in Poland, there would be approximately 5.1 million individuals aged 65 years or older eligible for coverage, of whom 274,320 would be 65 years-old and targeted for vaccination during the first year (based on estimations for 2010).Citation14 It is expected that the coverage rate in this first year of program implementation would be low (approximately 5%) and therefore only 13,716 of the targeted individuals would be vaccinated that year. The Polish population aged 65 years and older is assumed to increase over time (based on demographic data from the International Database of the US Census Bureau)Citation14, to up to 6.8 million individuals 10 years after the start of the vaccination program. The total number of vaccinated individuals aged 65 would range from 27,833 in year 2 (at a coverage rate of 10%) to 157,362 in year 10 (based on a 50% coverage rate, which was assumed to remain constant from year 10).
The implementation of a PPV23 vaccination policy would avoid a total of 8,935 pneumococcal infections, 2,542 hospitalizations and 671 deaths (of which 1,531, 1,468 and 391, respectively, would be IPD-related). In comparison with no pneumococcal vaccination, a PPV23 vaccination policy would save 5,004 QALYs. From the NFZ scenario, this policy would result in hospitalization savings of PLN10.83 million, ambulatory savings of PLN0.60 million and long-term sequelae savings of PLN0.37 million. The cost of vaccination would be PLN28.73 million. Therefore, the additional costs related to vaccination would be PLN16.92 million. From the HCS scenario, the additional cost of vaccination would be PLN11.29 million (as a result of an expenditure in PPV23 vaccines of 28.73 millions and of savings of PLN16.42 million in hospitalizations, PLN0.60 million in outpatient care and PLN 0.43 million in the management of long-term sequelae).
From the NFZ scenario, the incremental cost per additional QALY gained was PLN 3,382, compared to PLN 2,255 per QALY gained when costs borne by the HCS are considered.Citation10 According to the World Health Organization (WHO), an intervention resulting in a cost per life year gained of one times the gross domestic product (GDP) per capita of the region is considered cost-effective, while below that number it would be considered very cost-effective.Citation15 In the case of Poland, the GDP per capita was PLN37,505 or US$11,311 in 2009.Citation16,Citation17 Therefore a PPV23 vaccination policy targeting the elderly in Poland would be a very cost-effective strategy to implement ().
Table 2. Base-case results for all elderly using NFZ and HCS scenarios (price year: PLN 2009)
Analysis in at-risk elderly
In view of the results of our analyses focusing on the elderly at high risk of developing pneumococcal infections (i.e., 50% of the total elderly population, based on the assumptions formulated in the model, derived from expert opinion and from the literature),Citation18-Citation22 no PPV23 vaccination policy would result in 504,130 pneumococcal infections. In addition, 99,239 hospitalisations and 25,985 deaths would be estimated to occur. In total, 6% of the total number of infections, 31% of the hospitalizations and 31% of the deaths would be due to IPD. In this situation, 118,289 QALYs would be lost due to pneumococcal infections.
If a PPV23 vaccination program was to target only the elderly population at high-risk, there would be approximately 2.59 million of individuals aged 65 years or older at high risk of developing pneumococcal infections, 137,160 of them would be 65 years-old and targeted for vaccination during the first year.Citation14 Based on an expected 5% coverage rate for this first year, 6,858 of the targeted high-risk elderly would be vaccinated that year. The total number of individuals who would be vaccinated at age 65 would increase gradually from 13,917 in the second year (given a coverage rate of 10%) to 157,362 in year 10 (based on a 50% coverage rate).
Under these conditions, a PPV23 vaccination policy would avoid a total of 5,886 pneumococcal infections, 1,673 hospitalizations and 441 deaths (of which 1,006, 964 and 257, respectively, would be IPD-related). The implementation of this pneumococcal vaccination program would result in a reduction of the number of discounted QALYs (3,076 in total). When considering the NFZ perspective, vaccinating only the high-risk elderly would require fewer resources in terms of vaccine costs (PLN14.37 million), but the potential to reduce treatment costs would also be limited, resulting in expected cost savings of PLN7.76 million (i.e. PLN7.13 million in hospitalizations, PLN0.40 million in outpatient care and 0.23 million in care related to post-meningitis sequelae). Therefore, vaccination would result in incremental costs of PLN6.61 million when compared to no vaccination, and the resulting incremental cost-effectiveness ratio (ICER) would be PLN2,148 per additional QALY gained. From the HCS scenario, PLN10.82 million would be saved in hospitalizations, PLN0.39 million in out-patient costs, and PLN0.26 million in the management of post-meningitis long-term sequelae. The total cost of vaccinating the high-risk elderly would be PLN14.37 million resulting in an incremental cost per QALY gained of PLN939 ().
Table 3. Base-case results for high-risk elderly using NFZ and HCS scenarios (price year: PLN 2009)
Sensitivity analyses
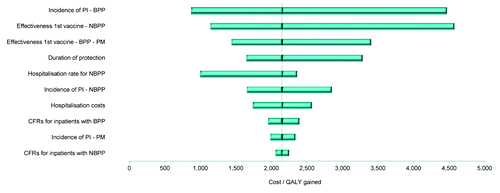
The impact of the individual parameters on cost-effectiveness was evaluated by conducting deterministic sensitivity analyses (DSAs) based on a plausible range of variation within the NFZ scenario (see ). Based on these analyses, results were most sensitive to the effectiveness of the vaccine against NBPP, BPP and PM, the duration of vaccine protection, the incidence of pneumococcal infections (mainly incidence of BPP) and the NBPP hospitalization rate.
Table 4. Parameter values and ranges used in sensitivity analyses
Using minimum and maximum values of vaccine effectiveness against NBPP infections for the NFZ scenario (base case) resulted in ICERs between PLN2,040 and PLN6,593 per QALY gained when targeting all elderly, and between PLN1,139 and PLN4,474 per QALY gained when targeting only high-risk elderly. Even when the most conservative effectiveness values were considered, the ICERs remained well below the accepted WHO cost-effectiveness threshold ( and ).
Figure 1. Deterministic sensitivity analysis results: Tornado diagram of the ICERs (i.e. incremental cost per QALY gained) from the NFZ scenario in all elderly. The 10 most sensitive parameters are represented, and the X axis reflects the absolute change in the ICER compared to the baseline value.
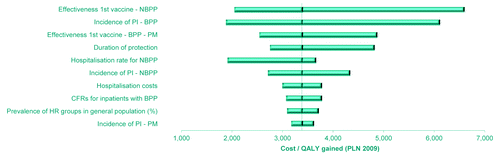
Figure 2. Tornado diagram of the deterministic sensitivity analysis of the ICERs related to pneumococcal infections from the NFZ scenario in high-risk elderly. The 10 most sensitive parameters are represented, and the X axis reflects the absolute change in the ICER compared to the baseline value.
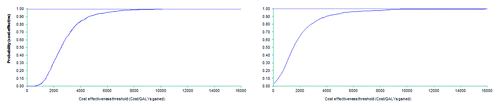
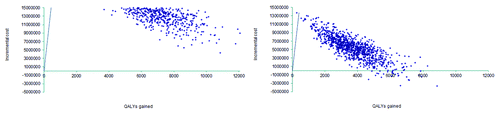
A probabilistic sensitivity analysis (PSA) was conducted that used the NFZ scenario (base case scenario) and assumed the vaccination policy would target all elderly. The main parameters modified in the PSA included the incidence of pneumococcal infections, hospitalization rates due to NBPP, the probability of developing long-term sequelae after PM, case fatality rates for inpatients and the effectiveness of the vaccine in preventing NBPP and IPD. Parameters and distributions are shown in . On the one hand, when all elderly were targeted in the vaccination program, PPV23 would have a 75% likelihood of being cost-effective compared to no vaccination at a willingness threshold of PLN3,500 per QALY gained. This probability would be close to 100% for a threshold of PLN10,000 or higher. On the other hand, for a vaccination policy targeting elderly at high risk only, PPV23 would be 75% cost-effective at a willingness to pay of PLN2,467 and 100% cost-effective at a willingness to pay threshold of PLN15,170 or higher (). Therefore the implementation of a PPV23 vaccination policy can be considered cost-effective as the ICER would be lower than the WHO threshold of PLN37,505 ( and ).
Table 5. Parameters and distributions considered in the PSA
Discussion
The results of our cost-effectiveness analysis showed that, according to the WHO threshold, the implementation of a PPV23 vaccination program in Poland would be highly cost-effective, independent of the population considered (all elderly or at-risk elderly) or the costs adopted (NFZ and HCS). All ICERs estimated including the base case and the sensitivity analyses were well below the established WHO threshold i.e. PLN37,505.
To date no cost-effectiveness studies of pneumococcal vaccination have been conducted for Poland,. therefore, direct comparison of results is not possible. Several published economic analyses of pneumococcal vaccines in the elderly have shown pneumococcal vaccination to be cost-effective or even cost-saving in countries such as Belgium, France, Scotland, Spain and Sweden compared to no vaccination. ICERs ranged from approximately ?11,000 per QALY in Spain to more than ?33,000 per QALY in Sweden for prevention of IPD in adults aged 65 years and older. A study assessing the cost-effectiveness of pneumococcal vaccination in the prevention of hospitalisation in persons aged 65 years or older reported ICERs that ranged from ?9,239 to ?23,657 per QALY. This study was conducted across 10 European countries (Belgium, France, Scotland, Spain, Sweden, Denmark, the UK (specifically, England and Wales), Germany, Italy and The Netherlands).Citation23
These results are consistent with the results of the present analysis and are not surprising since many costly clinical interventions may be avoided due to pneumococcal vaccination. In the present study, PPV23 appears to be more cost-effective than in other countries may be due to our assumption that the NFZ would reimburse only 50% of the vaccine cost, whereas in other countries the full cost is usually reimbursed.
ICERs are provided here for the total population aged 65 and over. From the NFZ scenario, an incremental cost per QALY gained was estimated to be PLN3,382 when vaccinating all elderly, and PLN2,148 when targeting only HR elderly. This difference is notably due to the fact that the incidence rates of pneumococcal infections were assumed to be higher among high-risk elderly. However, risk-based vaccination strategies are often much more difficult to implement and result in a lower vaccine coverage rates than age-based strategies. Therefore, it may be expected that the ICER for vaccinating the very old is less attractive than that for the total 65+ population.Citation6,Citation24,Citation25
When the HCS scenario was analysed, vaccination was seen to be an even more cost-effective strategy (PLN838 and PLN2,160 per QALY gained for high-risk elderly HR and all elderly respectively), than with the NFZ scenario, since the actual costs of hospitalizations were taken into account. This resulted in higher cost savings related to the avoidance of hospitalizations, out-patient care, and long-term sequelae.
A herd protection has been demonstrated with the 7-valent pneumococcal conjugate vaccine (PVC7) which resulted in the prevention of pneumococcal disease in unvaccinated children and adults (including the elderly).Citation26 Currently, although PCV7 is recommended for children at high risk of pneumococcal infections, it is not used routinely in Poland and has a low coverage rate, which is the reason why herd protection was not considered in the base case of this study. If PPV23 vaccination of the elderly were to be adopted in Poland in addition to a pneumococcal conjugate vaccine programme for children, the PCV7 vaccination programme would likely provide benefits to the larger population, including the elderly, thus necessitating the inclusion of herd protection in future economic assessments of PPV23. To gauge possible herd protection effects, an additional analysis was conducted, that assessed the effect of herd protection on the incidence rates of pneumococcal infections in the elderly and on the corresponding cost-effectiveness results. For this analysis, US data were used for the incidence of pneumococcal infections before and after the introduction of the PCV7, consideration of serotype distribution in Europe, and PCV7 coverage rates in children in Poland.Citation27,Citation28 The proportions of infections attributed to PCV7 serotypes, PPV23 serotypes not included in the PCV7 vaccine, and non-vaccine serotypes were derived from a population-based survey of IPD conducted in adults in North-Rhine Westphalia (Germany).Citation29 Based on this study, 47.8% of the IPD cases in Polish individuals older than 65 years were caused by PCV7 serotypes, 82.1% by PPV23 serotypes and the remaining cases were due to non-vaccine serotypes. Given the lack of information about the serotype distribution for NBPP infections, the same distribution was assumed across all types of pneumococcal infections. From the NFZ scenario and considering all elderly as the target population, the estimated ICER was PLN6,315. This ICER was higher than the value estimated in the base case analysis, which did not include herd protection effects (i.e., PLN3,382 per QALY gained). The reason for this is the expected reduction over time in the number of pneumococcal infections related to PCV7 serotypes, not only in vaccinated children but also in other populations including unvaccinated children, adults, and the elderly that decrease the medical and economic burden of pneumococcal infections.Citation30-Citation34 However even in such circumstances, we would expect PPV23 to remain cost-effective as indicated by the present analyses and by the results of previously published studies.Citation35
A limitation of this model is that not all data were obtained specifically from the Poland due to the lack of local data. Extensive deterministic sensitivity analyses were conducted to assess uncertainty related to these parameters. However, even when the most pessimistic values were used in the sensitivity analyses, the ICERs remained well below the accepted Polish thresholds. These results indicate a high likelihood that PPV23 vaccination policy in Poland would be cost-effective.Citation36,Citation37
In the base case analysis it was assumed that PPV23 would be effective in reducing not only IPD infections but also NBPP infections. It should be noted that meta-analyses of randomized clinical trials have shown inconclusive results regarding the efficacy of PPV23 against NBPP. However, these inconclusive results may be due to the heterogeneity of the results or the lack of power of the studies.Citation51,Citation52 Indeed, observational studies have demonstrated that PPV23 may be effective against NBPP, as concluded by Conaty et alCitation38 in a systematic review of observational studies, which reported a combined effectiveness of 32% against all pneumonia infections. Moreover, two recent clinical studies conducted in Spain reported an efficacy of PPV23 against NBPP of 42% in people aged 50 or older (95% CI: 14 to 61) and 39% (95% CI: -6 to 65) in those over 65 irrespective of their risk status.Citation37,Citation38 To assess the uncertainty surrounding this parameter in the present study, extensive sensitivity analyses were conducted. The results of these analyses strongly suggest that PPV23 would remain highly cost-effective even when it was assumed that the vaccine would not protect against NBPP infections (from the NFZ scenario, ICERs per QALY gained are equal to PLN6,593 for all elderly and PLN4,574 for HR elderly).
Based on the findings of the study, a strategy to vaccinate all people aged 65 and over regardless of risk status against pneumococcal infections using a 23-valent polysaccharide vaccine, would be highly cost-effective and therefore should be implemented. Substantial savings in out-patient care, hospitalisation and death are produced. Considering the difficulties in implementing risk-based strategies, targeting all elderly would be preferable.
Materials & Methods
Model description
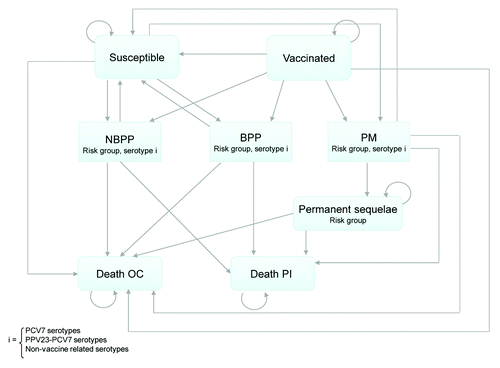
A Markov model with a 1-year cycle length was developed that could accommodate the complexity related to the substantial number of transitions associated with pneumococcal infections (i.e., susceptibility, protection, infections by type, risk levels, serotype groups, and the probability of developing sequelae or death). The model was semi-dynamic, allowing up to 10 new cohorts to enter the model to account for the assumption that the maximum vaccine coverage would be attained within the first 10 years after vaccine implementation. Two different scenarios were adopted. The so called “base case” scenario reflected the reimbursement paid by the National Health Fund, NFZ, to providers of in-patient or out-patient care. The second scenario, referred to here as the “HCS scenario”, considered the HCS costs that included the actual cost of hospitalisations (i.e., tests, procedures and drugs during the inpatient stay and accounted for length of stay). Since hospitalisation costs are not fully covered by the NFZ benefit package, the total hospitalization costs of pneumococcal infections are higher using the HCS scenario.Citation10 To evaluate the best strategy, analyses were conducted for two different target populations, these being all elderly and the elderly at high-risk of developing pneumococcal infections. Therefore, four analyses were conducted and compared to no vaccination: (1) routine vaccination of all elderly; (2) routine vaccination of all elderly using the HCS costs; (3) routine vaccination of HR elderly; and (4) routine vaccination of HR elderly using the HCS costs. No vaccination was selected as the comparator given the current low vaccine coverage in Poland. A lifetime horizon was considered for the estimation of health benefits and costs, which were discounted at a 5% rate as suggested by the Polish guidelines.Citation39 Costs were expressed in 2009 Polish Zloty (EUR 1= PLN 4.1045, December 2009 exchange rate).
Patients entered the model in one of two “healthy” states, i.e., vaccinated or susceptible. Over time, patients could develop pneumococcal disease (NBPP, BPP and PM) or they could remain in one of the two “healthy” states. The model assumed that while healthy, patients could die only from causes other than pneumococcal infections. The likelihood of transitioning from a healthy state to a pneumococcal disease-related state depended on the susceptibility of infection, which in turn depended on individuals risk level and their vaccination status. Only patients with PM could develop permanent sequelae. Transitions from the vaccinated state to the susceptible state could occur as vaccine efficacy waned over time (see ).
Model inputs
Demographic data for the elderly population by age group between 2010 and 2019 were obtained from the International Database of the US Census Bureau. These data were used to represent the 10 cohorts simulated in this open-population model.Citation40 Age-dependent mortality rates were obtained from the Life Tables from Poland.Citation7 The age-dependent probability of dying was adjusted to reflect that, based on expert opinion, high-risk elderly aged 65–74 are 1.75 times more likely to die from any cause and 2.5 times more likely to die if aged 75 years or older as compared to their low risk counterparts. No correction was made to the overall mortality to account for the fact that this included pneumococcal mortality since this is a relatively rare cause of mortality in the general population.Citation41
In line with estimates from the WHO and with values observed across Europe,Citation23,Citation29,Citation42 the incidence of IPD was assumed to be 54.50 per 100,000 in the population aged 65 or older in Poland. From these, 50.41 cases were BPP and 4.09 were PM.Citation42,Citation43 The incidence of NBPP was estimated as 804.27 cases per 100,000 elderly population. These values were calculated using the average incidence rates of pneumococcal pneumonia hospitalised cases observed across five European countriesCitation12 (i.e., 301.4 per 100,000 elderly population) accounting for the fact that hospitalised cases represented 40% of all cases of pneumoniaCitation44 and assuming that 80% of pneumococcal pneumonia cases are NBPP,Citation43 and that 13.6% of NBPP cases would require hospitalisation (confirmed by Polish experts). Annual incidence rates were further adjusted to reflect that high-risk elderly in Poland are twice as likely to develop pneumococcal infections as those at lower risk (based on expert opinion).
The impact of antibiotic resistance was also considered in view of the high resistance levels reported in the Polish literature. Patients initially treated as out-patients and who were subsequent found to be infected by a resistant organism had a probability of 7.69% of being hospitalized.Citation45
A 26.6% case-fatality rate (CFR) was assumed for the elderly persons hospitalized with IPD; this value was estimated using the average of the CFRs reported across 16 studies (values ranged from 11% to 44%).Citation9 For hospitalized NBPP cases, a CFR of 15% was assumed (derived from CAP patients),Citation46 while among out-patients a 2% CFR was assumed.Citation47
Elderly patients with PM were considered to have a 33% probability of developing long-term sequelae severe enough to affect their daily life and ability to work.Citation48 Due to lack of evidence, mortality rates for individuals developing long-term sequelae were conservatively assumed to be the identical to those observed in the general population.
In terms of efficacy, it was assumed that PPV23 was effective in reducing the number of both invasive and non-invasive pneumococcal infections. Little evidence was found in the literature differentiating PM and BPP in that most available effectiveness data pertained to IPD in general. Effectiveness against IPD was assumed to be 64% for all elderly (irrespective to underlying conditions) and was calculated as the mean effectiveness observed across multiple observational studies.Citation47,Citation49,Citation50 Data on NBPP-specific efficacy were scarce and inconclusive. In the base case analysis it was assumed that PPV23 is 21% effective in reducing the number of NBPP infections in the elderly, which represents the average of the efficacy observed from the available evidence (range 0Citation51,Citation52 to 42%).Citation46,Citation47 To assess the uncertainty of this assumption, we conducted sensitivity analyses that assumed no efficacy against NBPP infections. Regarding the duration of protection, we assumed that the efficacy of PPV23 peaked during the first year with protection waning over timeCitation53,Citation54 until no protection remained after 9 years as previously described.Citation11,Citation53
The annual PPV23 vaccination rate was assumed to increase linearly after the implementation of the vaccination program, i.e., from 5% in year 1 to 50% in year 10 (maximum uptake), and that it would remain stable at 50% in subsequent years.
From the NFZ scenario, estimated costs were based on the direct medical costs reimbursed by the Polish NFZ, such as costs of acquiring the vaccine, hospitalization costs of infected individuals treated as in-patients for pneumonia, streptococcus bacteremia and meningitis, out-patient costs for clinical influenza and the costs of treating long-term sequelae resulting from meningitis. The expected vaccine acquisition costs were provided by sanofi pasteur. It was considered that half of the cost would be reimbursed by the NFZ with the remainder being paid by the patient. Vaccine administration costs were assumed to be null since it was expected that the vaccine would be administered during a routine visit to the general practitioner. Hospitalization and outpatient cost data were obtained from a study recently conducted in PolandCitation10 that showed that the actual hospitalization costs incurred by the health care providers are not fully covered by the reimbursement paid by the NFZ.Citation10 Under the HCS scenario, the actual costs of hospitalization were considered rather than just those reimbursed by the NFZ.Citation10
Based on the results from a Canadian study, the annual cost of treating long-term sequelae was 3.46 times less than the costs of treatment for an acute meningococcal infection. This ratio was applied to the average cost of patients requiring hospitalization due to PM.Citation10 The resulting cost was PLN 1,099.
Health benefits of vaccination were estimated as the number of pneumococcal infections, hospitalizations and deaths avoided, in total, by infection type (NBPP, BPP and PM). The years of life lost (LYL) due to death following an infection and the number of quality-adjusted life years (QALYs) lost due to pneumococcal infections were estimated in the model. The number of LYLs due to infections was based on the life expectancy by age. Life expectancy data were obtained from the Life Tables of Poland, Polish Central Statistics Office 2007).Citation55 QALYs were estimated by applying a reduction in utility to pneumococcal infections (based on the duration of these infections), long-term (life long) sequelae and death. Polish-specific utility norms for the general population were not available, therefore we used utilities from Hungary, a neighboring countryCitation56
ICERs were estimated as the ratio of the difference in costs over the difference in health benefits between both strategies (vaccination versus no vaccination) for the four analyses defined previously.
Deterministic and probabilistic sensitivity analyses (DSA and PSA, respectively) were conducted to assess the robustness of the cost-effectiveness results (see and ) The PSA was conducted from the NFZ scenario for both target populations. The parameters used in the PSA were selected based on the level of uncertainty and their influence on results (as determined through the DSA). Probabilistic distributions were assigned to the selected parameters based on the type of input parameter (see ), and 1,000 simulations were run. Results were represented as a cost-effectiveness acceptability curve (CEAC).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgements
The authors would like to thank their colleagues Sebastian SOLUCH and Katarzyna ADAMCZYK and Monique MARTIN from OptumInsight for their assistance and their comments on previous drafts.
References
- Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 1997; 46:RR-8 1 - 24; PMID: 9132580
- Wisløff T, Abrahamsen TG, Bergsaker MA, Løvoll Ø, Møller P, Pedersen MK, et al. Cost effectiveness of adding 7-valent pneumococcal conjugate (PCV-7) vaccine to the Norwegian childhood vaccination program. Vaccine 2006; 24:5690 - 9; http://dx.doi.org/10.1016/j.vaccine.2006.04.042; PMID: 16735083
- 23-valent pneumococcal polysaccharide vaccine. WHO position paper. Wkly Epidemiol Rec 2008; 83:373 - 84; PMID: 18927997
- Kyaw MH, Rose CE Jr., Fry AM, Singleton JA, Moore Z, Zell ER, et al, Active Bacterial Core Surveillance Program of the Emerging Infections Program Network. The influence of chronic illnesses on the incidence of invasive pneumococcal disease in adults. J Infect Dis 2005; 192:377 - 86; http://dx.doi.org/10.1086/431521; PMID: 15995950
- Pebody RG, Hellenbrand W, D’Ancona F, Ruutu P, European Union funded Pnc-EURO contributing group. Pneumococcal disease surveillance in Europe. Euro Surveill 2006; 11:171 - 8; PMID: 17075159
- Melegaro A, Edmunds WJ. The 23-valent pneumococcal polysaccharide vaccine. Part I. Efficacy of PPV in the elderly: a comparison of meta-analyses. Eur J Epidemiol 2004; 19:353 - 63; http://dx.doi.org/10.1023/B:EJEP.0000024701.94769.98; PMID: 15180106
- Polish Central Statistical Office. . see: http://www.stat.gov.pl/gus/5840_655_ENG_HTML.htm
- ECDC surveillance report 2009; antimicrobial resistance surveillance in Europe 2009, http://www.ecdc.europa.eu/en/publications/Publications/1011_SUR_annual_EARS_Net_2009.pdf
- Fedson DS, Musher DM. Pneumococcal polysaccharide vaccine. In: Vaccines (Plotkin,SA, Orenstein,WA, eds), 4th edn. 2004: 543.
- Jahnz-Rózyk K. . [Health economic impact of viral respiratory infections and pneumonia diseases on the elderly population in Poland]. Pol Merkuriusz Lek 2010; 29:37 - 40
- Sisk JE, Whang W, Butler JC, Sneller VP, Whitney CG. Cost-effectiveness of vaccination against invasive pneumococcal disease among people 50 through 64 years of age: role of comorbid conditions and race. Ann Intern Med 2003; 138:960 - 8; PMID: 12809452
- Ament A, Baltussen R, Duru G, Rigaud-Bully C, de Graeve D, Ortqvist A, et al. Cost-effectiveness of pneumococcal vaccination of older people: a study in 5 western European countries. Clin Infect Dis 2000; 31:444 - 50; http://dx.doi.org/10.1086/313977; PMID: 10987703
- Akin L, Kaya M, Altinel S, Durand L. Cost of pneumococcal infections and cost-effectiveness analysis of pneumococcal vaccination at risk adults and elderly in Turkey. Hum Vaccin 2011; 7:441 - 50; http://dx.doi.org/10.4161/hv.7.4.14188; PMID: 21441776
- U.S. Census Bureau. International Data Base (IDB), http://www.census.gov/ipc/www/idb/ country.php, accessed in March 2011.
- WHO. CHoosing Interventions that are Cost Effective http://www.who.int/choice/costs/CER_levels/en/index.html
- WHO. Social indicators. http://unstats.un.org/unsd/demographic/products/socind/inc-eco.htm
- XE. http://www.xe.com/ict/ 19th July 2011
- de Andres AL, Garrido PC, Hernández-Barrera V, Del Pozo SV, de Miguel AG, Jiménez-García R. Influenza vaccination among the elderly Spanish population: trend from 1993 to 2003 and vaccination-related factors. Eur J Public Health 2007; 17:272 - 7; http://dx.doi.org/10.1093/eurpub/ckl242; PMID: 17071634
- Fleming DM, Elliot AJ. Estimating the risk population in relation to influenza vaccination policy. Vaccine 2006; 24:4378 - 85; http://dx.doi.org/10.1016/j.vaccine.2006.02.053; PMID: 16574281
- Mullooly JP, Bennett MD, Hornbrook MC, Barker WH, Williams WW, Patriarca PA, et al. Influenza vaccination programs for elderly persons: cost-effectiveness in a health maintenance organization. Ann Intern Med 1994; 121:947 - 52; PMID: 7978721
- Ryan J, Zoellner Y, Gradl B, Palache B, Medema J. Establishing the health and economic impact of influenza vaccination within the European Union 25 countries. Vaccine 2006; 24:6812 - 22; http://dx.doi.org/10.1016/j.vaccine.2006.07.042; PMID: 17034909
- Rychlik R, Heinen-Kammerer T, Rusche H, Piercy J, Scuffham P, Zöllner Y. [Cost-effectiveness of prophylaxis and treatment of influenza]. Dtsch Med Wochenschr 2003; 128:2267 - 70; PMID: 14574642
- Evers SM, Ament AJ, Colombo GL, Konradsen HB, Reinert RR, Sauerland D, et al. Cost-effectiveness of pneumococcal vaccination for prevention of invasive pneumococcal disease in the elderly: an update for 10 Western European countries. Eur J Clin Microbiol Infect Dis 2007; 26:531 - 40; http://dx.doi.org/10.1007/s10096-007-0327-z; PMID: 17570001
- Weaver M, Krieger J, Castorina J, Walls M, Ciske S. Cost-effectiveness of combined outreach for the pneumococcal and influenza vaccines. Arch Intern Med 2001; 161:111 - 20; http://dx.doi.org/10.1001/archinte.161.1.111; PMID: 11146707
- Baltussen R, Ament A, Leidl RM, et al. Cost-effectiveness of vaccination against pneumococcal pneumonia in the Netherlands. Eur J Public Health 1997; 7:153 - 61; http://dx.doi.org/10.1093/eurpub/7.2.153
- Fedson DS, Nicolas-Spony L, Klemets P, van der Linden M, Marques A, Salleras L, et al. Pneumococcal polysaccharide vaccination for adults: new perspectives for Europe. Expert Rev Vaccines 2011; 10:1143 - 67; http://dx.doi.org/10.1586/erv.11.99; PMID: 21810065
- Grijalva CG, Griffin MR. Population-based impact of routine infant immunization with pneumococcal conjugate vaccine in the USA. Expert Rev Vaccines 2008; 7:83 - 95; http://dx.doi.org/10.1586/14760584.7.1.83; PMID: 18251696
- Center for Disease Control and Prevention –CDC- 2007 National Immunization Survey
- Reinert RR, Haupts S, van der Linden M, Heeg C, Cil MY, Al-Lahham A, et al. Invasive pneumococcal disease in adults in North-Rhine Westphalia, Germany, 2001-2003. Clin Microbiol Infect 2005; 11:985 - 91; http://dx.doi.org/10.1111/j.1469-0691.2005.01282.x; PMID: 16307552
- Pneumococcal Vaccines Workgroup ACIP. , 25.06.2008
- Kellner JD, Vanderkooi OG, MacDonald J, Church DL, Tyrrell GJ, Scheifele DW. Changing epidemiology of invasive pneumococcal disease in Canada, 1998-2007: update from the Calgary-area Streptococcus pneumoniae research (CASPER) study. Clin Infect Dis 2009; 49:205 - 12; http://dx.doi.org/10.1086/599827; PMID: 19508165
- Vestrheim DF, Høiby EA, Bergsaker MR, Rønning K, Aaberge IS, Caugant DA. Indirect effect of conjugate pneumococcal vaccination in a 2+1 dose schedule. Vaccine 2010; 28:2214 - 21; http://dx.doi.org/10.1016/j.vaccine.2009.12.054; PMID: 20056192
- Ardanuy C, Tubau F, Pallares R, Calatayud L, Domínguez MA, Rolo D, et al. Epidemiology of invasive pneumococcal disease among adult patients in barcelona before and after pediatric 7-valent pneumococcal conjugate vaccine introduction, 1997-2007. Clin Infect Dis 2009; 48:57 - 64; http://dx.doi.org/10.1086/594125; PMID: 19035779
- Rodenburg GD, de Greeff SC, Jansen AG, de Melker HE, Schouls LM, Hak E, et al. Effects of pneumococcal conjugate vaccine 2 years after its introduction, the Netherlands. Emerg Infect Dis 2010; 16:816 - 23; http://dx.doi.org/10.3201/eid1605.091223; PMID: 20409372
- Smith KJ, Lee BY, Nowalk MP, Raymund M, Zimmerman RK. Cost-effectiveness of dual influenza and pneumococcal vaccination in 50-year-olds. Vaccine 2010; 28:7620 - 5; http://dx.doi.org/10.1016/j.vaccine.2010.09.053; PMID: 20887828
- Redekop WK, Orlewska E, Maciejewski P, Rutten FF, Niessen LW. Costs and effects of secondary prevention with perindopril in stable coronary heart disease in Poland: an analysis of the EUROPA study including 1251 Polish patients. Pharmacoeconomics 2008; 26:861 - 77; http://dx.doi.org/10.2165/00019053-200826100-00006; PMID: 18793033
- Simon K, Gladysz A, Rotter K, et al. Cost effectiveness of replacing recombinated interferon alpha-2b with its pegylated form in combination with ribavirin for the therapy of chronic HCV infection in Poland. Advances in Clinical and Experimental Medicine 2006; 15:453 - 62
- Conaty S, Watson L, Dinnes J, Waugh N. The effectiveness of pneumococcal polysaccharide vaccines in adults: a systematic review of observational studies and comparison with results from randomised controlled trials. Vaccine 2004; 22:3214 - 24; http://dx.doi.org/10.1016/j.vaccine.2003.08.050; PMID: 15297076
- Orlewska, Ewa and Mierzejewski, Piotr. Polish guidelines for conducting pharmacoeconomic evaluations. 1-15. 2009.
- U.S. Census Bureau. International Data Base (IDB), http://www.census.gov/ipc/www/idb/ country.php, accessed in March 2011.
- Weinstein MC, O’Brien B, Hornberger J et al. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR Task Force on Good Research Practices--Modeling Studies.
- 23-valent pneumococcal polysaccharide vaccine. WHO position paper. Wkly Epidemiol Rec 2008; 83:373 - 84; PMID: 18927997
- Fedson DS. Pneumococcal vaccination for older adults: the first 20 years. Drugs Aging 1999; 15:Suppl 1 21 - 30; http://dx.doi.org/10.2165/00002512-199915001-00003; PMID: 10690792
- Ament A, Baltussen R. Duru G and al. Cost-Effectiveness of Pneumococcal Vaccination of Older People : A Study in 5 Western European Countries. Clin Infect Dis 2000; 33:444 - 50; http://dx.doi.org/10.1086/313977
- Fantin B, Aubert JP, Unger P, Lecoeur H, Carbon C. Clinical evaluation of the management of community-acquired pneumonia by general practitioners in France. Chest 2001; 120:185 - 92; http://dx.doi.org/10.1378/chest.120.1.185; PMID: 11451836
- Vila-Corcoles A, Ochoa-Gondar O, Rodriguez-Blanco T, Raga-Luria X, Gomez-Bertomeu F, EPIVAC Study Group. Epidemiology of community-acquired pneumonia in older adults: a population-based study. Respir Med 2009; 103:309 - 16; http://dx.doi.org/10.1016/j.rmed.2008.08.006; PMID: 18804355
- Vila-Corcoles A, Salsench E, Rodriguez-Blanco T, Ochoa-Gondar O, de Diego C, Valdivieso A, et al. Clinical effectiveness of 23-valent pneumococcal polysaccharide vaccine against pneumonia in middle-aged and older adults: a matched case-control study. Vaccine 2009; 27:1504 - 10; http://dx.doi.org/10.1016/j.vaccine.2009.01.013; PMID: 19171174
- Bohr V, Rasmussen N, Hansen B, Gade A, Kjersem H, Johnsen N, et al. Pneumococcal meningitis: an evaluation of prognostic factors in 164 cases based on mortality and on a study of lasting sequelae. J Infect 1985; 10:143 - 57; http://dx.doi.org/10.1016/S0163-4453(85)91585-3; PMID: 4008963
- Mooney JD, Weir A, McMenamin J, Ritchie LD, Macfarlane TV, Simpson CR, et al. The impact and effectiveness of pneumococcal vaccination in Scotland for those aged 65 and over during winter 2003/2004. BMC Infect Dis 2008; 8:53; http://dx.doi.org/10.1186/1471-2334-8-53; PMID: 18433473
- Butler JC, Breiman RF, Campbell JF, Lipman HB, Broome CV, Facklam RR. Pneumococcal polysaccharide vaccine efficacy. An evaluation of current recommendations. JAMA 1993; 270:1826 - 31; http://dx.doi.org/10.1001/jama.1993.03510150060030; PMID: 8411526
- Huss A, Scott P, Stuck AE, Trotter C, Egger M. Efficacy of pneumococcal vaccination in adults: a meta-analysis. CMAJ 2009; 180:48 - 58; http://dx.doi.org/10.1503/cmaj.080734; PMID: 19124790
- Moberley SA, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev 2008; CD000422; PMID: 18253977
- Middleton DB, Lin CJ, Smith KJ, Zimmerman RK, Nowalk MP, Roberts MS, et al. Economic evaluation of standing order programs for pneumococcal vaccination of hospitalized elderly patients. Infect Control Hosp Epidemiol 2008; 29:385 - 94; http://dx.doi.org/10.1086/587155; PMID: 18521990
- Shapiro ED, Berg AT, Austrian R, Schroeder D, Parcells V, Margolis A, et al. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Engl J Med 1991; 325:1453 - 60; http://dx.doi.org/10.1056/NEJM199111213252101; PMID: 1944423
- Demographic Yearbook of Poland 2008. 2008. Polish Central Statistic Office.
- Szende A, Németh R. [Health-related quality of life of the Hungarian population]. Orv Hetil 2003; 144:1667 - 74; PMID: 14528840
- Dominguez A, Salleras L, Fedson DS, Izquierdo C, Ruiz L, Ciruela P, et al. Effectiveness of pneumococcal vaccination for elderly people in Catalonia, Spain: a case-control study. Clin Infect Dis 2005; 40:1250 - 7; http://dx.doi.org/10.1086/429236; PMID: 15825026
- Vila-Córcoles A, Ochoa-Gondar O, Hospital I, Ansa X, Vilanova A, Rodríguez T, et al, EVAN Study Group. Protective effects of the 23-valent pneumococcal polysaccharide vaccine in the elderly population: the EVAN-65 study. Clin Infect Dis 2006; 43:860 - 8; http://dx.doi.org/10.1086/507340; PMID: 16941367
- De Graeve D, Lombaert G, Goossens H. Cost-effectiveness analysis of pneumococcal vaccination of adults and elderly persons in Belgium. Pharmacoeconomics 2000; 17:591 - 601; http://dx.doi.org/10.2165/00019053-200017060-00005; PMID: 10977396
- Sisk JE, Moskowitz AJ, Whang W, Lin JD, Fedson DS, McBean AM, et al. Cost-effectiveness of vaccination against pneumococcal bacteremia among elderly people. JAMA 1997; 278:1333 - 9; http://dx.doi.org/10.1001/jama.1997.03550160053038; PMID: 9343464
- Robinson KA, Baughman W, Rothrock G, Barrett NL, Pass M, Lexau C, et al, Active Bacterial Core Surveillance (ABCs)/Emerging Infections Program Network. Epidemiology of invasive Streptococcus pneumoniae infections in the United States, 1995-1998: Opportunities for prevention in the conjugate vaccine era. JAMA 2001; 285:1729 - 35; http://dx.doi.org/10.1001/jama.285.13.1729; PMID: 11277827
- Jacobs MR, Felmingham D, Appelbaum PC, Grüneberg RN, Alexander Project Group. The Alexander Project 1998-2000: susceptibility of pathogens isolated from community-acquired respiratory tract infection to commonly used antimicrobial agents. J Antimicrob Chemother 2003; 52:229 - 46; http://dx.doi.org/10.1093/jac/dkg321; PMID: 12865398
- Lloyd A, Patel N, Scott DA, Runge C, Claes C, Rose M. Cost-effectiveness of heptavalent conjugate pneumococcal vaccine (Prevenar) in Germany: considering a high-risk population and herd immunity effects. Eur J Health Econ 2008; 9:7 - 15; http://dx.doi.org/10.1007/s10198-006-0013-6; PMID: 17333089
- Jackson LA, Neuzil KM, Yu O, Benson P, Barlow WE, Adams AL, et al, Vaccine Safety Datalink. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N Engl J Med 2003; 348:1747 - 55; http://dx.doi.org/10.1056/NEJMoa022678; PMID: 12724480
- Smith KJ, Roberts MS. Cost-effectiveness of newer treatment strategies for influenza. Am J Med 2002; 113:300 - 7; http://dx.doi.org/10.1016/S0002-9343(02)01222-6; PMID: 12361816
- Smith KJ, Zimmerman RK, Lin CJ, Nowalk MP, Ko FS, McEllistrem MC, et al. Alternative strategies for adult pneumococcal polysaccharide vaccination: a cost-effectiveness analysis. Vaccine 2008; 26:1420 - 31; http://dx.doi.org/10.1016/j.vaccine.2008.01.007; PMID: 18272262
- De Wals P, Petit G, Erickson LJ, Guay M, Tam T, Law B, et al. Benefits and costs of immunization of children with pneumococcal conjugate vaccine in Canada. Vaccine 2003; 21:3757 - 64; http://dx.doi.org/10.1016/S0264-410X(03)00361-X; PMID: 12922109