Abstract
In 2002, the first Chinese domestic preservative-free inactivated hepatitis A vaccine, Healive®, was introduced in China. It is highly immunogenic, and provides lasting protection in healthy individuals and generates protective levels of antibodies in other at-risk individuals. Over 10 years since its first licensure, postmarketing surveillance data have confirmed the outstanding safety profile of the vaccine. Comparative clinical trials indicated that Healive® induce equal or similar immunogenicity with other currently available inactivated hepatitis A vaccines and are interchangeable for the course of HAV immunization in Chinese children. The vaccine is effective in curbing outbreaks of hepatitis A due to rapid seroconversion and the long incubation period of the disease. Additional issues surrounding the use of the vaccine are also reviewed.
In 2001, a new preservative-free inactivated vaccine against hepatitis A virus (HAV) infection Healive® produced by Sinovac Biotech Co. Ltd., was licensed in China. Since then, this vaccine has covered 31 provinces, autonomous regions and municipalities directly under the Central Government and nearly 30 million doses have been given to over 15 million vaccinees. More than 20 clinical trials of its immunogenicity, reactogenicity, efficacy and safety have been conducted in more than 7,000 Chinese subjects, making it the most widely-studied Chinese domestic hepatitis A vaccine.
This review summarizes the data accumulated during more than a decade of clinical experience with the vaccine in China. Original research papers, review articles, meeting report and editorials in the public domain were reviewed. The literature included data from clinical trials published since 2001, reviews of the epidemiology of HAV disease and risk-factors for infection, cost-effectiveness studies and current recommendation for vaccination. Information on safety was obtained from the safety monitoring system of Sinovac Biotech through reporting by healthcare professionals. Immunogenicity is measured by the elicited seroconversion rate and the geometric mean concentration (GMC) of antibody to HAV antigen (anti-HAV) measured at intervals after vaccine administration. Seroconversion is defined as the achievement of anti-HAV levels of ≥ 20 mIU/ml, as determined by enzyme-linked immunosorbent assay (ELISA) using commercial Abbott’s AXSYM® HAVAB 2.0 quantification kit. To define a protective antibody response, clinical trials with Healive® have used the level ≥ 20 mIU/ml, reference to other inactivated hepatitis A vaccines.Citation1,Citation2
Hepatitis an Epidemiology in China
Hepatitis A, one of the major public health problems worldwide, is an acute infection of liver caused by the hepatitis A virus (HAV). HAV is responsible for 1.4 million cases of hepatitis A worldwide annually. In China, hepatitis A has long been a public health concern. As early as in 1988, the largest documented hepatitis A outbreak in the world occurred in Shanghai, with more than 300,000 persons infected, and with estimated direct costs of $58 million and indirect costs of $64 million.Citation3 During 1990 to 1992, between 584 353 and 637 717 cases of hepatitis A have been reported, with incidence in these years of 52.6/100,000, 55.7/100,000, and 52.1/100,000, respectively.Citation4 Surveillance data from the early 1990s also show that the highest incidence was in children aged < 10 y (between 100–140/105/yr), followed by persons aged 10–19 y and 20–39 y (less than 40/105/yr).Citation5 Thus, hepatitis A represents a substantial healthcare and economic burden.
In China, the most common recognized modes of transmission of hepatitis A outbreaks are ingestion of food and water, contaminated by hepatitis A virus,Citation6 as is the case in other countries.Citation7 However, many individuals are likely infected through close personal contact with an infected person.Citation8 In order to control hepatitis A infections, an effective live, attenuated hepatitis A vaccine was first licensed for private use (Class 2 vaccine, i.e., not routinely recommended for childhood vaccination, and was available to persons willing to pay for vaccination.) since 1992,Citation9 and inactivated vaccines became available in 2002.Citation10 In 2008, China has integrated hepatitis A vaccines into the National Expanded Immunization Programme and provided free of charge to eligible children beginning at the age of 18 mo. National vaccination against hepatitis A not only is highly effective in preventing both clinical hepatitis and in reducing disease spread, but also resulted in robust health and economic benefits.Citation11 On the other hand, with continuing economic development of China during the past 20 y, the quality of water, food, and sanitation has substantially improved. Therefore, fewer people became infected through water and food, and transmission through close contact was also reduced due to improved sanitation and life style. The economic growth also enhanced the national strength to afford large-scale vaccination for hepatitis A.
According to Emergency Events Reporting System by Chinese CDC, a dramatic decrease in reported hepatitis A cases in China was observed during a recent 20 y period.Citation4 The reported incidence of hepatitis A has decreased rapidly from 56/100,000 in 1991 (584,353 cases) to 2.3/100,000 in 2011 (31,456 cases).Citation11 Areas of high endemicity became areas of intermediate endemicity and regions of intermediate endemicity have prevalent rates previously associated with areas of low endemicity. During 1990 to 1994, the wealthier eastern provinces of China reported higher incidence than those in western China, but the current incidence in eastern China then declined more dramatically and was lower than in western China. In addition, there were no significant national differences in incidence by month or season; however, there was a seasonal increase in hepatitis A outbreaks in April and September each year. For age distribution of hepatitis A incidence, the decline in incidence was seen in all age groups, most dramatically among children younger than 10 y. Hepatitis A incidence remains highest among children younger than 10 y, but is only slightly lower among older age groups. Among reported cases in 2004 to 2007, 118,214 cases were farmers, 75,369 were students, 28,459 were preschoolers, and 90,693 were classified as other, accounting for 37.8%, 24.1%, 9.1% and 29% of cases, respectively.Citation5
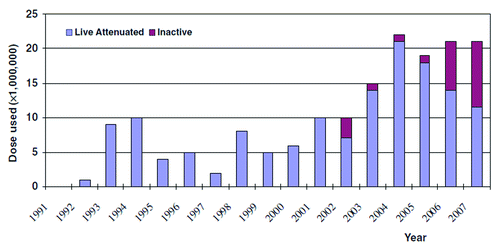
The last two decades have shown that hepatitis A vaccination is now playing a substantial role in the obvious epidemiological shift in HAV infection in China. Between 1992 and 2007, 156 million doses of hepatitis A vaccine were distributed ().Citation5 These vaccines have been used increasingly since 2000 to prevent hepatitis A, mostly for school-aged children. The total vaccine distributed from 2004 through 2007 (18 to 22 million doses each year) was sufficient to vaccinate approximately 1 child birth cohort each year (approximately 16 million children). Studies of population vaccination with hepatitis A vaccine have highlighted the importance of herd immunity, which becomes apparent with coverage of 66% or higher.Citation12,Citation13 Thus, hepatitis A vaccine may have provided significant protection to children, and to the entire population as well.
Introduction of Chinese domestic inactivated hepatitis A vaccine Healive®
Healive® was the first Chinese domestically produced inactivated hepatitis A vaccine and was licensed in China in 2001. It filled a domestic gap in which the commercially inactivated hepatitis A vaccine is entirely dependent on imports before 2001. Sinovac Biotech was the first in China to adopt the international advanced level of the Cell Factory technology for viral culture. The strain TZ84 of hepatitis A virus used for manufacture of Healive® was cultivated in 2BS human fetal lung diploid fibroblast, then harvested, purified by chromatography, inactivated by formalin, and adsorbed onto aluminum hydroxide. This allows large-scale production, and the existence of a single serotype means that one vaccine is effective against all wide type strains. The composition of the vaccine includes HAV whole-virion antigen, aluminum hydroxide, disodium hydrogen phosphate, sodium dihydrogen phosphate, sodium chloride, and water for injection. Healive® was preservative-free, which can eliminate potential health damage of ethyl mercury contained in thimerosal widely used as vaccine preservative and increase public confidence and compliance to immunization practice.
In China, Healive® was licensed at a dose of HAV 250 U/0.5 ml for children from 1 to 16 y of age and 500 U/0.5 ml for subjects aged 16 y and over in a two-dose regimen. A booster dose is recommended at any time between 6 and 12 mo after the primary immunization. There are two commercial packages of the vaccine, including pre-filled syringes and vials. Pre-filled syringes are user-friendly, enable exact dosing, and support safe administration; however, vial packages reduce the cost of the vaccine in order to make it available for children in economically underdeveloped regions.
In 2002, Sinovac Biotech designed, constructed and validated the production plant of the inactivated hepatitis A vaccine in accordance with US. Food and Drug Administration (FDA) and EU Good Manufacture Practices (GMPs) requirements, and finally obtained the GMP certification and production approval by the State Food and Drug Administration (SFDA), China. Since then, Sinovac Biotech continued to develop and improve internal production and quality management system of the manufacture, and again fulfilled the requirement for the manufacture of biological products by revised GMP guideline (2010 Edition) by SFDA. In 2011, the Chinese vaccine regulatory system passed the assessment by World Health Organization (WHO) and was recognized to comply with international standards for vaccine regulation. This means that vaccine manufacturers in China are now eligible to apply to WHO for prequalification of vaccines, and that vaccines produced in China will be eventually supplied through United Nations agencies to developing countries. Therefore, Sinovac Biotech is engaged in putting in place stricter quality management system for vaccine production and plans to apply Healive® for the prequalification by WHO in the next 1–2 y.
Immunogenicity of Healive®
The application for clinical trials of the inactivated hepatitis A vaccine was approved by SFDA in 1998, following shortly the imported inactivated hepatitis A vaccine (Havrix™, GlaxoSmithKline Biologicals.) launched in China. The phase I-III studies have evaluated the immunogenicity of different dosages (1,000 U, 500 U, 250 U and 125 U) of Healive® in healthy adults (aged: 18–22 y old) and children (aged: 5–15 y old) (). The eligibility criteria of subjects included axillary temperature ≤ 37°C, normal alanine aminotranserase (ALT) as well as negative HBsAg and anti-HAV test. The results have shown that ≥ 250 U of this vaccine is highly immunogenic in healthy adults and children, conferring protective immunity against the disease 4 weeks after first administration. When first introduced in 2002, Healive® was recommended at a dose of 250 U/ml for children and 500U/ml for adults in a two-dose administration regimen. And a second dose was recommended at any time 6–12 mo after the primary immunization in order to ensure long-term protection.
Table 1. Representative studies showing the immunogenicity of different dosages of Healive® administered according to different vaccination schedules
Recent data of the seroconversion rates following a single administration of Healive® have indicated that the vast majority of subjects seroconvert within 2 weeks of vaccination,Citation19 well within the 28 d incubation period of the virus. In this clinical trial to evaluate early sero-conversions in the first month following immunization in children, seroconversion rates were 35%, 93% and 100% at 7 d, 14 d and 28 d, respectively. And GMC of anti-HAV antibodies were 7.9, 73.3, and 71.3 mIU/ml, respectively. This rapid response means that protection against HAV can be provided within the incubation period for the infection, which average 28 d. Consequently, successful prevention of disease may also be achieved when the vaccine is administered shortly after exposure.
The immunogenicity of Healive® has been compared with Chinese live attenuated hepatitis A (H2 strain) vaccines within controlled clinical trials. In a randomized, controlled clinical trial in which children received a single dose of either H2 vaccine or Healive®, seroconversion rates were 25% and 35%, respectively, at 7 d; 90% and 93%, respectively, at 14 d; 98% and 100%, respectively, at 28 d, with no significant difference.Citation19 GMC in recipients of H2 vaccine or Healive® were 6.3 and 7.9 mIU/ml, respectively, at 7 d; 42.6 and 73.3 mIU/ml, respectively, at 14 d; 46.8 and 71.3 mIU/ml, respectively, at 28 d. Similarly, both vaccines induce equal immunogenicity in young adults.Citation20 Another trial compared the immunogenicity of Healive® with H2 vaccine in children one year after one injection.Citation21 Twelve months following immunization, 95.2% in the Healive® group had sero-converted, compared with 91.1% in the H2 vaccine group. However, GMC was significantly higher in the children vaccinated with the inactivated vaccine (101.7 mIU/ml) than that in the children vaccinated with the attenuated vaccine (65.5 mIU/ml). These results illustrate that both vaccines are similarly immunogenic in children; however, whether there is the difference in long lasting immunity is still unknown.
Except live attenuated hepatitis A vaccines, a number of clinical trials have also compared the immunogenicity of Healive® with imported inactivated hepatitis A vaccine, Havrix™ (720 EU, GlaxoSmithKline). Currently, both vaccines are the major licensed inactivated vaccine in China. The excellent and similar immunogenicity of the two vaccines has been demonstrated (). Considering that both Healive® and Havrix™ have a good safety profile and relative immunity protection, a randomized clinical trial evaluated the interchangeability of both vaccines in Chinese children.Citation24 Vaccine was administered to 303 healthy children at 0 and 6 mo in one of four vaccine regimens: Healive- Healive Healive- Havrix Havrix- Healive or Havrix- Havrix. Following two-dose regimen, the seroconversion rate was 100% among all groups. The GMC after two doses of Healive was 8,905.5 mIU/ml compared with 1,900.9 mIU/ml after two doses of Havrix (p < 0.001). The GMC in the Healive- Havrix group was 3,275.8 mIU/ml compared with 4,165.8 mIU/ml in the Havrix- Healive group (p = 0.058). The results of this study supported Healive® and Havrix™ are interchangeable for the course of HAV immunization in Chinese children and therefore provide latitude in vaccine choice. Vaccination can be accomplished using the same vaccine for both doses or a combination of vaccines may be administered.
Table 2. Representative studies comparing the immunogenicity of Healive® with Havrix ™
Long-Term Protection
With the availability of Healive® 10 y ago, the persistence of antibodies was estimated to be at least 5 y. Initial serological data from 81 children vaccinated with different two-dose regimen (0, 3 mo; and 0, 6 mo) and subsequently followed to study anti-HAV antibody persistence confirmed that all (100%) subjects remained seropositive 3 y after the first vaccination, with GMCs of 285 and 496 mIU/ml, respectively.Citation25 In a recent follow-up study of 410 healthy children given either 250 U of Healive® or 720 EU of Havrix™ at 0 and 6 mo, all subjects were seropositve at 5 y following the last dose and GMCs were 341 and 412 mIU/ml, respectively, at 3 y, 339 and 222 mIU/ml, respectively, at 4 y, and 261 and 180 mIU/ml, respectively, at 5 y (unpublished data). Considering that many mathematical models have predicted antibodies induced by Havrix™ to persist for at least 20–25 y without the need for periodic boosters,Citation26,Citation27 it is reasonable to deduce the long-term persistence of protection of Healive® in children.
Booster Immunization of Healive®
In China, a live attenuated vaccine against hepatitis A has been developed in 1988 and was then launched in 1992. Live, attenuated hepatitis A vaccine is recommended as a single dose to children older than 18 mo. Until now, more than 100 million Chinese received live attenuated vaccines. Although having low cost and high immunogenicity, they have been gradually replaced by inactivated vaccines due to concerns about the safety. Because live attenuated vaccines induce 44.9–100% of seroconversion rates and vaccine-induced antibodies with only 1 dose were lost rapidly,Citation28,Citation29 an additional booster dose with inactivated vaccines perhaps might be considered as a viable backup plan, especially in the regions with higher HAV prevalence. In an open-labeled study, 70 HAV-seronegative children who had received the live attenuated hepatitis A vaccine within one year were administered a booster dose of Healive®. The results showed that the longer the interval between live attenuated hepatitis A vaccine administration and the Healive® boosting was, the higher the GMC levels of anti-HAV antibodies were.Citation30 This was in accordance with the immune effect of dose of different schedules of Healive®. So, the inactivated hepatitis A vaccine can be used as booster vaccination to children who had received live attenuated hepatitis A vaccine before to induce persistence of vaccine prevention.
Halting Outbreak of HAV Infection
Although hepatitis disease incidence has decreased, local outbreaks remained common, particularly in western provinces. Of the 154 reported outbreaks that occurred in 2004 to 2007, 76 occurred in high-incidence provinces, 44 in intermediate-incidence provinces, and 34 in low-incidence provinces.Citation5 Moreover, with vaccine protection loss, childhood vaccination would postpone natural infections commonly occurring in children to adults, especially older individuals, leading to an increased the probability of HAV outbreak.Citation11 Thus, despite enhanced awareness of preventive measures, hepatitis A outbreaks have been difficult to control. Although widespread post-exposure prophylaxis with IgG may decrease HAV transmission, it does not stop the outbreak.Citation31
In a previous study to investigate the prophylactic use of attenuated hepatitis A vaccine during an outbreak in a village, the live vaccine did not show protective effect, as the infection rate was not significantly different between the vaccinated and control groups. The reason for this might be that the antibody induction period of the attenuated hepatitis A vaccine is relatively long and the seroconversion reaches a peak 2 or 3 mo after injection.Citation32 In contrast, the antibody induction period of inactivated hepatitis A vaccine is as short as 2 weeks, and the seroconversion reaches a peak one month after administration.Citation19 It is generally recognized that inactivated hepatitis A vaccine could provide good protection against hepatitis A after exposure to HAV.
The successful use of Healive® to control an outbreak of HAV in underdeveloped cities and rural areas has been reported.Citation33 In Sep 2004, 172 cases of acute hepatitis A were reported in 15 rural communities in Xiangshan country. Among these, 66.9% (115/172) were school-age children. An epidemiological survey showed the majority of students were estimated to be at risk of acquiring HAV infection, with only 22.7% being seropositive. Within 15 d, 51 305 susceptible children (75.5% of in-school students) were vaccinated and then the peak of HAV outbreak began to decrease in 2 weeks after the primary injection. Thus, a single dose of Healive® can halt community outbreak of hepatitis A if enough susceptible individuals are vaccinated and can also prevent an epidemic from becoming established in communities where only few cases of HAV infection have occurred.
The effectiveness of the vaccine was further evaluated during an epidemic in Jiaxing country in 2006.Citation34 Two nonrandomized controlled trials were conducted. In the first trial, inactivated hepatitis A vaccine, Healive® was administered to the 108 individuals with close contacts of 25 hepatitis A patients reported in September as post-exposure measure. Meanwhile, 115 close contacts of 44 hepatitis A patients reported from June to August were regarded as the untreated control. Within the 60 d observation period, 4 subjects (4/115) in the non-intervention group developed clinical symptoms and were diagnosed with hepatitis A. In the intervention group, the earliest and latest emergency injection of the vaccine was given 1 d and 31 d post contact (the median was 12 d). No hepatitis A cases (0/108) were observed in the intervention group during the 60 d observation period. The protective efficacy was 100% in those with close contacts. The second trial was to evaluate the ability of a massive vaccination of students to prevent the outbreak. The vaccination program was performed in 3,365 students who were given one dose of Healive® served as the vaccine group, with 2,572 students who did not receive the vaccines as the control group. After the vaccination for 3,365 students in early September 2006, only one hepatitis A case was reported 3 d after injection, which was excluded from the incidence analysis. Another 4 cases were detected between September and December 2006 in the control group of 2,572 students. The protective efficacy was 100% in students who received vaccination.
Protection of HBsAg Positive Children
Acute HAV infection superimposed on chronic hepatitis B infection is associated with more severe disease and a higher case fatality rate than acute HAV infection alone. During the epidemic in Shanghai, China in 1988, 15 deaths were reported due to HAV superimposed on HBV infection.Citation3 A clinical trial has evaluated the safety and immunogenicity of Healive® in HBsAg positive and anti-HAV antibody negative children, without ALT abnormality and clinical symptoms.Citation35 42 children aged 4–10 y old were divided into four groups to be administered the 250U dose vaccine according to 0 and 1, 0 and 3, 0 and 6, 0 and 12 mo schedules, respectively. The positive seroconversion rates of anti-HAV antibody were 100% in all four groups one month after the first dose. No significantly statistical differences were observed in the immune response between HBsAg positive children and the healthy children of the same age one month after two-dose vaccination. Most adverse reactions were mild and ALT levels were not significantly increased at any time during follow-up. Thus, Healive® was indicated to be safe and effective to HBsAg positive children.
Cost-Effectiveness
Economic model assessments in some developed and developing countries showed that widespread childhood vaccination could be cost-effective,Citation36-Citation38 and immunization programs in several populations have significantly reduced hepatitis A rates. In 2008, an analysis using the Markov model was performed to evaluate whether universal childhood hepatitis A vaccination is advisable now in China.Citation11 In the study, a single cohort of 1,000,000 healthy children was enrolled in 2005 and administered the Chinese inactivated vaccine with vaccination scheduled at ages 12 and 18 mo. The authors compared vaccination with no vaccination using the identical model structure, and evaluated the cost-effectiveness of vaccination from the health system and the societal perspectives. The analysis was run separately in five regions (covering all the 31 provinces of Mainland China) defined by anti-HAV prevalence (around 50%, 50–69%, 70–79%, 80–89% and 90%-). The study projects that with the Chinese low-cost vaccine, vaccination could gain quality adjusted life years (QALYs) through the whole country and save health system or societal costs in the lowest, lower, intermediate and higher infection regions. Vaccination should also be cost-effective in the highest infection region because of low additional costs per QALY gained. The trend that the lower infection level the region has, the more cost-effective vaccination would be is obvious.
Safety and Tolerability of Healive®
The majority of vaccinees experience no undesirable effects. In 21 postlicensure studies conducted in 16 cities, involving 6,736 subjects given 12,162 doses of vaccine, 287 vaccinees reported systemic and local adverse reactions and no serious adverse reactions were observed in the 3 d following injection (). Most reactions were mild and resolved spontaneously and included fever and injection site pain. The post-marketing safety monitoring system of Sinovac Biotech noted 70 spontaneous reports of adverse reactions from 2002 May to 2011 Dec during which approximately 15 million subjects received 2.85 million doses, with the frequency of adverse reactions less than 0.25/106 doses. Of these, 68 adverse reaction reports, including 11 serious adverse reactions, came from children given 2.72 million doses; and 2 reports without serious adverse reactions came from adults given 0.13 million doses.
Table 3. Representative studies evaluating the safety and tolerability of Healive® with Havrix™
Domestic and International Market Expansion
Since the implementation of the batch release by SFDA, China, in 2006, the total amount of hepatitis A vaccine batch release substantially increased from 8.89 million in 2006 to 32.64 million in 2009. The amount of inactivated hepatitis A vaccine batch release was 5.88 million doses in 2006 and 13.93 million doses in 2009, with an increase of 1.5 times. During the period, a total of 24.16 million (i.e., 3.17 million in 2006, 7.76 million in 2007, 5.93 million in 2008, and 7.30 million in 2009) Healive® has been approved for batch release, accounting for 57.1% percent of the total amount of inactivated hepatitis A vaccines, and the batch release amount of four consecutive years ranked the first. The production capacity of inactivated hepatitis A vaccine Healive® has reached 20 million / year.
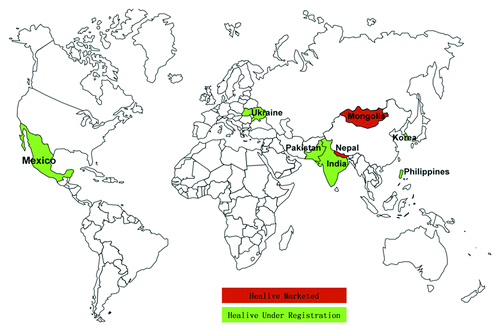
With the successful increases in domestic market, Sinovac started to explore international market opportunities with Healive® since 2008. Presently Healive® has been marketed in Nepal and Mongolia in 2009. Since then, more than 250,000 doses of Healive® have been distributed in these two countries. No severe adverse reactions have been reported to date. In addition, Healive® is being registered in other seven countries (as shown in ), most of which are developing or less developed countries in South East Asia, Africa, Southern America and West Pacific regions.
The recommendations For Use in The Future
Over the past decade, China has been experiencing an epidemiological shift from high- to intermediate-hepatitis A endemicity. Fewer young children are infected due to universal vaccination, but because relatively high levels of circulating hepatitis A virus persist, the disease burden from hepatitis A paradoxically increases as greater numbers of older children and adults who lack immunity become infected and have symptomatic illness. This is a global occurrence and disease outbreaks associated with this phenomenon have been seen in South America, central Asia, and elsewhere. In some areas with low incidences in China, hepatitis A incidence peak begins to shift to individuals with 20–40 y old.Citation48,Citation49 Infection in young children is generally asymptomatic, while in older children and adults, infection is usually accompanied by symptoms, and can lead to severe illness. Previous studies have shown that HAV infection in adulthood is symptomatic in 75% to 90% of cases, and case fatality rates exceed 2.1% among those aged 40 y or older.Citation50,Citation51 Thus, although levels of endemicity fall, hepatitis A can impose a serious healthcare and economic burden. Strategies for catch-up immunization may be considered as components of universal immunization programs. However, currently available data from the United States, Israel, and some European countries suggest that there is a significant herd-immunity effect associated with immunization of young children against hepatitis A. The programmers for catch-up immunization will await further surveillance to determine if the indirect effect on older, nonimmunized groups continues.
Overall, future directions in the development of Healive® are therefore likely to focus on: continued evaluation of safety for at-risk individuals, maximizing the potential for flexibility of the vaccination schedule, exploring the effectiveness of a single-dose administration and the continuing development of combination vaccines, such as the combined hepatitis A and hepatitis B vaccine, Bilive™. Such combination vaccines increase the comfort of vaccines, have the potential to reduce costs associated with vaccination, and contribute to further expanding the potential applications of this vaccine.
| Abbreviations: | ||
| ALT | = | alanine aminotranserase |
| ELISA | = | enzyme-linked immunosorbent assay |
| FDA | = | Food and Drug Administration |
| GMC | = | geometric mean concentration |
| GMP | = | good manufacture practices |
| HAV | = | hepatitis A virus |
| QALY | = | quality adjusted life years |
| SFDA | = | State Food and Drug Administration |
| WHO | = | World Health Organization |
Acknowledgments
The authors would like to express their special thanks to all department of Sinovac who contributed the original data that has been compiled since 2002. All authors were currently employed by Sinovac Biotech Co., LTD.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Reference
- André F, Van Damme P, Safary A, Banatvala J. Inactivated hepatitis A vaccine: immunogenicity, efficacy, safety and review of official recommendations for use. Expert Rev Vaccines 2002; 1:9 - 23; http://dx.doi.org/10.1586/14760584.1.1.9; PMID: 12908508
- Van Herck K, Van Damme P. Prevention of hepatitis A by Havrix: a review. Expert Rev Vaccines 2005; 4:459 - 71; http://dx.doi.org/10.1586/14760584.4.4.459; PMID: 16117704
- Xu ZY, Li ZH, Wang JX, Xiao ZP, Dong DX. Ecology and prevention of a shellfish-associated hepatitis A epidemic in Shanghai, China. Vaccine 1992; 10:Suppl 1 S67 - 8; http://dx.doi.org/10.1016/0264-410X(92)90547-W; PMID: 1335663
- National Bureau of Statistical of China. China Statistical Yearbook in 2007. China Statistic Press, Beijing, 2008.
- Cui F, Hadler SC, Zheng H, Wang F, Zhenhua W, Yuansheng H, et al. Hepatitis A surveillance and vaccine use in China from 1990 through 2007. J Epidemiol 2009; 19:189 - 95; http://dx.doi.org/10.2188/jea.JE20080087; PMID: 19561383
- Zhang LJ, Wang XJ, Bai JM, Fang G, Liu LG, Zhang Y, et al. An outbreak of hepatitis A in recently vaccinated students from ice snacks made from contaminated well water. Epidemiol Infect 2009; 137:428 - 33; http://dx.doi.org/10.1017/S0950268808001337; PMID: 18817585
- Barrimah E, Salem KA, Gabal MS. An outbreak of hepatitis A associated with treated waste water used for irrigation. J Egypt Public Health Assoc 1999; 74:227 - 39; PMID: 17219868
- Zheng H, Lu Y, Wang FZ, Cui FQ. Epidemiological Analysis on hepatitis A in China during 2004–2006. Chinese Journal of Vaccines and Immunization. 2007; 13:336 - 40
- Mao JS, Chai SA, Xie RY, Chen NL, Jiang Q, Zhu XZ, et al. Further evaluation of the safety and protective efficacy of live attenuated hepatitis A vaccine (H2-strain) in humans. Vaccine 1997; 15:944 - 7; http://dx.doi.org/10.1016/S0264-410X(96)00304-0; PMID: 9261939
- Liu CB, Ren YH, Zhang YC, Wu WT, Li SP, Kang WX, et al. The study on immunogenicity, safety and vacciantion schedule of a new inactivated hepatitis A vaccine in Chinese Children. [Chinese] Chinese Journal of Vaccine and Immunization 2002; 8:1 - 3
- Zhuang GH, Pan XJ, Wang XL. A cost-effectiveness analysis of universal childhood hepatitis A vaccination in China. Vaccine 2008; 26:4608 - 16; http://dx.doi.org/10.1016/j.vaccine.2008.05.086; PMID: 18597903
- Wasley A, Samandari T, Bell BP. Incidence of hepatitis A in the United States in the era of vaccination. JAMA 2005; 294:194 - 201; http://dx.doi.org/10.1001/jama.294.2.194; PMID: 16014593
- Averhoff F, Shapiro CN, Bell BP, Hyams I, Burd L, Deladisma A, et al. Control of hepatitis A through routine vaccination of children. JAMA 2001; 286:2968 - 73; http://dx.doi.org/10.1001/jama.286.23.2968; PMID: 11743837
- Ren YH, Wu WT, Zhang YC, Xue WH, Kang WX, Ren YF, et al. The study on immunogenicityof low-dose inactivated Chinese hepatitis A vaccine. Chinese Journal of Vaccine and Immunization 2003; 9:114 - 6
- Ren YH, Chen JT, Wu WT, Gong XJ, Zhang YC, Xue WH, et al. [The study on the 0, 12 month vaccination schedule’ of Healive inactivated hepatitis A vaccine in children]. [Chinese] Zhonghua Liu Xing Bing Xue Za Zhi 2003; 24:1013 - 5; PMID: 14687502
- Ren YH, Chen JT, Ma SD, Xue WH, Zhang YC, Wu WT, et al. The study on immunogenicity of the low-dose inactivated domestic HAV vaccine. [Chinese] Chinese Journal of Vaccine and Immunization 2002; 8:327 - 9
- Ren AG, Ma JR, Feng FM, Xu YJ, Liu CB. Safety and immunogenicity of a new inactivated hepatitis A caccine. [Chinese] Chinese J Exp Clin Virol 2001; 15:357 - 9
- Ren AG, Feng FM, Ma JR, Xu YJ, Liu CB. Immunogenicity and safety of a new inactivated hepatitis A vaccine in young adults: a comparative study. [Chinese] Chin Med J (Engl) 2002; 115:1483 - 5; PMID: 12490092
- Zheng H, Chen Y, Wang F, Gong X, Wu Z, Miao N, et al. Comparing live attenuated and inactivated hepatitis A vaccines: an immunogenicity study after one single dose. Vaccine 2011; 29:9098 - 103; http://dx.doi.org/10.1016/j.vaccine.2011.08.078; PMID: 21875638
- Liao Z, Liu XE, Wang X, Chen HY, Liu Y, Feng XW, et al. Immunogenicity and safety of inactivated and live attenuated hepatitis A vaccines in Chinese young adults. [Chinese] Chin J Viral Dis 2012; 2:242 - 6
- Zhang ZL, Zhu XJ, Wang X, Liang M, Liu Y, Yang Q, et al. Anti-HAV immune response in young children vaccinated with an inactivated and a live attenuated hepatitis A vaccine respectively. [Chinese] Chin J Viral Dis 2011; 1:64 - 6
- Ren YH, Chen JT, Wu WT, Gong XJ, Zhang YC, Wang JH, et al. Evaluation on different immunization schedules of hepatitis A vaccine Healive®. [Chinese] Chinese Journal of Vaccine and Immunization 2003; 9:219 - 21
- Jiang WP, Chen JT, Wang X, Wang YL, Liu Y, Chen WY, et al. Immunogenicity and safety of three consecutive lots of a new preservative-free inactivated hepatitis A vaccine (Healive): a double-blind, randomized and controlled trial. Vaccine 2008; 26:2297 - 301; http://dx.doi.org/10.1016/j.vaccine.2007.11.008; PMID: 18395305
- Zhang ZL, Zhu XJ, Wang X, Liang M, Sun J, Liu Y, et al. Interchangeability and tolerability of two inactivated hepatitis A vaccines in Chinese children. Vaccine 2012; 30:4028 - 33; http://dx.doi.org/10.1016/j.vaccine.2012.04.038; PMID: 22537990
- Ren YH, Chen JT, Zhang YC, Ma SD, Xue WH, Gong XJ, et al. A 3-year observation of a Healive inactivated hepatitis A vaccine in young children. [Chinese] Chin J Prev Med 2004; 5:102 - 3
- Van Herck K, Van Damme P. Inactivated hepatitis A vaccine-induced antibodies: follow-up and estimates of long-term persistence. J Med Virol 2001; 63:1 - 7; http://dx.doi.org/10.1002/1096-9071(200101)63:1<1::AID-JMV1000>3.0.CO;2-U; PMID: 11130881
- Bovier PA, Bock J, Loutan L, Farinelli T, Glueck R, Herzog C. Long-term immunogenicity of an inactivated virosome hepatitis A vaccine. J Med Virol 2002; 68:489 - 93; http://dx.doi.org/10.1002/jmv.10244; PMID: 12376955
- Zhang SY, Liu XL, Ding YX, Xia JL, Hou T, Zhang Y. Comparetive study on the immune effects of three kinds of China-made live attenuated hepatitis A vaccines. [Chinese] Chinese Journal of Vaccines and Immunization 2001; 7:38 - 9
- Dong DX, Cao YY. Research and development of live attenuated hepatitis A vaccine (H2 strain). [Chinese] Chinese Journal of Vaccines and Immunization 2001; 7:178 - 82
- Ren YH, Kang WX, Ren YF, Wu WT, Han LJ, Xue WH, et al. Results of boosting of inactivated hepatitis A vaccine Healive. [Chinese] Chinese Journal of Vaccines and Immunization 2003; 9:291 - 2
- Shaw FE Jr., Sudman JH, Smith SM, Williams DL, Kapell LA, Hadler SC, et al. A community-wide epidemic of hepatitis A in ohio. Am J Epidemiol 1986; 123:1057 - 65; PMID: 3706276
- Wang X, Ma J, Xu Z, Liu H, Zhang Y, Han C. [Effectiveness of post-exposure prophylaxis using live attenuated hepatitis Alpha vaccine (H(2) strain) among schoolchildren]. [Chinese] Zhonghua Yi Xue Za Zhi 2002; 82:955 - 7; PMID: 12181087
- Xu JR, Ye Z, Yang CJ, Zheng GY, Gao L. Research on control of the outbreak of hepatitis A using inactivated hepatitis A vaccine. [Chinese] Chinese Journal of Vaccine and Immunization 2007; 13:66 - 8
- Shen YG, Gu XJ, Zhou JH. Protective effect of inactivated hepatitis A vaccine against the outbreak of hepatitis A in an open rural community. World J Gastroenterol 2008; 14:2771 - 5; http://dx.doi.org/10.3748/wjg.14.2771; PMID: 18461664
- Ren YH, Chen JT, Ren YF, Gong XJ, Wu WT, Wang JH, et al. Vaccination of Healive inactivated hepatitis A vaccine to HBsAg positive children. [Chinese] Chinese Journal of Vaccine and Immunization 2004; 2:44 - 6
- Rein DB, Hicks KA, Wirth KE, Billah K, Finelli L, Fiore AE, et al. Cost-effectiveness of routine childhood vaccination for hepatitis A in the United States. Pediatrics 2007; 119:e12 - 21; http://dx.doi.org/10.1542/peds.2006-1573; PMID: 17200237
- Lopez E, Debbag R, Coudeville L, Baron-Papillon F, Armoni J. The cost-effectiveness of universal vaccination of children against hepatitis A in Argentina: results of a dynamic health-economic analysis. J Gastroenterol 2007; 42:152 - 60; http://dx.doi.org/10.1007/s00535-006-1984-x; PMID: 17351805
- Rosenthal P. Cost-effectiveness of hepatitis A vaccination in children, adolescents, and adults. Hepatology 2003; 37:44 - 51; http://dx.doi.org/10.1053/jhep.2003.50016; PMID: 12500187
- Jia XY, Liu D, Lv ZZ, Zhang P, Zhang XM, Gao XQ, et al. Study on safety and immunogenicity of Healive inactiveted hepatitis A vaccine used among children. [Chinese] Chinese Journal of Vaccine and Immunization 2004; 10:213 - 4
- Yao J, Wang CZ, Fang CF, Li Q, Xu X. Study on the safety and immunogenicity of China-made inactivated hepatitis A vaccine Healive. [Chinese] Chinese Journal of Vaccine and Immunization 2004; 10:89 - 90
- Zhao YL, Chen YG, Li J, Han GX, Tian C, Liang JL, et al. Safety and immunogeicity of BiliveTM combined hepatitis A and B vaccine. [Chinese] Chin J Epidemiol 2004; 25:470 - 3
- Wan ZJ, Zhang HY, Liang ZL, Li RC, Zhao YL, Bi SL. Safety and immunogenicity of a combined hepatitis A and Hepatitis B-BiliveTM vaccine in healthy adult volunteers. [Chinese] Chinese Journal of Vaccine and Immunization 2004; 10:129 - 32
- Liang GQ, Guo ZB, Zhang ML. Observation on tolerability and immunogenicity of Healive inactivated hepatitis A vaccine. [Chinese] China Preventive Medicine 2006; 7:336 - 8
- Qin YD, Jiang XK. Safety observation of domestic inactivated hepatitis A vaccine in young children. [Chinese] China Medical Herald 2007; 4:132
- Fei XZ. Observation on safety and immunogenicity of Healive inactivated hepatitis A vaccine. [Chinese] Chin J Nat Med 2007; 9:333 - 5
- Liang J, Tang Y, Liu XE, Liu Y, Xia YH, Su JL, et al. Study on Safety and Immunogenicity of Supplementary Immunization of both Inactivated Hepatitis A Vaccine and Combined Hepatitis A and Hepatitis B Vaccine. [Chinese] S China J Prev Med 2012; Accepted
- Jiang M, Li B, Peng SQ, Wang YL, Wang CH, Cui WH. Study on Safety and Immunogenicity of Supplementary Immunization of both Inactivated Hepatitis A Vaccine and Combined Hepatitis A and B Vaccine of 24 to 38-month-old healthy children. [Chinese] Chin J Prev Med 2012; Accepted
- Qin JN, Gao XY, Gao B, Lu Y, Zhao XX. A seroprevalence of anti-HAV IgG in the adults of Beijing city. [Chinese] Chinese Journal of Health Laboratory Technology 2011; 21:1775 - 6
- Wu XD, Li WF, Yang SY, Liu XE, Yang J, Zhou B, et al. The prevalent rate of anti-HAV IgG in normal adults over 20 years old in Guangzhou in 2008. [Chinese] J Trop Med 2010; 10:551 - 2
- Ansaldi F, Bruzzone B, Rota MC, Bella A, Ciofi degli Atti M, Durando P, et al, Serologic Study Group. Hepatitis A incidence and hospital-based seroprevalence in Italy: a nation-wide study. Eur J Epidemiol 2008; 23:45 - 53; http://dx.doi.org/10.1007/s10654-007-9198-y; PMID: 17978852
- Lopez E, Debbag R, Coudeville L, Baron-Papillon F, Armoni J. The cost-effectiveness of universal vaccination of children against hepatitis A in Argentina: results of a dynamic health-economic analysis. J Gastroenterol 2007; 42:152 - 60; http://dx.doi.org/10.1007/s00535-006-1984-x; PMID: 17351805