Abstract
Staphylococcus aureus is a major cause of healthcare-associated infections and is responsible for a substantial burden of disease in hospitalized patients. Despite increasingly rigorous infection control guidelines, the prevalence and corresponding negative impact of S. aureus infections remain considerable. Difficulties in controlling S. aureus infections as well as the associated treatment costs are exacerbated by increasing rates of resistance to available antibiotics. Despite ongoing efforts over the past 20 years, no licensed S. aureus vaccine is currently available. However, learnings from past clinical failures of vaccine candidates and a better understanding of the immunopathology of S. aureus colonization and infection have aided in the design of new vaccine candidates based on multiple important bacterial pathogenesis mechanisms. This review outlines important considerations in designing a vaccine for the prevention of S. aureus disease in healthcare settings.
The Complexity of Staphylococcal Disease
Staphylococcus aureus (S. aureus) are commensal, Gram-positive bacteria which colonize the nares, axilla, pharynx and other mucosal and skin surfaces of ~30% of human subjects. S. aureus is estimated to be responsible for 20–25% of all healthcare-associated infections,Citation1,Citation2 resulting in three times the length of hospital stay and a 5-fold higher risk of in-hospital death for infected patients compared with patients without such infections.Citation3 Furthermore, S. aureus infections can be associated with in-hospital mortality rates of up to 25%.Citation4 Although S. aureus is the etiological agent of a diverse number of diseases, including necrotizing pneumonia, septic arthritis and osteomyelitis, 90% of all infections are a result of skin and soft tissue structure breaches.Citation5-Citation7 Historically, S. aureus has been associated mainly with nosocomial infections. Over recent decades, however, there has been a dramatic increase in S. aureus infection associated with antibiotic resistances throughout the community, most notably in the United States.Citation8-Citation11
Customized Pathogenicity
S. aureus utilizes distinct mechanisms tailored for survival in different microenvironments encountered during host colonizationCitation12-Citation15 or invasion, such as inhibition of phagocytic uptake and killing,Citation16-Citation18 dissemination in the bloodstream, and formation of abscessesCitation19,Citation20 or biofilms.Citation21,Citation22 This versatility arises from the large number of virulence factors which S. aureus deploys in distinct spatial and temporal patterns to optimize its chances of survival.Citation20 These virulence factors include surface proteins that allow adhesion to host components such as fibrinogen (Clumping Factors, ClfAand B) or fibronectin (fibronectin binding proteins, FnBP A and B),Citation23,Citation24 and proteins which scavenge nutrients that are normally sequestered in vivo (such as iron-responsive surface determinants, IsdA and B).Citation25 In addition, the bacteria can express an impressive array of factors specifically designed to avoid the immune system, including an anti-opsonic extracellular capsule that protects the bacteria from non-anticapsular polysaccharide antibodies and innate immune components, protein inhibitors of neutrophil chemotaxis and the complement cascade, immunoglobulin binding proteins (such as staphylococcal protein A or Spa) and enzymes which aid its survival within the phagosome of neutrophils (such as the superoxide dismutases SodA and M).Citation26 Most strains of S. aureus also elaborate a number of different invasins (such as hyaluronidase and staphylokinase) and/or toxins (such as enterotoxins A and B, α toxin and Panton-Valentine leukocidin) which promote tissue damage and play an important role in septic shock.Citation27,Citation28
A significant amount of research has explored how S. aureus is capable of transitioning from a harmless commensal organism to a life threatening infectious agent.Citation15,Citation29-Citation31 It has been observed that people colonized with S. aureus are at higher risk of infection than non-carriersCitation32,Citation33 and that those infections usually arise from the colonizing strain,Citation34 yet colonized individuals have a greater chance of recovering from these infections. Interestingly, recovery from a S. aureus infection does not appear to confer immunity to subsequent infections.Citation35 Although somewhat paradoxical, these observations could be interpreted to mean that humans who are naturally exposed to S. aureus through asymptomatic carriage or previous infection may mount a sufficient immune response to the carriage strain to reduce the severity of infection but not to other strains circulating in the hospital or the community. In most cases, S. aureus infection occurs after breaches in the skin or mucosal barriers through wounds, trauma or surgical intervention which give the organism direct access to tissues or the bloodstream.Citation36 Once the skin or mucosa has been penetrated, infection can spread to the blood, causing bacteremia, or disseminate to other sites throughout the body.Citation37 Foreign devices surgically inserted into the body, such as joint prostheses, ventilators or catheters, can also become sites of S. aureus infection; ~20% of infections of implanted devices have been found to be caused by this pathogen.Citation38-Citation40 The surface characteristics of the device may facilitate bacterial adhesion, thereby increasing the risk of infection. As mentioned above, community-associated infections are increasing in incidence and initial superficial infections as a result of small skin abrasions or other minor skin lesions have the propensity to develop and spread.Citation41
Attack/Counterattack: Mechanisms of immunity that control S. aureus
Humans can be permanently colonized with S. aureus;as such, there can be a constant interplay between the bacteria and the host immune system. This is supported by the observation that all humans have pre-existing antibodies to S. aureus antigens. However, as discussed below, functional antibodies that facilitate the clearing of staphylococci from the site of an infection, which is through uptake and killing by professional phagocytes, especially neutrophils,Citation17,Citation42 or functional antibodies that neutralize virulence factors, are absent in the majority of the human population. The importance of immune clearance is underscored by the increased rates of infection that are observed for subjects with immunological disorders as summarized below; the close link between defects in clearance and the risk of disease provides confidence that if a vaccine generates adequate levels of functional antibodies in subjects with competent effector cells, those individuals are likely to be protected at times when they are at risk of infection.
Polymorphonuclear neutrophils, or PMNs, are the primary cellular defense against staphylococcal infections. These effector cells are highly efficient at killing phagocytosed pathogens by engaging in a complex cascade of cellular events involving multiple defense mechanisms to eradicate bacteria. Phagocytosis is the binding and ingestion of bacteria which can be facilitated by opsonization of the microbial surfaces with antibody and/or complement.Citation43 Neutrophils recognize many molecules produced by S. aureus such as lipoteichoic acid (LTA) and CpG DNA which interact with TLRs on the neutrophils to promote phagocytosis. Additionally, uptake of bacteria is enhanced if they are opsonized by complement and/or antibodies present in serum. Neutrophils express on their cell surfaces both complement receptors that bind complement-opsonized bacteria and Fc receptors that bind the Fc region of antibody-coated bacteria. Once it is bound to the neutrophils, a phagosome is formed to engulf the bacteria. This is followed by neutrophil-initiated mechanisms to kill the bacteria. Phagocytosis activates membrane bound NADPH-dependent oxidase to initiate a “respiratory burst,” which generates high levels of reactive oxygen species (ROS), i.e., superoxide radicals, hydrogen peroxide and hydroxyl radicals, to kill microorganisms. In addition, degranulation, the fusion of cytoplasmic granules with the bacteria-containing phagosome, results in the release of anti-microbial peptides, proteases and α-defensins with potent microbicidal activity.Citation26
Individuals with PMN defects have a higher incidence of S. aureus disease. Examples of neutrophil dysfunction include frank neutropenia,Citation44 Chronic Granulomatous Disease, in which neutrophils are unable to generate a functional respiratory burstCitation45-Citation47 and Chediak-Higashi Syndrome, in which individuals have neutrophils with reduced chemotaxis and phagolysosome function.Citation48 Subjects with defects in non-antibody-mediated clearance mechanisms, such as those with mutations in the pattern recognition receptor TLR2,Citation49 or defects in the complement pathwayCitation50 cannot effectively clear staphylococci and thus are more susceptible to S. aureus infections. Individuals with defective STAT3 signaling proteins have also been shown to be more prone to S.aureus infections due to their inability to generate IL-17-producing Th17 cells, which results in diminished neutrophil recruitment and function.Citation51-Citation53 Th17 cells have been shown to be important in response to natural infection with S. aureus, and some studies have shown Th17 responses may contribute to effective vaccine responses.Citation54,Citation55 In addition, S. aureus infection in the absence of vaccination has been shown to induce a Th17/Th1 response.Citation56,Citation57 Th17 cells may therefore play an important role in the control of S. aureus infections by serving as an link between neutrophil function and cell-mediated immunity.
Antibody-mediated clearance mechanisms driven by the adaptive immune system, in which antibody-secreting B cells and cytokine-secreting and cytolytic T cells play a key role, are also important in the prevention of staphylococcal disease. For example, individuals with immune defects impairing the ability to produce functional antibodies, such as AIDS,Citation58 immature immune systems due to premature birth,Citation59 or defects in immunoglobulin production all have increased susceptibility to staphylococcal infections.Citation60,Citation61 In males with X-linked agammaglobulinemia, recurrent pyogenic infections, often caused by S. aureus, begin to occur within the first year of life, after maternal IgG has been exhausted.Citation62 Overall, these findings support the requirement for functional antibodies and a functional neutrophil effector cell function to prevent staphylococcal infections and to prevent disease.
Learnings from Previous Clinical Experience
Several reviews have recently been published which summarize the clinical experience to date for S. aureus vaccines and discuss future prospects for the field.Citation35,Citation63-Citation67 The following sections describe key learnings from previous clinical experiences that could explain why clinical success may have been elusive to date and how Pfizer has incorporated the acquired knowledge into the design of its current vaccine candidates for the prevention of S. aureus infection and disease.
Single vs. multiple targets
Nearly all S. aureus vaccine candidates that have undergone clinical testing to date have targeted single antigens.Citation35,Citation65,Citation67 Although this has been a very effective strategy for vaccines against other human bacterial pathogens such as Streptococcus pneumoniae,Citation68 Hemophilus influenzaeCitation69 and Neisseria meningitidis,Citation70 it has not been effective for S. aureus. NABI conducted clinical studies for the prevention of S. aureus infection in hemodialysis patients using StaphVAX, a bivalent polysaccharide conjugate vaccine using E. coli enterotoxin as a carrier protein.Citation71 Despite promising preclinical data and demonstrated immunogenicity and safety in humans, administration of StaphVAX did not result in a decrease in the incidence of S. aureus infection in a pivotal phase III trial.Citation66 Another more recent example of a vaccine failure using a single S. aureus antigen is Merck’s monovalent vaccine based on IsdB, a conserved iron-scavenging protein expressed on the surface of S. aureusCitation72 which was identified using a novel screen for antigens which are highly expressed during infection.Citation73 While IsdB showed efficacy in several preclinical murine models of S. aureus infectionCitation72,Citation74,Citation75 it did not demonstrate efficacy in preventing S. aureus infections in cardiothoracic surgery populations.Citation76 These and other clinical failures summarized elsewhereCitation64,Citation65,Citation67 underscore the complex nature of S. aureus pathogenicity and suggest that a multiantigen vaccine designed to address major bacterial pathogenesis mechanisms will be required for efficacy. In support of this strategy, a recent preclinical study showed that vaccination of mice with a combination of four S.aureus cell wall-anchored adhesins resulted in 100% survival in a sepsis model of infection whereas immunization with each individual component only resulted in 50–70% protection.Citation77 Accordingly, as described below, Pfizer’s vaccine candidate contains multiple components that are designed to combat key pathogenic mechanisms deployed by S. aureus.
Selection of the antigen composition
Capsular polysaccharides are anti-phagocytic and key for immune evasion
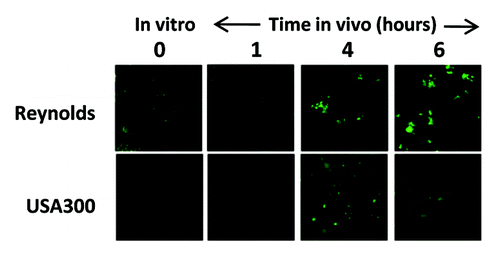
Expression of capsular polysaccharides is a common mechanism by which pathogenic bacteria, including S. aureus, evade opsonophagocytosis.Citation36 All S. aureus clinical isolates have the genetic pathway for either capsular polysaccharide (CP) type 5 or 8Citation78 and serotyping has shown that the vast majority of S. aureus strains express one or the other.Citation79 Although CP5 and CP8 are comprised of identical monosaccharides (L-FucNAc, D-FucNAc and D-ManNAc), they are serologically distinct due to differences in the linkages between sugars and the sites of O-acetylation.Citation80 Encapsulated S. aureus strains are more virulent in bacteremia models compared with capsule-defective isogenic mutants.Citation3 The increased virulence of capsule-expressing strains correlates with increased resistance to in vitro opsonophagocytic (OP) killing by human PMNsCitation81,Citation80. In addition, as shown in . aureus capsular polysaccharides are expressed early after infection of test animals, indicating that these structures appear to convey an in vivo survival benefit to the bacteria.
Figure 1. Expression of capsule during infection. Immunofluorescence analysis was performed on the laboratory S. aureus strain Reynolds and the USA300 strain CDC3 to determine the timing and degree of capsule expression in a murine infection model of bacteremia. CD1 mice (n = 3) were infected by intraperitoneal injection of approximately 2 × 108 colony-forming units. Immunofluorescence was performed on bacteria harvested at various time points using primary rabbit antibodies and an ALEXA488-conjugated goat-α-rabbit antibody. Both strains express high levels of capsule after four hours of infection (reproduced with permission from (107). Note this is in contrast to several reports that suggest USA300 strains are acapsular due to the lack of observed expression in vitro.Citation119,Citation120
Though capsular polysaccharides provide a good immune evasion strategy for the bacteria, anti- polysaccharide antibodies efficiently bind to bacterial cell surfaces and facilitate phagocytosis. To determine whether S. aureus capsular polysaccharides can induce opsonophagocytic antibody responses, Pfizer conducted preclinical studies with the CP5 and CP8 polysaccharides conjugated to CRM197, the same protein carrier also used in other polysaccharide conjugate vaccines such as Prevnar and Prevnar 13® 68. Efficacy of CP5/CP8 conjugates have previously been observed after immunization with the conjugates in preclinical animal models such as murine pylonephritis, rat and rabbit endocarditis and infant rat studies.Citation82-Citation84 Pfizer recently demonstrated that vaccination with both CP5 and CP8 conjugates in preclinical animal models and in humans resulted in high levels of antibodies that killed S. aureus clinical isolates in opsonophagocytosis assays (OPAs) as described below.
ClfA is a multifunctional virulence factor
Another component that has been selected by Pfizer for inclusion in its multivalent S. aureus vaccine is Clumping Factor A (ClfA). ClfA is a surface adhesin that binds to the C-terminus of the plasma fibrinogen γ chain.Citation85,Citation86 This interaction is central to the multifunctional activities of this well-characterized virulence factor. It promotes fibrin cross-linking and mediates the binding of the pathogens to platelets,Citation87,Citation88 resulting in thrombus (blood clot) formation. ClfA has also been shown to play a key role in the agglutination of staphylococci in the blood during infection, which leads to thromboembolic lesions in heart tissue and sepsis.Citation89 The binding of ClfA to fibrinogen may also subvert opsonophagocytic killing by enhancing the binding of complement factor I to ClfA on the bacterial surface, which in turn promotes cleavage of opsonin C3b into inactive fragments.Citation90,Citation91 The fibrinogen binding activity of ClfA is linked to the ability of S. aureus to cause disease, as S. aureus strains with ClfA point mutations that prevent fibrinogen binding show reduced virulence.Citation92 The ClfA antigen is detected in over 99% of S. aureus clinical isolates and is highly conserved (> 88% sequence identity).
The importance of ClfA as a virulence factor is illustrated by its ability to confer virulence, independent of other surface antigens, to an otherwise harmless strain of bacteria, Lactococcus lactis. L. lactis is able to process recombinant S. aureus ClfA, expressing it on its own surface at levels similar to that seen on S. aureus.Citation93 Heterologous expression of the ClfA adhesin in L. lactis allows the bacterium to then successfully colonize damaged valves in an experimental rat endocarditis model.Citation94 Recent unpublished data from Magnus Hook’s lab (U. Texas A and M) demonstrate that a L. lactis ClfA-expressing strain is capable of killing mice in a mouse bacteremia survival model (data not shown). In contrast, control strains expressing a ClfA mutant that cannot bind to fibrinogen (ClfAm) or that harbor the empty plasmid vector are completely avirulent (p = < 0.001 compared with L. lactis with intact ClfA). Therefore, the ability of S. aureus cells to bind to fibrinogen via ClfA is an important virulence mechanism of the bacteria. This phenotype has been captured in the Fibrinogen Binding Inhibition (FBI) Assay, which, as described below, measures the ability of antibodies to prevent S. aureus from binding to human fibrinogen.
Numerous studies suggest that ClfA is a promising component of an efficacious S. aureus vaccine. Preclinically, it was shown to be protective in murine models of arthritis, sepsis and endocarditis.Citation92,Citation95,Citation96 A particularly relevant study recently demonstrated the benefit of targeting both CP5 and ClfA antigens in a mouse mastitis model, in which passive immunization with antibodies to the two antigens had an additive effect on reducing bacterial burden.Citation97 In the clinic, ClfA was indirectly evaluated in studies with Veronate, a pool of plasma-derived, donor-selected, polyclonal antistaphylococcal human IgG with high titers against staphylococcal fibrinogen-binding proteins, Ser-Asp dipeptide repeat G (SdrG) and clumping factor A (ClfA), to prevent invasive S. aureus infections in neonates.Citation98 Despite preliminary promising clinical results, Veronate failed to reduce the incidence of neonatal late-stage staphylococcal sepsis in phase III.Citation99 It has been suggested that this failure might have been due to the fact that Veronate consisted of a human total IgG preparation and that the ClfA specific IgG concentration may have not been potent enough to neutralize the detrimental effects of ClfA. Alternatively, the ClfA binding antibodies in Veronate were possibly not functional,Citation35 a point which will be addressed further below.
MntC transports essential nutrients during infection
The fourth antigen in the Pfizer S. aureus vaccine is the Manganese Transporter C (MntC), a highly conserved (> 98% sequence identity) lipoprotein that is the surface-exposed metal binding subunit of MntABC, a heterotrimeric membrane transporter responsible for the acquisition of manganese. A primary host defense mechanism against bacterial invasion is the sequestration of metal ions that are essential for bacterial survival.Citation100 S. aureus and other bacteria have developed approaches to rapidly scavenge divalent cations like manganese and iron from the host when the bacterium establishes infection. As a cofactor for a number of diverse enzymes, manganese plays important roles in bacterial metabolism, cell wall synthesis and virulence.Citation101,Citation102 Most notably, it is the sole cofactor for superoxide dismutases, which neutralize superoxide radicals generated during the oxidative burst in the phagosome of macrophages and neutrophils.Citation103 S. aureus strains which lack functional MntC display increased sensitivity to superoxide radicals.Citation104 Therefore antibodies that target MntC have the potential to interfere with two distinct S. aureus virulence mechanisms: nutrient acquisition and phagosome survival.
Pfizer first identified MntC as a potential vaccine target after it was observed that the analogous S. epidermidis protein (SitC) was only expressed in vivo or in the presence of human serum.Citation105 SitC showed > 75% amino acid similarity to MntC and was efficacious in an S. epidermidis bacteremia model.Citation105 In vivo expression analysis of bacteria harvested from the blood at various times revealed that MntC is rapidly upregulated by S. aureus in a murine model of infection (). Expression profiles were compared with those for another cation-sequestering protein, IsdB. MntC was found to be consistently expressed earlier during infection in vivo in comparison to other antigens including the IsdB antigen (). Thus, MntC is an important antigen to include in a multicomponent vaccine as it is expressed earlier than many of the surface adhesion molecules.
Table 1.In vivo Surface Expression of Ion Scavenging Proteins in S. aureus Clinical Isolates
Preclinical studies demonstrated that MntC could induce a protective immune response.Citation106 Active immunization studies in a S. aureus bacteremia model of infection showed that MntC was effective in reducing the bacterial load of S. aureus in the blood. The same antigen also protected against S. epidermidis infection ().Citation106 This is the first example of a protein antigen that has the potential to provide protection against both S. aureus and S. epidermidis. In addition, anti-MntC monoclonal antibodies were identified that could bind both S. aureus and S. epidermidis cells and were protective in an infant rat passive protection model (). These monoclonal antibodies can be used in serological assays to measure antibodies generated against MntC that compete for binding with the monoclonal antibody to MntC, thereby providing a semifunctional assessment of the potency of antibodies elicited by the MntC vaccine.
Table 2. Immunization with MntC reduces the S. aureus bacterial load in a Murine Bacteremia Model (adapted with permission from 106)
Figure 2. Reduction of S. aureus Bacteremia in Infant Rats By Passive Administration of an Anti-MntC Monoclonal Antibody [adapted with permission from Table 4 in (106)]. Groups of Sprague-Dawley infant rats were immunized intraperitoneally with 0.4 mg of either test mAb or isotype control mAb. Sixteen hours after immunization, rats were challenged intraperitoneally with 1 × 108 colony forming unites of a S. aureus clinical isolate, PFESA0140. Four hours later, blood was collected, and serial dilutions were plated to enumerate recovered bacteria. Statistical significance was determined via the Student t-test, and a P value of ≤ 0.05 was considered significant.
![Figure 2. Reduction of S. aureus Bacteremia in Infant Rats By Passive Administration of an Anti-MntC Monoclonal Antibody [adapted with permission from Table 4 in (106)]. Groups of Sprague-Dawley infant rats were immunized intraperitoneally with 0.4 mg of either test mAb or isotype control mAb. Sixteen hours after immunization, rats were challenged intraperitoneally with 1 × 108 colony forming unites of a S. aureus clinical isolate, PFESA0140. Four hours later, blood was collected, and serial dilutions were plated to enumerate recovered bacteria. Statistical significance was determined via the Student t-test, and a P value of ≤ 0.05 was considered significant.](/cms/asset/8de513ca-5bef-4768-8364-4dba299020bc/khvi_a_10921872_f0003.gif)
Other S. aureus antigens
Toxins
A number of S. aureus vaccines composed of inactivated toxins or their subunits have been evaluated preclinically.Citation35,Citation63 Due to the heterogeneous nature of toxins produced by S. aureus, and the fact that toxins are usually released after an infection has been already established, the value of adding toxin components into multicomponent prophylactic vaccine formulation is questionable.
Protein A
Protein A is a highly conserved S. aureus cell wall protein encoded by the spa gene that binds with high affinity to the Fc region of human IgG1 and IgG2 and mouse IgG2a and IgG2b, thereby inhibiting functional antibodies from blocking key functions such as opsonophagocytoss and adhesion. In addition, Protein A binds to VH3 IgM on the surface of B-cell receptors, resulting in reduced antibody production.Citation18 Many experts have raised concerns about the possibility that protein A and related proteins, such as Sbi, could render vaccination futile.Citation18,Citation65,Citation67 However, it is important to keep in mind that protein A appears to be most relevant for abscess formation and has less impact on the early stages of S. aureus infection and dissemination during which time it is not expressed in vivo.Citation19 Therefore its inclusion in a multi-antigen vaccine designed to prevent infection in the first place may not be appropriate, although it could be considered for therapeutic vaccines.
Suitable antigen expression
Another key feature of a successful vaccine that may not have been sufficiently demonstrated for previous S. aureus candidate vaccines is evidence that target antigens are expressed to sufficient levels during infection 64,67, 72. Although the genetic factors influencing the expression of S. aureus virulence factors during infection have been described in depth,Citation117 relatively few studies have directly measured antigen expression in vivo. Recent studies using immunofluorescence microscopy in both bacteremia and wound models of infections have been conducted to fully characterize the in vivo expression patterns of capsule and ClfA.Citation107 It was demonstrated that the expression of these antigens can be temporally different, and is dependent on both the challenge strains examined and the microenvironment encountered during infection. The results also showed that antibody access to ClfA is not impeded by capsule expression.
Demonstration of S. aureus killing and virulence inhibition by vaccine-elicited immune sera
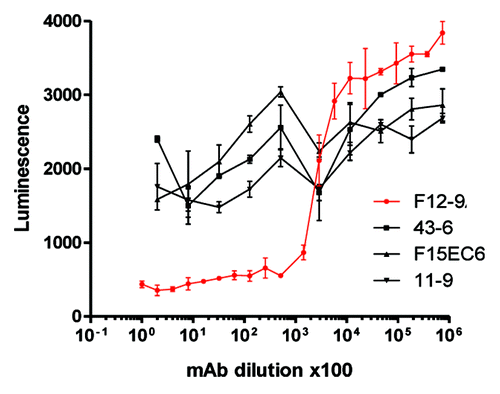
A critical feature of a successful vaccine, which experts believe may not have been suitably addressed in previous S. aureus vaccine clinical trials, is the demonstration that vaccine-elicited antibodies are functional and have sufficient activity and avidity to support opsonophagocytic killing and/or neutralization of important virulence factors.Citation108,Citation109 Vaccination with CP5 or CP8 CRM197 conjugates elicit robust OPA responses in non-human primates () and in humans.Citation110 Furthermore, anti-ClfA antibodies generated after immunization with a ClfA-containing vaccine inhibited the binding of S. aureus to fibrinogen in a Fibrinogen Binding Inhibition Assay (FBI assay,Citation111 adapted from (86)). The FBI assay monitors the inhibition of live S. aureus cells binding to fibrinogen as shown schematically in . Such an assay provides a useful readout regarding the effectiveness of a ClfA vaccine component to inhibit this important virulence mechanism. The FBI assay was tested for specificity using a number of S. aureus anti-ClfA monoclonal antibodies (mAbs) that bind with high affinity to native ClfA on the surface of S. aureus. shows the data for a subset of four mAbs, including mAb12–9, the only monoclonal antibody that was protective in an animal model of staphylococcal infection and, importantly, the only antibody in this subset that could inhibit the binding of S. aureus to the fibrinogen. Despite similar binding affinities, only mAb 12–9 shows binding inhibition in a dilution-dependent manner. These results (1) further confirm the important role of ClfA in virulence and (2) underscore the important distinction between binding affinity and functional activity.
Figure 3. Immune sera from CP-conjugates induce opsonophagocytosis. Sera from three different cynomolgous monkeys vaccinated with either CP8 or CP5 conjugates (each shown with a different symbol) were evaluated for their ability to promote opsonophagocytic killing as described in (82). Preimmune sera is shown in dashed lines and post-immune sera, harvested at six weeks post dose, is shown in solid lines. The titer is defined as the reciprocal serum dilution that kills 50% of the test bacteria. The CFU associated with 50% bacterial killing is indicated by the dotted line.
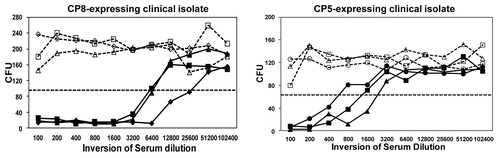
Figure 4. Schematic representation of the S. aureus Fibrinogen Binding Inhibition Assay. Microplate wells were coated with fibrinogen (Fg), incubated with blocking solution to prevent non-specific binding and rinsed. Live S. aureus cells (1 × 106 colony forming units) anti-sera were mixed and added to the plate. After 30 min incubation at 37°C adherent cells were washed and quantified using the Luciferase-based BacTiter-Glo® reagent, which measures ATP of live bacteria and associated luminescence.
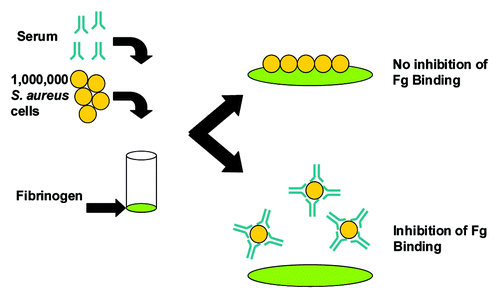
Figure 5.S. aureus FBI assay is Specific for Anti-ClfA Functional Monoclonal Antibodies. Serial dilutions of monoclonal antibodies (mAb) were pre-incubated with S. aureus strain PFESA0237 and their binding to immobilized fibrinogen was assessed as described in the legend to .Citation111 Despite similar binding affinities,only the functional mAb 12–9 shows binding inhibition in a dilution-dependent manner.
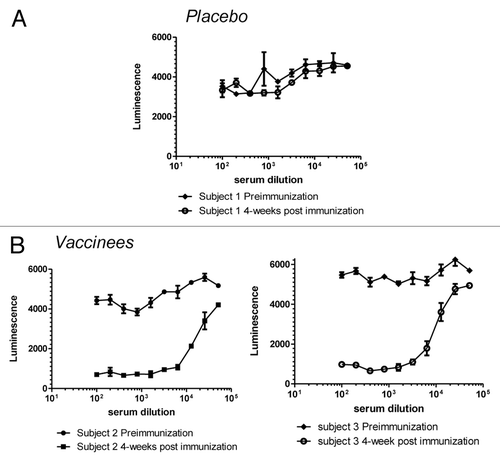
Clinical sera from the Pfizer SA3Ag vaccine study (described below) were evaluated using the exploratory FBI assay. No FBI activity was detected in unvaccinated subjects and complete inhibition of Fg binding was observed for most vaccinated subjects (28/32) (). It is important to note that, despite the frequency of S. aureus colonization and the presence of pre-existing antibodies, most individuals do not have circulating functional antibodies to this important virulence factor. Encouragingly, immunization with a ClfA-containing vaccine does induce high titers of such functional antibody, illustrating the critical importance of demonstrating the functionality of antibodies generated during vaccine development.
Figure 6. Human Immune Sera from the Clinical Tri-antigen Vaccine Study (NCT01018641) Prevents S. aureus (strain PFESA0237) from Binding Fibrinogen. Serial dilutions of serum taken from human subjects either before or four weeks after immunization with either a placebo (A) or the trivalent vaccine (B) were tested for activity in the FBI assay as described in the legend to .
Current status in the clinic
As described on www.clinicaltrials.gov, Pfizer recently completed a phase I trial to evaluate a trivalent vaccine composed of two capsular polysaccharide (CP) conjugates and Clumping Factor A (CP5, CP8 and rmClfA) in healthy adults (18 to 85 y of age) (ID#NCT01018641) and currently has phase I/II trials in progress to evaluate three ascending doses of a tetravalent vaccine (consisting of CP5, CP8, rmClfA and MntC) (ID#NCT01364571 and ID#NCT01643941) in the same target population. Although CPs are produced by bacteria to be antiphagocytic, Pfizer and others have shown in S. aureus, anti-CP antibodies mediate killing by opsonophagocytosis. ClfA is associated with bloodstream infections and facilitates the binding of S. aureus to fibrinogen.Citation89 The vaccine in the initial study was reported to be well-tolerated and able to elicit robust functional antibodies after a single dose.Citation110 Of significance was the use of the FBI assay described above to demonstrate that healthy humans do not have functional antibodies that can prevent deployment of this virulence mechanism. In contrast, high inhibitory titers were observed after vaccination.Citation111
Other Considerations
Clinical trial design
Several recent reviews have included detailed discussions on key considerations for the design of a successful clinical trial for S. aureus vaccines.Citation35,Citation64,Citation66 In addition to the relevant, robust functional assays to characterize initial immune responses and a careful selection of patient populations that would be most appropriate for initial proof-of-concept efficacy evaluations, the definition and timing of the disease endpoint is also critical.
Risk factors
It is almost certain that selection of the appropriate target population for a S. aureus vaccine is a key determinant for the successful demonstration of its efficacy. The risk factors for invasive S.aureus infection are well-defined for both community and health-care settingsCitation66 and notably distinct from each other, and with the former consisting of chronically immunocompromised patients and the latter consisting of patients in the ICU or undergoing surgery or recipients of foreign bodies such as catheters, implants or other medical devices.Citation112,Citation113 It is therefore implicit that vaccines targeting these distinct populations would differ in their development strategies.Citation64
HA-MRSA vs. CA-MRSA
Strains causing infections differ between hospital and community. Methicillin-resistant S. aureus (MRSA), historically associated with nosocomial infections, is now highly prevalent in both healthcare and community settings.Citation114,Citation115 However, MRSA strains causing disease in the community are not only phylogenetically and phenotypically distinct from those found in the hospital,Citation116 but also differ in the degreeCitation27 and siteCitation117 of colonization and often demonstrate different clinical disease manifestations.Citation118 These differences must also be taken into account during the design of an effective vaccine.
Conclusion
S. aureus is a challenging vaccine target due to complexity of its pathogenesis involving a multitude of virulence factors. However, these pathogenic mechanisms can be addressed by eliciting functional antibodies to an appropriate selection of S. aureus antigens that are expressed in vivo early during the infection cycle. Pfizer’s S. aureus investigational vaccine has been designed to address three major virulence mechanisms associated with staphylococcal disease: immune evasion (capsule), binding to host cells and immune evasion (ClfA) and nutrient acquisition (MntC). The need to generate functional antibodies that are capable of mediating measurable opsonophagocytic killing has been addressed by inclusion of conjugated capsular polysaccharide antigens CP5 and CP8. We have demonstrated that anti-ClfA is an important virulence factor and that vaccine-elicited antibodies can completely inhibit S. aureus binding to human fibrinogen which is required to prevent ClfA mediated pathology. We have also shown that MntC is protective in preclinical models of infection and others have demonstrated that S. aureus lacking MntC are more susceptible to oxidative stress. Unlike monovalent vaccine candidates described in the past that were not successful in preventing S. aureus infections, it is hoped that inclusion of multiple S. aureus antigen targets involved in several pathogen defense mechanisms and that are expressed early in the infectious cycle, will be sufficient in preventing infection and disease with this important pathogen.
| Abbreviations: | ||
| PMN | = | polymorphonuclear neutrophil |
| HA | = | hospital-acquired |
| CA | = | community-acquired |
| MRSA | = | methicillin-resistance Staphylococcus aureus |
| ClfA | = | Clumping Factor A |
| Fg | = | Fibrinogen |
| MSCRAMM | = | microbial surface component recognizing adhesive matrix molecule |
| MntC | = | Manganese Transporter C |
| IdsB | = | Iron-responsive Surface Determinant B |
| CP | = | Capsular Polysaccharide |
| OPA | = | Opsonophagocytosis |
| OPA | = | OP Assay |
| FBI | = | Fibrinogen Binding Inhibition |
Acknowledgments
This report was sponsored by Pfizer. The authors would like to acknowledge the following individuals for their contribution to this work: Yekaterina Timofeyeva, Julio Cesar, Pam Fink and Sandra Buitrago from Pfizer Vaccines; Magnus Hook and Emanual Smeds from Texas A&M.
Disclosure of Potential Conflicts of Interest
All authors are employees of Pfizer and as such were paid to do this work and may have shares in the company. ASA was a previously employed by Merck and may have shares in the company.
References
- Lowy FD. Staphylococcus aureus infections. N Engl J Med 1998; 339:520 - 32; http://dx.doi.org/10.1056/NEJM199808203390806; PMID: 9709046
- Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 2004; 39:309 - 17; http://dx.doi.org/10.1086/421946; PMID: 15306996
- Noskin GA, Rubin RJ, Schentag JJ, Kluytmans J, Hedblom EC, Smulders M, et al. The burden of Staphylococcus aureus infections on hospitals in the United States: an analysis of the 2000 and 2001 Nationwide Inpatient Sample Database. Arch Intern Med 2005; 165:1756 - 61; http://dx.doi.org/10.1001/archinte.165.15.1756; PMID: 16087824
- Rubin RJ, Harrington CA, Poon A, Dietrich K, Greene JA, Moiduddin A. The economic impact of Staphylococcus aureus infection in New York City hospitals. Emerg Infect Dis 1999; 5:9 - 17; http://dx.doi.org/10.3201/eid0501.990102; PMID: 10081667
- Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, Albrecht V, Limbago B, et al, EMERGEncy ID NET Study Group. Prevalence of methicillin-resistant staphylococcus aureus as an etiology of community-acquired pneumonia. Clin Infect Dis 2012; 54:1126 - 33; http://dx.doi.org/10.1093/cid/cis022; PMID: 22438343
- Nimmo GR. USA300 abroad: global spread of a virulent strain of community-associated methicillin-resistant Staphylococcus aureus. Clin Microbiol Infect 2012; 18:725 - 34; http://dx.doi.org/10.1111/j.1469-0691.2012.03822.x; PMID: 22448902
- Tong SY, Chen LF, Fowler VG Jr.. Colonization, pathogenicity, host susceptibility, and therapeutics for Staphylococcus aureus: what is the clinical relevance?. Semin Immunopathol 2012; 34:185 - 200; http://dx.doi.org/10.1007/s00281-011-0300-x; PMID: 22160374
- Rivera AM, Boucher HW. Current concepts in antimicrobial therapy against select gram-positive organisms: methicillin-resistant Staphylococcus aureus, penicillin-resistant pneumococci, and vancomycin-resistant enterococci. Mayo Clin Proc 2011; 86:1230 - 43; http://dx.doi.org/10.4065/mcp.2011.0514; PMID: 22134942
- Karamatsu ML, Thorp AW, Brown L. Changes in community-associated methicillin-resistant Staphylococcus aureus skin and soft tissue infections presenting to the pediatric emergency department: comparing 2003 to 2008. Pediatr Emerg Care 2012; 28:131 - 5; PMID: 22270497
- Skov R, Christiansen K, Dancer SJ, Daum RS, Dryden M, Huang Y-C, et al. Update on the prevention and control of community-acquired meticillin-resistant Staphylococcus aureus (CA-MRSA). Int J Antimicrob Agents 2012; 39:193 - 200; http://dx.doi.org/10.1016/j.ijantimicag.2011.09.029; PMID: 22226649
- Del Giudice P, Tattevin P, Etienne J.. Community-acquired methicillin-resistant Staphylococcus aureus. Review Presse Medicale 2011;
- Sivaraman K, Venkataraman N, Tsai J, Dewell S, Cole AM. Genome sequencing and analysis reveals possible determinants of Staphylococcus aureus nasal carriage. BMC Genomics 2008; 9:433; http://dx.doi.org/10.1186/1471-2164-9-433; PMID: 18808706
- Muthukrishnan G, Quinn GA, Lamers RP, Diaz C, Cole AL, Chen S, et al. Exoproteome of Staphylococcus aureus reveals putative determinants of nasal carriage. J Proteome Res 2011; 10:2064 - 78; http://dx.doi.org/10.1021/pr200029r; PMID: 21338050
- Krishna S, Miller LS. Host-pathogen interactions between the skin and Staphylococcus aureus. Curr Opin Microbiol 2012; 15:28 - 35; http://dx.doi.org/10.1016/j.mib.2011.11.003; PMID: 22137885
- Edwards AM, Massey RC, Clarke SR. Molecular mechanisms of Staphylococcus aureus nasopharyngeal colonization. Mol Oral Microbiol 2012; 27:1 - 10; http://dx.doi.org/10.1111/j.2041-1014.2011.00628.x; PMID: 22230461
- Malachowa N, Whitney AR, Kobayashi SD, Sturdevant DE, Kennedy AD, Braughton KR, et al. Global changes in Staphylococcus aureus gene expression in human blood. PLoS One 2011; 6:e18617; http://dx.doi.org/10.1371/journal.pone.0018617; PMID: 21525981
- Rigby KM, DeLeo FR. Neutrophils in innate host defense against Staphylococcus aureus infections. Semin Immunopathol 2012; 34:237 - 59; http://dx.doi.org/10.1007/s00281-011-0295-3; PMID: 22080185
- Kim HK, Thammavongsa V, Schneewind O, Missiakas D. Recurrent infections and immune evasion strategies of Staphylococcus aureus. Curr Opin Microbiol 2012; 15:92 - 9; http://dx.doi.org/10.1016/j.mib.2011.10.012; PMID: 22088393
- Cheng AG, Kim HK, Burts ML, Krausz T, Schneewind O, Missiakas DM. Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues. FASEB J 2009; 23:3393 - 404; http://dx.doi.org/10.1096/fj.09-135467; PMID: 19525403
- Cheng AG, DeDent AC, Schneewind O, Missiakas D. A play in four acts: Staphylococcus aureus abscess formation. Trends Microbiol 2011; 19:225 - 32; http://dx.doi.org/10.1016/j.tim.2011.01.007; PMID: 21353779
- Otto M. Staphylococcal biofilms. Curr Top Microbiol Immunol 2008; 322:207 - 28; http://dx.doi.org/10.1007/978-3-540-75418-3_10; PMID: 18453278
- Harro JM, Peters BM, O’May GA, Archer N, Kerns P, Prabhakara R, et al. Vaccine development in Staphylococcus aureus: taking the biofilm phenotype into consideration. FEMS Immunol Med Microbiol 2010; 59:306 - 23; PMID: 20602638
- Clarke SR, Foster SJ. Surface Adhesins of Staphylococcus aureus. In: Robert KP, ed. Advances in Microbial Physiology: Academic Press, 2006:187-224.
- Heilmann C. Mechanisms of Staphylococci Bacterial Adhesion. In: Linke D, Goldman A, eds.: Springer Netherlands, 2011:105-23.
- Cassat JE, Skaar EP. Metal ion acquisition in Staphylococcus aureus: overcoming nutritional immunity. Semin Immunopathol 2012; 34:215 - 35; http://dx.doi.org/10.1007/s00281-011-0294-4; PMID: 22048835
- Foster TJ. Immune evasion by staphylococci. Nat Rev Microbiol 2005; 3:948 - 58; http://dx.doi.org/10.1038/nrmicro1289; PMID: 16322743
- Pinchuk IV, Beswick EJ, Reyes VE. Staphylococcal enterotoxins. Toxins (Basel) 2010; 2:2177 - 97; http://dx.doi.org/10.3390/toxins2082177; PMID: 22069679
- Otto M. Basis of virulence in community-associated methicillin-resistant Staphylococcus aureus. Annu Rev Microbiol 2010; 64:143 - 62; http://dx.doi.org/10.1146/annurev.micro.112408.134309; PMID: 20825344
- Otto M. How Staphylococcus aureus breaches our skin to cause infection. J Infect Dis 2012; 205:1483 - 5; http://dx.doi.org/10.1093/infdis/jis248; PMID: 22457276
- Gordon RJ, Lowy FD. Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis 2008; 46:Suppl 5 S350 - 9; http://dx.doi.org/10.1086/533591; PMID: 18462090
- Lindsay JA, Moore CE, Day NP, Peacock SJ, Witney AA, Stabler RA, et al. Microarrays reveal that each of the ten dominant lineages of Staphylococcus aureus has a unique combination of surface-associated and regulatory genes. J Bacteriol 2006; 188:669 - 76; http://dx.doi.org/10.1128/JB.188.2.669-676.2006; PMID: 16385056
- Wertheim HFL, Melles DC, Vos MC, van Leeuwen W, van Belkum A, Verbrugh HA, et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis 2005; 5:751 - 62; http://dx.doi.org/10.1016/S1473-3099(05)70295-4; PMID: 16310147
- Kluytmans J. A van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev 1997; 3:505 - 20
- von Eiff C, Becker K, Machka K, Stammer H, Peters G, Study Group. Nasal carriage as a source of Staphylococcus aureus bacteremia. N Engl J Med 2001; 344:11 - 6; http://dx.doi.org/10.1056/NEJM200101043440102; PMID: 11136954
- Schaffer AC, Lee JC. Staphylococcal vaccines and immunotherapies. Infect Dis Clin North Am 2009; 23:153 - 71; http://dx.doi.org/10.1016/j.idc.2008.10.005; PMID: 19135920
- Foster TJ. Colonization and infection of the human host by staphylococci: adhesion, survival and immune evasion. Vet Dermatol 2009; 20:456 - 70; http://dx.doi.org/10.1111/j.1365-3164.2009.00825.x; PMID: 20178484
- David A, Risitano DC, Mazzeo G, Sinardi L, Venuti FS, Sinardi AU. Central venous catheters and infections. Minerva Anestesiol 2005; 71:561 - 4; PMID: 16166918
- Rafiq I, Gambhir AK, Wroblewski BM, Kay PR. The microbiology of infected hip arthroplasty. Int Orthop 2006; 30:532 - 5; http://dx.doi.org/10.1007/s00264-006-0125-8; PMID: 16896877
- Chambers ST. Diagnosis and management of staphylococcal infections of vascular grafts and stents. Intern Med J 2005; 35:Suppl 2 S72 - 8; http://dx.doi.org/10.1111/j.1444-0903.2005.00981.x; PMID: 16271063
- Segawa H, Tsukayama DT, Kyle RF, Becker DA, Gustilo RB. Infection after total knee arthroplasty. A retrospective study of the treatment of eighty-one infections. J Bone Joint Surg Am 1999; 81:1434 - 45; PMID: 10535593
- Kale-Pradhan P, Johnson LB. Treatment and recurrence management of staphylococcal infections: community-acquired MRSA. Expert Rev Anti Infect Ther 2008; 6:909 - 15; http://dx.doi.org/10.1586/14787210.6.6.909; PMID: 19053903
- Krishna S, Miller LS. Innate and adaptive immune responses against Staphylococcus aureus skin infections. Semin Immunopathol 2012; 34:261 - 80; http://dx.doi.org/10.1007/s00281-011-0292-6; PMID: 22057887
- DeLeo FR, Diep BA, Otto M. Host defense and pathogenesis in Staphylococcus aureus infections. Infect Dis Clin North Am 2009; 23:17 - 34; http://dx.doi.org/10.1016/j.idc.2008.10.003; PMID: 19135914
- Bodey GP, Buckley M, Sathe YS, Freireich EJ. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med 1966; 64:328 - 40; PMID: 5216294
- Quie PG, White JG, Holmes B, Good RA. In vitro bactericidal capacity of human polymorphonuclear leukocytes: diminished activity in chronic granulomatous disease of childhood. J Clin Invest 1967; 46:668 - 79; http://dx.doi.org/10.1172/JCI105568; PMID: 6021213
- Johnston RB Jr., Keele BB Jr., Misra HP, Lehmeyer JE, Webb LS, Baehner RL, et al. The role of superoxide anion generation in phagocytic bactericidal activity. Studies with normal and chronic granulomatous disease leukocytes. J Clin Invest 1975; 55:1357 - 72; http://dx.doi.org/10.1172/JCI108055; PMID: 166094
- Heyworth PG, Cross AR, Curnutte JT. Chronic granulomatous disease. Curr Opin Immunol 2003; 15:578 - 84; http://dx.doi.org/10.1016/S0952-7915(03)00109-2; PMID: 14499268
- Dinauer MC. Disorders of neutrophil function: an overview. Methods Mol Biol 2007; 412:489 - 504; http://dx.doi.org/10.1007/978-1-59745-467-4_30; PMID: 18453130
- Lorenz E, Mira JP, Cornish KL, Arbour NC, Schwartz DA. A novel polymorphism in the toll-like receptor 2 gene and its potential association with staphylococcal infection. Infect Immun 2000; 68:6398 - 401; http://dx.doi.org/10.1128/IAI.68.11.6398-6401.2000; PMID: 11035751
- Figueroa JE, Densen P. Infectious diseases associated with complement deficiencies. Clin Microbiol Rev 1991; 4:359 - 95; PMID: 1889047
- de Beaucoudrey L, Puel A, Filipe-Santos O, Cobat A, Ghandil P, Chrabieh M, et al. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J Exp Med 2008; 205:1543 - 50; http://dx.doi.org/10.1084/jem.20080321; PMID: 18591412
- Ma CS, Chew GYJ, Simpson N, Priyadarshi A, Wong M, Grimbacher B, et al. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J Exp Med 2008; 205:1551 - 7; http://dx.doi.org/10.1084/jem.20080218; PMID: 18591410
- Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature 2008; 452:773 - 6; http://dx.doi.org/10.1038/nature06764; PMID: 18337720
- Lin L, Ibrahim AS, Xu X, Farber JM, Avanesian V, Baquir B, et al. Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog 2009; 5:e1000703; http://dx.doi.org/10.1371/journal.ppat.1000703; PMID: 20041174
- Joshi A, Pancari G, Cope L, Bowman E, Cua D, Proctor R, et al. Immunization with Staphylococcus aureus iron regulated surface determinant B (IsdB) confers protection via Th17/IL17 pathway in a murine sepsis model. Hum Vaccin Immunother 2012; 8:0 - 10; PMID: 22327491
- Cho JS, Pietras EM, Garcia NC, Ramos RI, Farzam DM, Monroe HR, et al. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J Clin Invest 2010; 120:1762 - 73; http://dx.doi.org/10.1172/JCI40891; PMID: 20364087
- Prabhakara R, Harro JM, Leid JG, Harris M, Shirtliff ME. Murine immune response to a chronic Staphylococcus aureus biofilm infection. Infect Immun 2011; 79:1789 - 96; http://dx.doi.org/10.1128/IAI.01386-10; PMID: 21282411
- Jacobson MA, Gellermann H, Chambers H. Staphylococcus aureus bacteremia and recurrent staphylococcal infection in patients with acquired immunodeficiency syndrome and AIDS-related complex. Am J Med 1988; 85:172 - 6; http://dx.doi.org/10.1016/S0002-9343(88)80337-1; PMID: 3400693
- Maraqa NF, Aigbivbalu L, Masnita-Iusan C, Wludyka P, Shareef Z, Bailey C, et al. Prevalence of and risk factors for methicillin-resistant Staphylococcus aureus colonization and infection among infants at a level III neonatal intensive care unit. Am J Infect Control 2011; 39:35 - 41; http://dx.doi.org/10.1016/j.ajic.2010.07.013; PMID: 21281885
- Mickenberg ID, Root RK, Wolff SM. Leukocytic function in hypogammaglobulinemia. J Clin Invest 1970; 49:1528 - 38; http://dx.doi.org/10.1172/JCI106370; PMID: 4194088
- Trakultivakorn M, Ochs HD. X-linked agammaglobulinemia in northern Thailand. Asian Pac J Allergy Immunol 2006; 24:57 - 63; PMID: 16913189
- Fauci A, ed. Harrison's Principles of Internal Medicine, 14th Ed.: McGraw-Hill Companies, 1998.
- Otto M. Novel targeted immunotherapy approaches for staphylococcal infection. Expert Opin Biol Ther 2010; 10:1049 - 59; http://dx.doi.org/10.1517/14712598.2010.495115; PMID: 20528609
- Broughan J, Anderson R, Anderson AS. Strategies for and advances in the development of Staphylococcus aureus prophylactic vaccines. Expert Rev Vaccines 2011; 10:695 - 708; http://dx.doi.org/10.1586/erv.11.54; PMID: 21604989
- Daum RS, Spellberg B. Progress toward a Staphylococcus aureus vaccine. Clin Infect Dis 2012; 54:560 - 7; http://dx.doi.org/10.1093/cid/cir828; PMID: 22186773
- Spellberg B, Daum R. Development of a vaccine against Staphylococcus aureus. Semin Immunopathol 2012; 34:335 - 48; http://dx.doi.org/10.1007/s00281-011-0293-5; PMID: 22080194
- Proctor RA. Challenges for a universal Staphylococcus aureus vaccine. Clin Infect Dis 2012; 54:1179 - 86; http://dx.doi.org/10.1093/cid/cis033; PMID: 22354924
- Jefferies JMC, Macdonald E, Faust SN, Clarke SC. 13-valent pneumococcal conjugate vaccine (PCV13). Hum Vaccin 2011; 7:1012 - 8; http://dx.doi.org/10.4161/hv.7.10.16794; PMID: 21941097
- Agrawal A, Murphy TF. Haemophilus influenzae infections in the H. influenzae type b conjugate vaccine era. J Clin Microbiol 2011; 49:3728 - 32; http://dx.doi.org/10.1128/JCM.05476-11; PMID: 21900515
- Anderson AS, Jansen KU, Eiden J. New frontiers in meningococcal vaccines. Expert Rev Vaccines 2011; 10:617 - 34; http://dx.doi.org/10.1586/erv.11.50; PMID: 21604983
- Shinefield H, Black S, Fattom A, Horwith G, Rasgon S, Ordonez J, et al. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N Engl J Med 2002; 346:491 - 6; http://dx.doi.org/10.1056/NEJMoa011297; PMID: 11844850
- Kuklin NA, Clark DJ, Secore S, Cook J, Cope LD, McNeely T, et al. A novel Staphylococcus aureus vaccine: iron surface determinant B induces rapid antibody responses in rhesus macaques and specific increased survival in a murine S. aureus sepsis model. Infect Immun 2006; 74:2215 - 23; http://dx.doi.org/10.1128/IAI.74.4.2215-2223.2006; PMID: 16552052
- Etz H, Minh DB, Henics T, Dryla A, Winkler B, Triska C, et al. Identification of in vivo expressed vaccine candidate antigens from Staphylococcus aureus. Proc Natl Acad Sci U S A 2002; 99:6573 - 8; http://dx.doi.org/10.1073/pnas.092569199; PMID: 11997460
- Ebert T, Smith S, Pancari G, Clark D, Hampton R, Secore S, et al. A fully human monoclonal antibody to Staphylococcus aureus iron regulated surface determinant B (IsdB) with functional activity in vitro and in vivo. Hum Antibodies 2010; 19:113 - 28; PMID: 21178283
- Kim HK, DeDent A, Cheng AG, McAdow M, Bagnoli F, Missiakas DM, et al. IsdA and IsdB antibodies protect mice against Staphylococcus aureus abscess formation and lethal challenge. Vaccine 2010; 28:6382 - 92; http://dx.doi.org/10.1016/j.vaccine.2010.02.097; PMID: 20226248
- Merck and Intercell AG Announce Termination of Phase II/III Clinical Trial of Investigational Staphylococcus aureus Vaccine, V710. Whitehouse Station, NJ, 2011. http://www.merck.com/newsroom/news-release-archive/research-and-development/2011_0608.html
- Stranger-Jones YK, Bae T, Schneewind O. Vaccine assembly from surface proteins of Staphylococcus aureus. Proc Natl Acad Sci U S A 2006; 103:16942 - 7; http://dx.doi.org/10.1073/pnas.0606863103; PMID: 17075065
- Murphy E, Lin SL, Nunez L, Andrew L, Fink PS, Dilts DA, et al. Challenges for the evaluation of Staphylococcus aureus protein based vaccines: monitoring antigenic diversity. Hum Vaccin 2011; 7:Suppl 51 - 9; http://dx.doi.org/10.4161/hv.7.0.14562; PMID: 21245656
- Sompolinsky D, Samra Z, Karakawa WW, Vann WF, Schneerson R, Malik Z. Encapsulation and capsular types in isolates of Staphylococcus aureus from different sources and relationship to phage types. J Clin Microbiol 1985; 22:828 - 34; PMID: 2932464
- O’Riordan K, Lee JC. Staphylococcus aureus capsular polysaccharides. Clin Microbiol Rev 2004; 17:218 - 34; http://dx.doi.org/10.1128/CMR.17.1.218-234.2004; PMID: 14726462
- Thakker M, Park J-S, Carey V, Lee JC. Staphylococcus aureus serotype 5 capsular polysaccharide is antiphagocytic and enhances bacterial virulence in a murine bacteremia model. Infect Immun 1998; 66:5183 - 9; PMID: 9784520
- Fattom AI, Sarwar J, Ortiz A, Naso R. A Staphylococcus aureus capsular polysaccharide (CP) vaccine and CP-specific antibodies protect mice against bacterial challenge. Infect Immun 1996; 64:1659 - 65; PMID: 8613375
- Lee JC, Park JS, Shepherd SE, Carey V, Fattom A. Protective efficacy of antibodies to the Staphylococcus aureus type 5 capsular polysaccharide in a modified model of endocarditis in rats. Infect Immun 1997; 65:4146 - 51; PMID: 9317020
- Cook J, Hepler R, Pancari G, Kuklin N, Fan H, Wang XM, et al. Staphylococcus aureus capsule type 8 antibodies provide inconsistent efficacy in murine models of staphylococcal infection. Hum Vaccin 2009; 5:254 - 63; http://dx.doi.org/10.4161/hv.5.4.6765; PMID: 18787395
- Liu CZ, Shih MH, Tsai PJ. ClfA(221-550), a fibrinogen-binding segment of Staphylococcus aureus clumping factor A, disrupts fibrinogen function. Thromb Haemost 2005; 94:286 - 94; PMID: 16113817
- McDevitt D, Nanavaty T, House-Pompeo K, Bell E, Turner N, McIntire L, et al. Characterization of the interaction between the Staphylococcus aureus clumping factor (ClfA) and fibrinogen. Eur J Biochem 1997; 247:416 - 24; http://dx.doi.org/10.1111/j.1432-1033.1997.00416.x; PMID: 9249055
- Bayer AS, Sullam PM, Ramos M, Li C, Cheung AL, Yeaman MR. Staphylococcus aureus induces platelet aggregation via a fibrinogen-dependent mechanism which is independent of principal platelet glycoprotein IIb/IIIa fibrinogen-binding domains. Infect Immun 1995; 63:3634 - 41; PMID: 7642301
- Siboo IR, Cheung AL, Bayer AS, Sullam PM. Clumping factor A mediates binding of Staphylococcus aureus to human platelets. Infect Immun 2001; 69:3120 - 7; http://dx.doi.org/10.1128/IAI.69.5.3120-3127.2001; PMID: 11292731
- McAdow M, Kim HK, Dedent AC, Hendrickx APA, Schneewind O, Missiakas DM. Preventing Staphylococcus aureus sepsis through the inhibition of its agglutination in blood. PLoS Pathog 2011; 7:e1002307; http://dx.doi.org/10.1371/journal.ppat.1002307; PMID: 22028651
- Hair PS, Ward MD, Semmes OJ, Foster TJ, Cunnion KM. Staphylococcus aureus clumping factor A binds to complement regulator factor I and increases factor I cleavage of C3b. J Infect Dis 2008; 198:125 - 33; http://dx.doi.org/10.1086/588825; PMID: 18544012
- Hair PS, Echague CG, Sholl AM, Watkins JA, Geoghegan JA, Foster TJ, et al. Clumping factor A interaction with complement factor I increases C3b cleavage on the bacterial surface of Staphylococcus aureus and decreases complement-mediated phagocytosis. Infect Immun 2010; 78:1717 - 27; http://dx.doi.org/10.1128/IAI.01065-09; PMID: 20100856
- Josefsson E, Hartford O, O’Brien L, Patti JM, Foster T. Protection against experimental Staphylococcus aureus arthritis by vaccination with clumping factor A, a novel virulence determinant. J Infect Dis 2001; 184:1572 - 80; http://dx.doi.org/10.1086/324430; PMID: 11740733
- Que Y-A, Haefliger J-A, Francioli P, Moreillon P. Expression of Staphylococcus aureus clumping factor A in Lactococcus lactis subsp. cremoris using a new shuttle vector. Infect Immun 2000; 68:3516 - 22; http://dx.doi.org/10.1128/IAI.68.6.3516-3522.2000; PMID: 10816506
- Que Y-A, Haefliger J-A, Piroth L, François P, Widmer E, Entenza JM, et al. Fibrinogen and fibronectin binding cooperate for valve infection and invasion in Staphylococcus aureus experimental endocarditis. J Exp Med 2005; 201:1627 - 35; http://dx.doi.org/10.1084/jem.20050125; PMID: 15897276
- Josefsson E, Higgins J, Foster TJ, Tarkowski A. Fibrinogen binding sites P336 and Y338 of clumping factor A are crucial for Staphylococcus aureus virulence. PLoS One 2008; 3:e2206; http://dx.doi.org/10.1371/journal.pone.0002206; PMID: 18493318
- Vernachio J, Bayer AS, Le T, Chai Y-L, Prater B, Schneider A, et al. Anti-clumping factor A immunoglobulin reduces the duration of methicillin-resistant Staphylococcus aureus bacteremia in an experimental model of infective endocarditis. Antimicrob Agents Chemother 2003; 47:3400 - 6; http://dx.doi.org/10.1128/AAC.47.11.3400-3406.2003; PMID: 14576094
- Tuchscherr LPN, Buzzola FR, Alvarez LP, Lee JC, Sordelli DO. Antibodies to capsular polysaccharide and clumping factor A prevent mastitis and the emergence of unencapsulated and small-colony variants of Staphylococcus aureus in mice. Infect Immun 2008; 76:5738 - 44; http://dx.doi.org/10.1128/IAI.00874-08; PMID: 18809660
- Bloom B, Schelonka R, Kueser T, Walker W, Jung E, Kaufman D, et al, INH-A21 Phase II Study Team. Multicenter study to assess safety and efficacy of INH-A21, a donor-selected human staphylococcal immunoglobulin, for prevention of nosocomial infections in very low birth weight infants. Pediatr Infect Dis J 2005; 24:858 - 66; http://dx.doi.org/10.1097/01.inf.0000180504.66437.1f; PMID: 16220082
- DeJonge M, Burchfield D, Bloom B, Duenas M, Walker W, Polak M, et al. Clinical trial of safety and efficacy of INH-A21 for the prevention of nosocomial staphylococcal bloodstream infection in premature infants. J Pediatr 2007; 151:260 - 5, 265, e1; http://dx.doi.org/10.1016/j.jpeds.2007.04.060; PMID: 17719934
- Hammer ND, Skaar EP. The impact of metal sequestration on Staphylococcus aureus metabolism. Curr Opin Microbiol 2012; 15:10 - 4; http://dx.doi.org/10.1016/j.mib.2011.11.004; PMID: 22153710
- Horsburgh MJ, Wharton SJ, Karavolos M, Foster SJ. Manganese: elemental defence for a life with oxygen. Trends Microbiol 2002; 10:496 - 501; http://dx.doi.org/10.1016/S0966-842X(02)02462-9; PMID: 12419613
- Papp-Wallace KM, Maguire ME. Manganese transport and the role of manganese in virulence. Annu Rev Microbiol 2006; 60:187 - 209; http://dx.doi.org/10.1146/annurev.micro.60.080805.142149; PMID: 16704341
- Karavolos MH, Horsburgh MJ, Ingham E, Foster SJ. Role and regulation of the superoxide dismutases of Staphylococcus aureus. Microbiology 2003; 149:2749 - 58; http://dx.doi.org/10.1099/mic.0.26353-0; PMID: 14523108
- Horsburgh MJ, Wharton SJ, Cox AG, Ingham E, Peacock S, Foster SJ. MntR modulates expression of the PerR regulon and superoxide resistance in Staphylococcus aureus through control of manganese uptake. Mol Microbiol 2002; 44:1269 - 86; http://dx.doi.org/10.1046/j.1365-2958.2002.02944.x; PMID: 12028379
- Sellman BR, Howell AP, Kelly-Boyd C, Baker SM. Identification of immunogenic and serum binding proteins of Staphylococcus epidermidis. Infect Immun 2005; 73:6591 - 600; http://dx.doi.org/10.1128/IAI.73.10.6591-6600.2005; PMID: 16177335
- Anderson AS, Scully IL, Timofeyeva Y, Murphy E, McNeil LK, Mininni T, et al. The Staphylococcus aureus transporter MntC is a highly conserved cell surface protein that elicits protective immunity against both S. aureus and Staphylococcus epidermidis. J Infect Dis 2012; 205:1688 - 96; http://dx.doi.org/10.1093/infdis/jis272; PMID: 22474033
- Nanra JS, Timofeyeva Y, Buitrago SM, Sellman BR, Dilts DA, Fink P, et al. Heterogeneous in vivo expression of clumping factor A and capsular polysaccharide by Staphylococcus aureus: implications for vaccine design. Vaccine 2009; 27:3276 - 80; http://dx.doi.org/10.1016/j.vaccine.2009.01.062; PMID: 19200819
- Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol 2010; 17:1055 - 65; http://dx.doi.org/10.1128/CVI.00131-10; PMID: 20463105
- Usinger WR, Lucas AH. Avidity as a determinant of the protective efficacy of human antibodies to pneumococcal capsular polysaccharides. Infect Immun 1999; 67:2366 - 70; PMID: 10225896
- Richmond P, Nissen M, Marshall H, Shakib S, Hodsman P, Jiang Q, et al. A randomised, placebo controlled Phase 1 first-in-human study of a novel 3-antigen Staphylococcus aureus vaccine in healthy adults. ECCMID. Milan, Italy, 2011.
- Hawkins J, Kodali S, Matsuka Y, McNeil L, Mininni T, Dodge I, et al. A recombinant Clumping factor A containing vaccine induces a functional response to Staphylococcus aureus that is not observed with natural exposure. Clin Vaccine Immunol 2012; In press http://dx.doi.org/10.1128/CVI.00354-12; PMID: 22896688
- Laupland KB, Church DL, Mucenski M, Sutherland LR, Davies HD. Population-based study of the epidemiology of and the risk factors for invasive Staphylococcus aureus infections. J Infect Dis 2003; 187:1452 - 9; http://dx.doi.org/10.1086/374621; PMID: 12717627
- Jensen AG, Wachmann CH, Poulsen KB, Espersen F, Scheibel J, Skinhøj P, et al. Risk factors for hospital-acquired Staphylococcus aureus bacteremia. Arch Intern Med 1999; 159:1437 - 44; http://dx.doi.org/10.1001/archinte.159.13.1437; PMID: 10399895
- DeLeo FR, Chambers HF. Reemergence of antibiotic-resistant Staphylococcus aureus in the genomics era. J Clin Invest 2009; 119:2464 - 74; http://dx.doi.org/10.1172/JCI38226; PMID: 19729844
- Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, et al, Active Bacterial Core surveillance (ABCs) MRSA Investigators. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 2007; 298:1763 - 71; http://dx.doi.org/10.1001/jama.298.15.1763; PMID: 17940231
- Thurlow LR, Joshi GS, Richardson AR. Virulence strategies of the dominant USA300 lineage of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA). FEMS Immunol Med Microbiol 2012; 65:5 - 22; http://dx.doi.org/10.1111/j.1574-695X.2012.00937.x; PMID: 22309135
- Faden H, Lesse AJ, Trask J, Hill JA, Hess DJ, Dryja D, et al. Importance of colonization site in the current epidemic of staphylococcal skin abscesses. Pediatrics 2010; 125:e618 - 24; http://dx.doi.org/10.1542/peds.2009-1523; PMID: 20156893
- Miller LG, Kaplan SL. Staphylococcus aureus: a community pathogen. Infect Dis Clin North Am 2009; 23:35 - 52; http://dx.doi.org/10.1016/j.idc.2008.10.002; PMID: 19135915
- Montgomery CP, Boyle-Vavra S, Adem PV, Lee JC, Husain AN, Clasen J, et al. Comparison of virulence in community-associated methicillin-resistant Staphylococcus aureus pulsotypes USA300 and USA400 in a rat model of pneumonia. J Infect Dis 2008; 198:561 - 70; http://dx.doi.org/10.1086/590157; PMID: 18598194
- Sutter DE, Summers AM, Keys CE, Taylor KL, Frasch CE, Braun LE, et al. Capsular serotype of Staphylococcus aureus in the era of community-acquired MRSA. FEMS Immunol Med Microbiol 2011; 63:16 - 24; http://dx.doi.org/10.1111/j.1574-695X.2011.00822.x; PMID: 21631600