Abstract
In Germany, routine RV-vaccination is not adopted into the national immunization schedule as of 2012. Because RV-vaccines were already on the market since 2006, in 2010 a moderate (58%) and low (22%) vaccine uptake was observed in the 5 eastern federal states (EFS) and the 11 western federal states (WFS), respectively. To assess the impact of RV-vaccination, we compared the incidence rates (IR) of RV-related hospitalizations before (2004‒2006) and in seasons after (2008/09–2010/11) RV-vaccine introduction in Germany by utilizing data from the national mandatory disease reporting system. In the EFS, the IR was significantly reduced in age-groups < 18 mo in 2008/09 and in age-groups < 24 mo in 2009/10–2010/11. In the WFS an IR-reduction was observed only in age-groups < 12 mo in 2008/09 and in age-groups < 18 mo in 2009/10–2010/11. Overall IR-reduction in age-groups < 24 mo comparing 2008–11 with 2004–06 was 36% and 25% in EFS and WFS, respectively. In addition, we computed IR-ratios (IRR) in the seasons after mid-2006 with negative binomial regression. The effect of vaccination was independent from the geographic region. Vaccination was associated with a significant reduction in RV-related hospitalizations in the age-groups 6–23 mo. Most prominently, vaccination of 50% of infants led to an estimated decrease in age group 6–11 mo by 42%. No significant reduction was observed in age-groups ≥ 24 mo. In conclusion, in the German setting with low to moderate vaccine uptake, RV-related hospitalization incidence decreased substantially depending on the achieved vaccination coverage, but only in the first two years of life.
Introduction
Rotavirus (RV) infection is the most common cause of gastroenteritis in children worldwide. It has been estimated that by the age of five years, nearly every child has experienced at least one episode of RV-infection.Citation1,Citation2 In Germany, where laboratory-confirmed RV disease is notifiable since 2001, RV was the third most frequently reported pathogen between 2005 and 2010 and the leading cause of diarrhea in children < 5 y of age between 2001 and 2010.Citation3,Citation4 In this latter age-group, RV was responsible on average for 70% of all reported acute gastroenteritis leading to hospitalization that were caused by one of the notable gastrointestinal pathogens in Germany.Citation3 Between 2001 and 2010, the total number of annually reported RV-cases ranged between 37,829 and 77,532, corresponding to an incidence of 46 and 95 cases per 100,000 population.Citation4 In 2010, approximately 60% of the reported RV-cases occurred among children < 5 y of age. Of these, 57% were hospitalized.Citation4 The total cost (including direct and indirect costs) per one RV gastroenteritis hospitalization in Germany was 2,085 Euros in 2005.Citation5
The two RV vaccines, Rotarix® and RotaTeq®, have been available on the German market since June 2006. In large clinical trials, both vaccines have demonstrated a good safety profile and a high efficacy (83 to 96%) in preventing RV-related hospitalization.Citation6-Citation11 Rotarix® is approved for the vaccination of infants until the age of 24 weeks and RotaTeq® until the age of 32 weeks. Since in Germany almost no RV-related deaths occur, the adoption of RV-vaccination into the national childhood immunization schedule was still under consideration by the German Standing Committee on Vaccination (STIKO) as of May 2012. While all vaccinations recommended by the STIKO are free of charge in Germany, a health insurance company can individually decide if they reimburse costs related to a specific vaccination not recommended by STIKO.
If a vaccine is available on the German market but a STIKO-recommendation is pending, a federal state can include this vaccine in its state-level guidance in addition to the national immunization schedule endorsed by STIKO. Starting in 2008, five of the 16 federal states have included RV vaccination already into their local guidelines. These states were: Saxony (January 2008), Brandenburg (January 2009), Mecklenburg-Western Pomerania (July 2009), Thuringia (October 2009) and Schleswig-Holstein (March 2011). All of these states except Schleswig-Holstein belong to the eastern federal states (EFS). Although it is more than 20 y since the two parts of Germany were reunified, there are still differences in the attitudes toward vaccination, and for most vaccines the uptake is significantly higher in the EFS compared with the 11 western federal states (WFS).Citation12-Citation14 For example, two-dose measles vaccination coverage in children at school entry was 92% in the EFS and 89% in the WFS in 2008, and seasonal influenza vaccination coverage in the total population was 59% in the EFS and 40% in the WFS in 2008/09.Citation12,Citation13 Vaccine sales data showed that there is also a clear difference in the RV vaccination coverage with an average 58% in the EFS and 22% in the WFS in 2010.Citation15
To estimate the impact of RV vaccination on the incidence of RV-related hospitalizations on a population level, we analyzed surveillance data on RV-infections derived from the mandatory disease reporting system before and after RV vaccine introduction in German federal states. The unique situation with different levels of vaccine uptake by federal state allowed us in particular to compare regions with different vaccination coverage and to quantify the impact of RV-vaccination as a function of the vaccine uptake.
Results
RV-related hospitalization by season, age-group and region
In the study period 2001–2011, the total number of reported laboratory-confirmed RV-infections leading to hospitalization was 234,310. Of these, 124,182 (53%) were among children < 2 y of age.
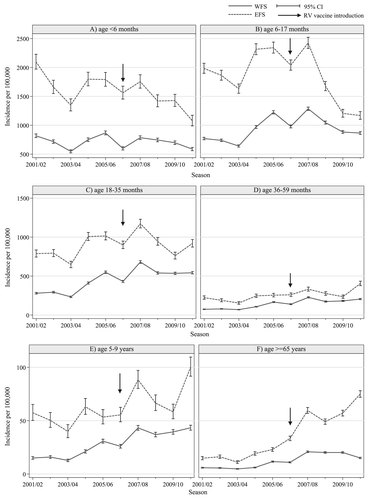
The annual number of reported hospitalized RV-cases ranged between 4,192 (2003/04) and 8,679 (2007/08) in the EFS, and between 10,186 (2003/04) and 24,076 (2007/08) in the WFS, corresponding to an annual incidence of 31 and 66 per 100,000 population in the EFS and of 15 and 35 per 100,000 population in the WFS, respectively. In both regions the incidence of RV-related hospitalizations increased after a change in the reimbursement system (season 2004/05). This increase was most dominant in the age-groups with the highest incidences (). Starting from season 2006/07, a decrease in RV hospitalization incidence was observed, which is in close temporal proximity to the introduction of RV-vaccination in June 2006. shows the annual incidence in different age-groups by region. In both regions a decrease in incidence was most prominently seen in age-group 6–17 mo, with a steeper decline in the EFS than in the WFS. A decline in incidence occurred also in age-group < 6 mo and 18–35 mo.
Assessment of RV vaccination coverage
A total of 4,402 households were contacted of which 3,711 returned a completed questionnaire (response rate 84.3%). As several households comprised more than one child under the age of 5 y (year of birth 2006 to 2010), data on 4,565 children was obtained. At the time of completing the questionnaire 403 children had been younger than 26 weeks and were therefore excluded from further analysis, leaving a total of 4,162 eligible subjects, of whom 2,156 were male (51.8%) and 2006 female (48.2%). In both regions of Germany, RV vaccination coverage continuously increased from 2006 (year of market authorization) to 2010. Nevertheless, there was a remarkable difference in vaccination coverage between EFS and WFS with consistently higher RV vaccine uptake in the EFS (). Vaccination coverage in 2010 was 56% [95% confidence interval (CI) 45–68%] in the EFS and 28% (95% CI 24–32%) in the WFS. In both regions of Germany, the completeness of immunization series increased and reached 91% (95% CI 82–100%) and 96% (95% CI 93–100%) in 2010 in the EFS and WFS, respectively. According to the current recommendations of the manufactures (vaccinations until age of 24 weeks for Rotarix® and 32 weeks for RotaTeq®), 87% of the immunization series were timely completed. Proportion of children vaccinated with Rotarix®, RotaTeq® or both vaccines did not significantly differ between regions and was 51.8%, 47.0%, 1.2% in EFS and 57.2%, 42.5%, 0.3% in WFS, respectively.
Table 1. Rotavirus vaccination coverage (in %) with 95% confidence intervals by geographic region in Germany (EFS and WFS) and year of birth
RV-related hospitalization in the pre- and post-vaccine introduction period (analysis A)
Compared with the mean incidence of RV-related hospitalizations in 2004/05–2005/06, in the EFS a significant reduction was observed among age-groups < 18 mo in 2008/09 and among age-groups < 24 mo in 2009/10–2010/11 (). In contrast, in all age-groups ≥ 36 mo there was a significant increase in the RV-related hospitalization incidences in 2010/2011. In the WFS a significant reduction was observed among age-groups < 12 mo in 2008/09, and among age-groups < 18 mo in 2009/10–2010/11. In contrast, for all other age-groups in 2009/10 and age-groups ≥ 24 mo in 2008/09 and 2010/11 a significant increase in the RV-related hospitalization incidence was observed when compared with the pre-vaccine introduction period (). The overall reduction in the annually reported RV-related hospitalization incidence in age-groups < 24 mo was 36% in the EFS and 25% in the WFS when comparing 2008–11 with 2004–06.
Table 2. Number and incidence (per 100,000 population) of reported RV-related hospitalizations by age-group in the pre-vaccination period (2004–2006) and changes (in %) in the post-vaccine introduction seasons when compared with the pre-vaccination period in the (A) EFS and (B) WFS of Germany
When using 2001/02–2005/06 as reference for the pre-vaccination period, the decrease in RV-related hospitalization incidences was still significant but with approximately 10–15 percentage points less pronounced (data not shown).
Nosocomial RV-infections in the pre- and post-vaccine introduction period
Over the study period for 27% (63,466/234,310) hospitalized cases information on the date of diseases onset or the date of hospital admission was missing. Among cases with available information on these dates, a total of 11% (18,053/170,844) RV-cases fulfilled the case-definition for nosocomial infections. The mean annual incidence of nosocomial RV-infections by age-group in the pre- and post-vaccine introduction period after adding the proportion attributable to nosocomial cases from the records with missing dates is displayed in . There was a decrease in incidence of nosocomial infections in most age-groups below 9 y, which was, however, significant only in the age-group 6–11 mo in both regions and in age-group < 6 mo in the WFS ().
Table 3. Mean annual incidence of nosocomial RV-infections (per 100,000 population) in the pre- and post-vaccine introduction period by age-group, Germany, 2004–06 compared with 2008–11
Vaccination impact estimated in negative binomial regression (analysis B)
The final model obtained from the model selection procedure contained region (EFS/WFS), age-group and the negative logarithm with base 2 of the proportion of unvaccinated children as main effects together with an interaction between the age-group and this logarithm. There was no seasonal trend, as well as no interaction between region and the logarithm of the proportion of unvaccinated children. Thus, there was no significant difference between the estimated impact of the vaccination for the EFS and WFS when the vaccination coverage is similar. The incidence rate ratio (IRR) for being hospitalized due to RV-infection by age-group each time 50% of the unvaccinated eligible population was vaccinated is shown in . In particular, the vaccination (which is possible only up to the age of 24 or 32 weeks according to the licensure) was significantly associated with a reduction in the incidence of RV-related hospitalizations in the age-groups 6–11 mo, 12–17 mo and 18–23 mo. The reduction was largest in the age-group 6–11 mo leading to an incidence reduction of 42% for each 50% of the unvaccinated population being vaccinated. There was a less pronounced reduction of the incidence in the age-groups < 6 mo and 24–29 mo, albeit not statistically significant. In the age-group 30–35 mo there was no incidence reduction. Note that for example, vaccination coverage of 75% would mean that the unvaccinated eligible population was halved twice and therefore the estimated IRR had to be applied two times. Furthermore, if the number of eligible unvaccinated children is halved only one half times, i.e., for coverage 29% (0.29≈1–0.50.5), the effect equals the estimated IRR to the power one half.
Table 4. Estimated incidence rate ratio (IRR) by age group when vaccinating 50% of the eligible unvaccinated population
Discussion
In Germany, RV vaccines have been available on the market since mid-2006. Our analyses, which compared the incidence of RV-related hospitalizations in seasons before and after RV-vaccines became available, demonstrated consistently the impact of RV-vaccination on RV-associated hospitalizations in age-groups < 24 mo on a population level both in regions with low and moderate vaccine uptake. The magnitude in incidence reduction was larger in EFS, which can be explained by a higher and earlier vaccine uptake since 2007 when compared with the WFS. These findings are further supported by the results of the regression analysis. Here, the decrease in incidence was significantly associated with the vaccination coverage in the eligible age-group independent from the geographic region. In contrast to randomized controlled trials, where the effectiveness of a specific vaccine is usually measured on an individual level, studies such as ours measure the impact of vaccination on the population level, which includes also individuals who were unvaccinated and who might even belong to an age-group not eligible or targeted for vaccination.
In both, the before-and-after analysis and the regression analysis, we were not able to detect a significant decrease in incidence in age-groups ≥ 24 mo. It remains unclear if this is caused by lower vaccination coverage rates early after introduction of RV-vaccines (and the fact that it needs some time for the accumulation of several vaccinated birth cohorts to establish herd protection effects) or also due to waning immunity in children vaccinated more than 2 y ago. In other settings with routine childhood RV-vaccination but also considerably higher vaccination coverage than in Germany the occurrence of herd protection effects was described,Citation16 but there are also indications for waning immunity 2–3 y after vaccination.Citation8,Citation17
A dramatic decrease in RV disease burden on the population level after introduction of routine childhood RV-vaccination was observed in the United States (US), Australia and several European countries.Citation18-Citation29 In the US for example, the proportion of RV-positive tests declined by 64% and 60% in seasons 2007/2008 and 2008/2009, respectively, when compared with the pre-vaccine introduction period (2000–2006).Citation20 Another study from the US reported the decrease in RV-related hospitalizations of 83% in 2007/2008 and 66% in 2008/2009 among children < 5 y of age in comparison to the 2003–2006 median.Citation24 In the US representative data regarding RV-vaccination coverage on a country-level are not available, but coverage with ≥ 1 dose for children aged < 24 mo was estimated at 31% by using data from 6 sentinel sites in 2007.Citation18 In Austria, where estimated vaccination coverage was 87% in 2008 and 74% in 2009, the hospital admission rates in post-vaccine introduction seasons (2008 and 2009) were reduced by nearly 75% in children eligible for the RV vaccination, while for older not vaccinated children a first slight increase (8%) was observed, that was followed by a decrease in subsequent season.Citation16,Citation23 Furthermore, RV reporting data modeling performed in France, where vaccination coverage was 47%, revealed a reduction by a factor of 2.04 in the number of hospitalizations among children < 2 y of age during the season 2008/2009 compared with the expected magnitude, while –similar to the observations in our study– hospitalizations among children 2–5 y of age tended to be static or even increasing.Citation30
In our data set we were able to define nosocomial RV-infections and demonstrate that the introduction of RV-vaccination of young children also had an impact on the incidence of nosocomial infections. A recently published meta-analysis demonstrated that nosocomially-acquired RV-infection is a significant problem among hospitalized infants during the winter months.Citation31 An impact of routine RV-vaccination on the incidence of nosocomial RV-infections was also shown in Austria, the US and Australia.Citation23,Citation25,Citation26
Our analysis revealed that IR before and after vaccine introduction was higher in EFS than in WFS and this difference remained stable over the study period. This phenomenon is well-known in the German infectious disease surveillance and can be observed in the majority of notifiable gastrointestinal diseases.Citation4 It has been explained by differences in the health seeking behavior in population and the testing practices of individual physicians.Citation32 It must be highlighted that we used passively-collected surveillance data for the identification of both RV-associated hospitalizations and nosocomial RV-infections and therefore our incidence estimates do not reflect the true disease incidence. However, we believe that under detection and underreporting of hospitalized RV cases, which were considered in our study, are considerably lower than for RV-cases treated as outpatients. And most importantly, if we assume that the degree of under detection or underreporting of cases remained stable over the study period, this case under-ascertainment and the different magnitude in case under-ascertainment between EFS and WFS will not influence our results since we only considered trends or the proportion in disease reduction.
There are a few limitations in our study that need to be considered. First, there is no immunization register in Germany and routine vaccination coverage data on a country-level are only collected at school entry in children aged 5–6 y. Therefore, precise data on RV vaccine uptake in children eligible for vaccination were not available. However, we conducted a large survey by utilizing two representative panels of households with young children. A high participation rate guaranteed that the risk for a selection bias is low. Second, we cannot be sure that the observed higher incidence since 2004 is completely attributable to the change in the reimbursement system. Hence, our estimate could be an overestimation of the impact of the vaccination. Therefore, we performed a sensitivity analysis by expanding the reference period to the 5 seasons (i.e., 2001/02–2005/06). As a result, a similar but less pronounced decrease was observed in the youngest age-groups. In both scenarios no incidence reductions were observed in older age-groups not vaccinated. Finally, we can still not fully exclude that the decrease in the last seasons were partly attributable to changes in circulating genotypes, seasonal factors, or changes in stool sampling practices. However, since the decreasing effect was shown in various analyses, since the effect was only present in age-groups that were vaccinated in the past 2 y (i.e., < 24 mo of age) and an opposite trend in incidence was observed in older age-groups, and since a clear dose-response-effect on a population level with a stronger IR-decline in regions with higher vaccination coverage was observed, it is very likely that the observed effect on the RV-related hospitalization incidence can be attributed to the use of RV vaccines in the population.
Major strength of our study was that we were able to use a very large data set with robust RV surveillance data covering a 10-y time period with the same RV case definition. The size of the population in Germany leads to a large number of annually reported RV cases in the data set, and different immunization policies provided the unique situation that the vaccination impact in 5 federal states with a moderate RV vaccination coverage could be compared with the impact of RV vaccination in 11 federal states with low vaccination coverage. With the availability of robust vaccination coverage data we were able to quantify the impact of RV-vaccination on a population level as a function of the achieved vaccine uptake.
We conclude that after the partially introduction of RV vaccines in Germany the absolute incidence rates in the post-vaccine introduction seasons decreased in EFS (among children younger than 2 y of age) and to a lesser magnitude in WFS (among children younger than 1 y of age). The decrease was associated with the level of the vaccination coverage found in the federal states. Furthermore, the decrease in the overall RV-related hospitalization incidence resulted in a significant decrease in nosocomial RV-infections in the age-group eligible for vaccination. We were not able to show an impact on age-groups ≥ 24 mo on the population level. However, we cannot rule out that indirect effects will occur with higher vaccination coverage in the following years. Therefore, a follow-up analysis using the same methodologies should be applied in two or three years to provide further evidence.
Methods
Study population and setting
The study population consisted of the total population in Germany. Demographic data on the general population, which were used as denominator to calculate incidences, were obtained from the German Federal Statistical Office.Citation33 Since for 2010 and 2011 the population data were not yet available; population data from 2009 were used instead.
RV cases were identified through the national disease reporting system, which contains mandatory notifications of RV-infections. In Germany, laboratory-confirmed symptomatic RV-infections are notifiable since 2001. The reporting system is regulated by the German Protection against Infection Act,Citation34 and reference case definitions are used to ensure comparability of surveillance data across federal states and over time. This law requests that all laboratories in Germany report a positive test result indicative for a RV-infection to the local public health office. Office staff contacts the patient to collect all relevant data to be entered in the electronic reporting system for communicable diseases, and the case-based standardized data set is transmitted via the state health authorities to the Robert Koch Institute, which is the national public health authority in Germany. For the analysis we used notifications of patients that met reference case definition, were hospitalized and for whom information on sex, date of birth, notifying federal state, date of disease onset and hospitalization status were available. Reference RV case definition is met when both clinical and laboratory criteria of the case definition are fulfilled. In order to meet the clinical criteria, at least one of the two clinical manifestations must be present: diarrhea (defined as three and more loose stools in 24 h) or vomiting. A RV-infection was laboratory-confirmed when in stool samples RV-antigen was detected (for example by ELISA, including rapid diagnostic tests), by detection of RV nucleic acid (by polymerase chain reaction methods) or by electron microscopy.
Between 2001 and 2011, the notification algorithm as well as the case definition did not change. However, in 2004 the German reimbursement system for hospitalization charges was reformed. Since then, hospitalized patients were classified based on diagnosis-related groups which serve as a basis for payment of services provided by the hospitals. As hospitals receive higher payments for each case of acute gastroenteritis with a confirmed causative agent than for microbiologically unconfirmed enteral infection it can be suspected that a higher number of stool samples might have been tested in German hospitals since 2004.
Study design and study periods
We utilized data from the mandatory disease reporting system in Germany with date of onset from week 35 in 2001 to week 34 in 2011. Previously conducted data analyses showed that in Germany RV-infections are notified over the entire year with a clear seasonal trend and a dominant late winter and early spring peak from January to May.Citation3 Therefore we defined time periods for annual RV seasons lasting from week 35 (beginning of September) in one year to week 34 (end of August) in the following year.
Pre- and post-vaccine introduction analysis (analysis A)
We conducted a “before-and-after analysis” that assumed that RV-related outcomes and reporting numbers would not change over time in the absence of vaccination. Due to the change in the reimbursement system in 2004, we defined the pre-vaccination period lasting from week 35 in 2004 to week 34 in 2006 (i.e., 2 seasons). To assess the influence of this change in reimbursement on our calculations, we conducted a sensitivity-analysis by including also the seasons 2001–2004 in the reference period (total 5 seasons). The post-vaccine introduction period was defined lasting from week 35 in 2008 to week 34 in 2011 (3 seasons). The period from week 35 in 2006 to week 34 in 2008 (2 seasons), when the introduction of RV vaccines in Germany started but vaccination coverage was below levels that are expected to have a substantial impact on the disease incidence, was defined as a transitional period.
To detect the impact on the incidence of the nosocomial cases we defined RV-case as a nosocomial case if onset of gastrointestinal symptom was at least 72 h (3 d) after being admitted to hospital and if there were no signs or symptoms of gastroenteritis at time of admission. To control for differences in proportion of records with available information on date of hospital admission between seasons, we presumed that the proportion of nosocomial cases among hospitalized cases with missing dates is the same as among hospitalized cases with information on respective dates. We extrapolated the final count of nosocomial cases by adding up nosocomial cases from hospitalized cases with required information on dates and proportion attributable to nosocomial cases from the records with missing dates.
Regression-analysis (analysis B)
As a second methodological approach to estimate the impact of RV vaccination on the population level, we quantified the relative reduction in RV-related hospitalization incidences in the seasons after vaccine introduction by using negative binomial regression models. For this analysis, we included seasonal incidence data between 2006/07 and 2010/11.
Sources of vaccination coverage data
A retrospective survey was performed in November and December 2010 in two representative panels of households with children aged 0–4 y. Parents were asked to fill in a self-administered questionnaire including questions on RV vaccination status (yes/no), dose number, vaccination date, trade name and batch number. Children under the age of 26 weeks were excluded from analysis, since the chance was given that these would still receive RV vaccination or had not yet completed the vaccination series at the time when completing the questionnaire. The vaccination coverage was calculated stratified by area (WFS and EFS) and year of birth (2006 to 2010). A weighted analysis was performed to account for disproportionate sampling with respect to federal state, age and gender. For both analyses point estimates and 95% CI were calculated. In addition, for all strata completeness of vaccination series (two doses of Rotarix® or three doses of RotaTeq®) was calculated.
Statistical analysis
We stratified the study population by age-groups: 6 mo age-groups were used for children < 5 y (< 6, 6–11, 12–17, 18–23, 24–29, 30–35, 36–41, 42–47, 48–53 and 54–59 mo of age), then 5–9 y, 10–64 y and ≥ 65 y of age. The analysis was stratified into two regions of Germany (EFS and WFS) because of the remarkable difference in RV vaccine uptake.
Pre- and post- vaccine introduction analysis (analysis A)
To compare the RV-related hospitalization incidences in the pre- and post-vaccine introduction period, we calculated IR together with 95% CI by season and age-group (cases/100,000 population). First, a comparison of RV hospitalization IR in the pre- and post-vaccine introduction periods was performed by age-group for each of the two regions (EFS/WFS). The impact was computed as the reduction in IR per seasons in the post-vaccine introduction period compared with the mean estimates from the pre-vaccine introduction period.
To compare the incidences of nosocomial cases we calculated and compared mean annual IR in the post-vaccine introduction period to the mean annual incidence in the pre-vaccination period.
Regression-analysis (analysis B)
To quantify the relative IR-reduction in RV-related hospitalizations in the post-vaccine introduction seasons, we performed negative binomial regression analyses on the annual IR data. We assumed that the changes in the reimbursement system in 2004 had leveled off in 2006 and therefore, we chose season 2006/07 as starting point for the analysis. Moreover, we restricted analysis to the age-groups that have had a chance to be vaccinated (< 6, 6–11, 12–17, 18–23, 24–30 and 31–36 mo of age).
We tested effects of secular trend, vaccination coverage, age-group, as well as region, together with interactions between these variables, on IR of RV-related hospitalizations. Vaccination coverage from the survey was applied for age-group 6–11 mo of age and then shifted according to aging, since only children younger than 6 mo were eligible for RV-vaccination in accordance to the vaccines’ licensure. For children younger than six months half of the estimated vaccination coverage for the age-group 6–11 mo was applied. We quantified the impact of the coverage by estimating the change in the expected hospitalization incidence rate each time 50% of the unvaccinated eligible children were vaccinated. To do this we computed the transformed coverage as the negative logarithm to base 2 of the proportion of vaccinated children in each age-group. Hence, the transformed coverage is equal to the number of times the number of unvaccinated eligible children was halved. This means that the estimated IRR should be applied as many times as the unvaccinated eligible population has been halved, e.g., if the unvaccinated eligible population has been halved twice (vaccine coverage 75%) the estimated IRR should be set in power of two. Note that coverage of less than 50% would lead to a transformed coverage less than one.
Model selection was performed using a manual stepwise forward selection procedure based on p-values from two-sided likelihood ratio tests. Significance level was set at p-value < 0.05.
| Abbreviations: | ||
| CI | = | confidence interval |
| EFS | = | eastern federal states |
| IR | = | incidence rates |
| IRR | = | incidence rate ratios |
| RV | = | rotavirus |
| STIKO | = | German Standing Committee on Vaccination |
| US | = | United States |
| WFS | = | western federal states |
Acknowledgments
We would like to thank Marta Valenciano, Alain Moren and Camelia Savulescu from EpiConcept for reviewing the study protocol and the final report of this study. The study was funded by a grant from the European Center for Disease Prevention and Control (ECDC) under the framework contract n# ECDC/09/041.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Soriano-Gabarró M, Mrukowicz J, Vesikari T, Verstraeten T. Burden of rotavirus disease in European Union countries. Pediatr Infect Dis J 2006; 25:Suppl S7 - 11; http://dx.doi.org/10.1097/01.inf.0000197622.98559.01; PMID: 16397431
- Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis 2003; 9:565 - 72; http://dx.doi.org/10.3201/eid0905.020562; PMID: 12737740
- Koch J, Wiese-Posselt M. Epidemiology of rotavirus infections in children less than 5 years of age: Germany, 2001-2008. Pediatr Infect Dis J 2011; 30:112 - 7; http://dx.doi.org/10.1097/INF.0b013e3181f1eb21; PMID: 20724954
- Robert Koch -Institut. Infektionsepidemiologisches Jahrbuch für 2010, Berlin, 2011.
- Giaquinto C, Van Damme P, Huet F, Gothefors L, Van der Wielen M, Group RS, REVEAL Study Group. Costs of community-acquired pediatric rotavirus gastroenteritis in 7 European countries: the REVEAL Study. J Infect Dis 2007; 195:Suppl 1 S36 - 44; http://dx.doi.org/10.1086/516716; PMID: 17539193
- Vesikari T, Karvonen A, Prymula R, Schuster V, Tejedor JC, Cohen R, et al. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet 2007; 370:1757 - 63; http://dx.doi.org/10.1016/S0140-6736(07)61744-9; PMID: 18037080
- Vesikari T, Itzler R, Matson DO, Santosham M, Christie CD, Coia M, et al. Efficacy of a pentavalent rotavirus vaccine in reducing rotavirus-associated health care utilization across three regions (11 countries). Int J Infect Dis 2007; 11:Suppl 2 S29 - 35; http://dx.doi.org/10.1016/S1201-9712(07)60019-8; PMID: 18162243
- Linhares AC, Velázquez FR, Pérez-Schael I, Sáez-Llorens X, Abate H, Espinoza F, et al, Human Rotavirus Vaccine Study Group. Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, double-blind, placebo-controlled phase III study. Lancet 2008; 371:1181 - 9; http://dx.doi.org/10.1016/S0140-6736(08)60524-3; PMID: 18395579
- Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, Abate H, Breuer T, Clemens SC, et al, Human Rotavirus Vaccine Study Group. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 2006; 354:11 - 22; http://dx.doi.org/10.1056/NEJMoa052434; PMID: 16394298
- Kawamura N, Tokoeda Y, Oshima M, Okahata H, Tsutsumi H, Van Doorn LJ, et al. Efficacy, safety and immunogenicity of RIX4414 in Japanese infants during the first two years of life. Vaccine 2011; 29:6335 - 41; http://dx.doi.org/10.1016/j.vaccine.2011.05.017; PMID: 21640780
- Phua KB, Lim FS, Lau YL, Nelson EA, Huang LM, Quak SH, et al. Safety and efficacy of human rotavirus vaccine during the first 2 years of life in Asian infants: randomised, double-blind, controlled study. Vaccine 2009; 27:5936 - 41; http://dx.doi.org/10.1016/j.vaccine.2009.07.098; PMID: 19679216
- Böhmer MM, Walter D, Krause G, Müters S, Gösswald A, Wichmann O. Determinants of tetanus and seasonal influenza vaccine uptake in adults living in Germany. Hum Vaccin 2011; 7:1317 - 25; http://dx.doi.org/10.4161/hv.7.12.18130; PMID: 22108034
- Siedler A, Mankertz A, Feil F, Ahlemeyer G, Hornig A, Kirchner M, et al. Closer to the goal: efforts in measles elimination in Germany 2010. J Infect Dis 2011; 204:Suppl 1 S373 - 80; http://dx.doi.org/10.1093/infdis/jir068; PMID: 21666187
- Böhmer MM, Walter D, Müters S, Krause G, Wichmann O. Seasonal influenza vaccine uptake in Germany 2007/2008 and 2008/2009: results from a national health update survey. Vaccine 2011; 29:4492 - 8; http://dx.doi.org/10.1016/j.vaccine.2011.04.039; PMID: 21545822
- IMS Pharmascope®. Online available: http://www.imshealth.de
- Paulke-Korinek M, Kundi M, Rendi-Wagner P, de Martin A, Eder G, Schmidle-Loss B, et al. Herd immunity after two years of the universal mass vaccination program against rotavirus gastroenteritis in Austria. Vaccine 2011; 29:2791 - 6; http://dx.doi.org/10.1016/j.vaccine.2011.01.104; PMID: 21320539
- Itzler R, Koch G, Matson DO, Gothefors L, Van Damme P, Dinubile MJ, et al. Robustness of the healthcare utilization results from the Rotavirus Efficacy and Safety Trial (REST) evaluating the human-bovine (WC3) reassortant pentavalent rotavirus vaccine (RV5). BMC Pediatr 2010; 10:42; http://dx.doi.org/10.1186/1471-2431-10-42; PMID: 20540778
- Tate JE, Panozzo CA, Payne DC, Patel MM, Cortese MM, Fowlkes AL, et al. Decline and change in seasonality of US rotavirus activity after the introduction of rotavirus vaccine. Pediatrics 2009; 124:465 - 71; http://dx.doi.org/10.1542/peds.2008-3528; PMID: 19581260
- Centers for Disease Control and Prevention (CDC). Delayed onset and diminished magnitude of rotavirus activity--United States, November 2007-May 2008. MMWR Morb Mortal Wkly Rep 2008; 57:697 - 700; PMID: 18583958
- Centers for Disease Control and Prevention (CDC). Reduction in rotavirus after vaccine introduction--United States, 2000-2009. MMWR Morb Mortal Wkly Rep 2009; 58:1146 - 9; PMID: 19847149
- Payne DC, Staat MA, Edwards KM, Szilagyi PG, Weinberg GA, Hall CB, et al, New Vaccine Surveillance Network (NVSN). Direct and indirect effects of rotavirus vaccination upon childhood hospitalizations in 3 US Counties, 2006-2009. Clin Infect Dis 2011; 53:245 - 53; http://dx.doi.org/10.1093/cid/cir307; PMID: 21705316
- Clark HF, Lawley D, Mallette LA, DiNubile MJ, Hodinka RL. Decline in cases of rotavirus gastroenteritis presenting to The Children’s Hospital of Philadelphia after introduction of a pentavalent rotavirus vaccine. Clin Vaccine Immunol 2009; 16:382 - 6; http://dx.doi.org/10.1128/CVI.00382-08; PMID: 19158283
- Paulke-Korinek M, Rendi-Wagner P, Kundi M, Kronik R, Kollaritsch H. Universal mass vaccination against rotavirus gastroenteritis: impact on hospitalization rates in austrian children. Pediatr Infect Dis J 2010; 29:319 - 23; PMID: 19935446
- Yen C, Tate JE, Wenk JD, Harris JM 2nd, Parashar UD. Diarrhea-associated hospitalizations among US children over 2 rotavirus seasons after vaccine introduction. Pediatrics 2011; 127:e9 - 15; http://dx.doi.org/10.1542/peds.2010-1393; PMID: 21172995
- Anderson EJ, Rupp A, Shulman ST, Wang D, Zheng X, Noskin GA. Impact of rotavirus vaccination on hospital-acquired rotavirus gastroenteritis in children. Pediatrics 2011; 127:e264 - 70; http://dx.doi.org/10.1542/peds.2010-1830; PMID: 21262887
- Buttery JP, Lambert SB, Grimwood K, Nissen MD, Field EJ, Macartney KK, et al. Reduction in rotavirus-associated acute gastroenteritis following introduction of rotavirus vaccine into Australia’s National Childhood vaccine schedule. Pediatr Infect Dis J 2011; 30:Suppl S25 - 9; http://dx.doi.org/10.1097/INF.0b013e3181fefdee; PMID: 21183837
- Clarke MF, Davidson GP, Gold MS, Marshall HS. Direct and indirect impact on rotavirus positive and all-cause gastroenteritis hospitalizations in South Australian children following the introduction of rotavirus vaccination. Vaccine 2011; 29:4663 - 7; http://dx.doi.org/10.1016/j.vaccine.2011.04.109; PMID: 21575665
- Atkins KE, Shim E, Pitzer VE, Galvani AP. Impact of rotavirus vaccination on epidemiological dynamics in England and Wales. Vaccine 2012; 30:552 - 64; http://dx.doi.org/10.1016/j.vaccine.2011.11.064; PMID: 22133508
- Zeller M, Rahman M, Heylen E, De Coster S, De Vos S, Arijs I, et al. Rotavirus incidence and genotype distribution before and after national rotavirus vaccine introduction in Belgium. Vaccine 2010; 28:7507 - 13; http://dx.doi.org/10.1016/j.vaccine.2010.09.004; PMID: 20851085
- Gagneur A, Nowak E, Lemaitre T, Segura JF, Delaperrière N, Abalea L, et al, IVANHOE investigators. Impact of rotavirus vaccination on hospitalizations for rotavirus diarrhea: the IVANHOE study. Vaccine 2011; 29:3753 - 9; http://dx.doi.org/10.1016/j.vaccine.2011.03.035; PMID: 21443962
- Bruijning-Verhagen P, Quach C, Bonten M. Nosocomial rotavirus infections: a meta-analysis. Pediatrics 2012; 129:e1011 - 9; http://dx.doi.org/10.1542/peds.2011-2779; PMID: 22392170
- 20 Jahre nach dem Fall der Mauer: Wie hat sich die Gesundheit in Deutschland entwickelt? Beiträge zur Gesundheitsberichterstattung des Bundes. Berlin: Robert Koch-Institut, 2009.
- German Federal Statistical Office. Online available: http://www.destatis.de.
- Krause G, Altmann D, Faensen D, Porten K, Benzler J, Pfoch T, et al. SurvNet electronic surveillance system for infectious disease outbreaks, Germany. Emerg Infect Dis 2007; 13:1548 - 55; http://dx.doi.org/10.3201/eid1310.070253; PMID: 18258005