Background
Infectious diseases are responsible for a large percentage of the burden of disease in any region, and contribute markedly to death rates worldwide.Citation1 Vaccines are effective prophylaxis and can assist in curtailing death, and burdens on health care providers. According to the World Health Organization, Department of Immunization, Vaccines and Biologicals, aside from water sanitation measures, no other method of prophylaxis or treatment has had as substantial an effect on overall mortality or population growth as vaccines do.Citation1 As such the importance of vaccine research cannot be over-stressed.
Recent research has highlighted the practice of dose extrapolation when prescribing medicines for children (ages 18 and below). This is due to a lack of toxicology, safety and efficacy data in this population and suggests that children are “therapeutic orphans.”†,Citation2-Citation5 Young people are developmentally different from adults, and therefore are likely to have reactions emerge in the course of treatments that cannot be anticipated from research done on adults.Citation5,Citation6 The fact that healthcare workers are often compelled to use medicines that are not licensed for use in children or to use medicines at a different dose, or for a different indication, or by an alternative route from that recommended has led clinicians to call on researchers to conduct more age-appropriate, evidence-based research of medical interventions and vaccines for young people.Citation2,Citation3,Citation7-Citation9
Attention and money are being directed toward addressing this knowledge gap with funding aimed at increasing child-focused trial activity through legislation (in the US, and the UK) granting pharmaceutical companies financial and patent extension incentives for clinical trials that include young people as participants.Citation10-Citation12
Trials are increasingly being conducted in resource constrained areas due the relatively low costs of conducting research in these settings, as well as the relative ease in recruiting participants.Citation13-Citation15 It should come as no surprise that young people in Africa are increasingly being recruited to participate in clinical research. And yet, a longitudinal analysis of the African trial landscape with regard to young people has not been undertaken as the data are spread across a number of registries, journals and institutions, and in some cases, not registered or published at all, but in the hands of principal investigators or in the format of unpublished research reports.
While an overview has yet to be reported, disease-specific analysis of research taking place in Africa does exist. Siegfried and colleagues’ analyzed African HIV/AIDS trials conducted on the continent prior to 2004Citation16; their data reveals a paucity of trials in children and adolescents.Citation17 In contrast to the HIV/AIDS trials, research on malaria trials conducted in Africa shows that the inclusion of children as participants occurred at higher percentages (almost 70%) than those focusing only on adults.Citation18 Aside from a lack of knowledge on the drugs and interventions used for young people, there is little understanding of what child-focused research is being conducted on the African continent.Citation7 If you pair this issue with that of under-registration of trials in Africa, this picture only partly outlines the landscape of clinical trials with regard to young people in Africa.Citation19
Clinical Trial Registries can provide a broad understanding of the current research landscape. In 2009, the Pan African Clinical Trials Registry became the first (and presently only) African member of the World Health Organization’s (WHO) Network of Primary Registries. A registry database can provide a resource for information on what trials are planned, ongoing or completed in the region. Registries that are members of the WHO Network provide data to a central search engine on the WHO International Clinical Trials Registry Platform (ICTRP). Searching the ICTRP and PACTR databases can assist researchers in determining where research they intend to undertake is repeating efforts already in progress, as well as providing a resource to explore potential collaborations, and to locate gaps in research efforts. A search of the PACTR database and the ICTRP can provide a more detailed picture of vaccine research on the continent, while data from qualitative studies on young people involved in vaccine research can add depth to our understanding of the experiences of young people participating in such vaccine trails.
The Outline—Vaccine Research in Africa
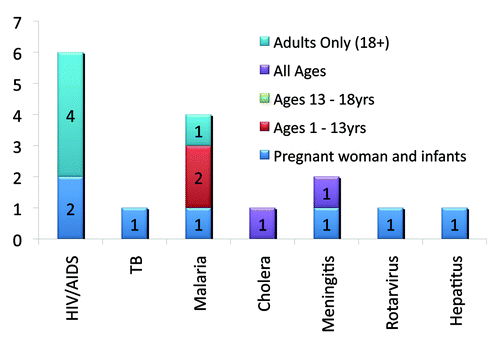
There are 16 vaccine trials registered on the PACTR database (14% of all registered trials), 11 of which are conducted in participants less than 18 y of age (see ). Of the remaining five trials, recruiting adult participants, four vaccine trials are HIV/AIDS related, and one explores malaria prophylaxis.
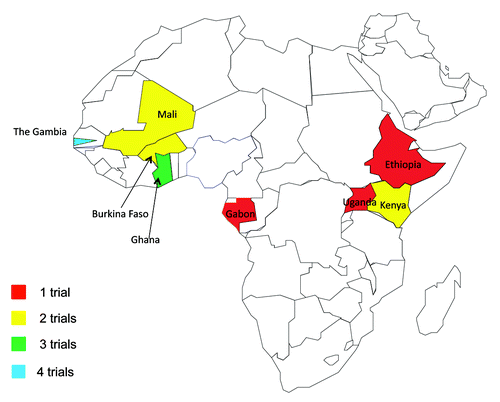
Of the 11 trials recruiting participants younger than 18, three trials research malaria, two trials each explore HIV/AIDS and meningitis prevention, and one trial each researching cholera, hepatitis B, rotavirus and tuberculosis, which is fairly reflective of the disease burden in the region. Nine of these trials are single-centered: Gambia (3), Kenya (2), Mali (2), Ethiopia and Ghana. The remaining two trials are multi-centered, with research sites in Burkina Faso (2), Ghana (2), Gabon, Gambia and Uganda (see Map 1, ).
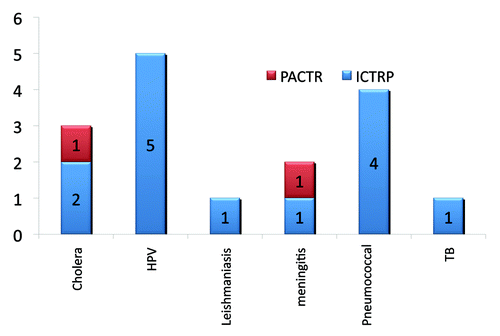
In searching the WHO ICTRP to locate further African-based trials starting from 2008, there are 46 registered vaccine trials with participants 18 y old or younger; 14 of which recruited participants between the ages of 10 and 18. Of the 14 trials recruiting adolescent participants five trials explore HPV vaccines, four research pneumococcal conjugate vaccines, two cholera vaccines, and one trial each exploring vaccines for tuberculosis, leishmaniasis and meningitis. Of 46 trials registered between 2008 and 2012, 16 trials are currently recruiting participants younger than 18 y that were not registered on the PACTR database. Of these 16 trials, only three recruit participants older than 10 y of age: two trials evaluate safety and tolerability of a pneumococcal conjugate vaccine and one pilot trial explores delivery, uptake and acceptability of an HPV vaccine.
By searching the PACTR database, and augmenting that by searching the ICTRP, one can develop a broad understanding of the trials underway and planned, thereby developing insight on the clinical trial landscape in Africa (see ). The results of our evaluation highlight that vaccine research is being conducted on participants younger than 18 y of age, and that approximately 30% of those trials recruit adolescents. Since research shows that the period of adolescence often coincides with sexual debut, and this period indicates risk of exposure to sexually transmitted diseases, it is consistent that the majority of vaccines in this sample explore prophylaxis for STDs and HIV-related co-morbidities (pneumonia).Citation20 Further, the other diseases researched reflect the disease burden in the region (cholera, tuberculosis) as well as the age of the participant population (meningitis). With this data on the vaccine trial landscape, we can confirm that the diseases researched reflect the population in question, however, there is very little reported about the experience of young people involved in clinical trials.
Figure 3. ICTRP and PACTR vaccine data—disease type researched. All trials conducted with participants between the ages of 10–18.
Qualitative exploration of the vaccine trial experience among adolescent participants can assist in developing a friendly environment for adolescent participants. This will assist in ensuring recruitment is feasible, retention levels are high, and the trial is appropriately understood by those participating.
Coloring it in—Qualitative Research on Adolescent Trial Experiences
Research focusing on adolescent participants in clinical trials is available for the sub-Saharan region.Citation21-Citation24 Qualitative data, in most cases, has focused on preparedness among adolescents to partake in vaccine research – exploring methods of, and potential capacity for recruitment which can provide valuable insight into the motivations for participation, and the viability of vaccine trials.Citation24,Citation25 Less common has been research on the actual experience of adolescents participating in a vaccine trial; this research can unpack important aspects of trial participation including not only motivations for participation, but perceived benefits, obstacles to adherence, the potential harms of trial participation, and details of the actual lived experience as a participant.
In a pilot study of adolescent vaccine trial participants, the topics and issues that were most pertinent to their experience were explored.Citation23 This research identified the importance of social connections for recruitment to trials, similar to other report findings.Citation24,Citation25 Adolescents were informed of the potential to participate and were motivated to participate through parental or peer encouragement. Motivations to participate in the vaccine trial were similar to previous reports, and included altruism, perceived access to care, and health seeking (blood testing).
Unlike preparedness studies, this study provided rich details of the emotions, thoughts and understandings of adolescent vaccine trial participants with regard to their participation.Citation23 Trial participants and researchers explored memories of emotions from the first day on the trial site, uncovering that fear and excitement were both salient to the experience.Citation23 Participants recalled fears of needles, and blood test results, leading trial staff to realize the importance of pre-test counseling for what they had previously understood as routine blood collection.
The research highlighted the importance of trial staff in participant retention, and provided insight into what trial participants learned about the disease being researched through their participation. The study provided further insight into the experience of these vaccine trial participants by uncovering misunderstanding among participants of the trial aims highlighting the potential importance of employing alternative consenting procedures like continuous consent.Citation26
The value of this research did not end with the findings of the pilot study, but rather extended to the information dissemination sessions where both participants and staff were able to have misunderstandings clarified, and could provide further feedback to ensure that the trial environment in which these adolescent trial participants were involved would aim to be sensitive to their needs, emotions, fears and level of understanding. With the increased attention directed toward clinical research with young people as participants, qualitative enquiry can ensure that the experiences of adolescent trial participants are taken into account when planning future trials.
The Whole Picture—Conclusion
Attaining a clear picture on the landscape of vaccine trials recruiting adolescent participants can be reached through the use of tools like the Pan African Clinical Trial Registry and the International Clinical Trials Registry Platform. These databases can be searched thereby assisting in the identification of gaps in trial research, and providing a resource to search for potential collaborators. For ongoing and planned vaccine trials to be successful, they need to be able to recruit and retain participants, by ensuring that participants’ needs are being met. For vaccine trial research, focused on adolescents, a successful trial environment will need to cater to the specific needs of adolescent trial participants, and qualitative feedback within a trial environment can assist in ensuring that those needs are met.
†Footnote: This term, coined by Dr H Shirkey, refers to the lack of labeling of drugs for uses in children and is now widely used to discuss the dilemmas faced by doctors when looking to prescribe interventions for children, highlighting the need for more research into the toxicology and pharmacokinetics of medication use in children.
References
- World Health Organization. Immunizations, V.a.B.I.f.V.R. State of the art of new vaccines: research and development. Geneva: World Health Organization; 2006.
- Choonara I. Clinical trials of medicines in children. BMJ 2000; 321:1093 - 4; http://dx.doi.org/10.1136/bmj.321.7269.1093; PMID: 11061718
- Conroy S, Choonara I, Impicciatore P, Mohn A, Arnell H, Rane A, et al, European Network for Drug Investigation in Children. Survey of unlicensed and off label drug use in paediatric wards in European countries. BMJ 2000; 320:79 - 82; http://dx.doi.org/10.1136/bmj.320.7227.79; PMID: 10625257
- Tan E, Cranswick NE, Rayner CR, Chapman CB. Dosing information for paediatric patients: are they really “therapeutic orphans”?. Med J Aust 2003; 179:195 - 8; PMID: 12914509
- Schreiner MS, Greeley WJ. Pediatric clinical trials: shall we take a lead?. Anesth Analg 2002; 94:1 - 3; PMID: 11772791
- Kurz R, Gill D. Practical and Ethical Issues in Paediatric Clinical Trials. Applied Clinical Trials Online 2003.
- Smyth R, Weindling A. Research in Children: Ethical and Scientific Aspects. Paediatrics 1999; 354:21 - 4
- Assael BM. Therapeutic orphans: European perspective. Pediatrics 1999; 104:591 - 2; PMID: 10469795
- Klassen TP, Hartling L, Craig JC, Offringa M. Children are not just small adults: the urgent need for high-quality trial evidence in children. PLoS Med 2008; 5:e172; http://dx.doi.org/10.1371/journal.pmed.0050172; PMID: 18700813
- Greener M. Bitter medicine. New regulations aim to address the dearth of clinical safety trials for drugs used in children. EMBO Rep 2008; 9:505 - 8; http://dx.doi.org/10.1038/embor.2008.95; PMID: 18516084
- Administration, U.S.F.a.D. Food and Drug Administration Modernization Act (FDAMA) of 1997. In: FDA U, ed. Public law 105-115. Washington DC; 1997.
- House of Representatives, U.S. International Pediatric HIV/AIDS Network Act of 2005 (HR164). In: House of Representatives tC, ed. HR 164. Washington, District of Columbia: United States Government; 2005.
- Tharyan P. Prospective registration of clinical trials in India: strategies, achievements & challenges. J Evid Based Med 2009; 2:19 - 28; http://dx.doi.org/10.1111/j.1756-5391.2009.01015.x; PMID: 21348978
- Angell M. The ethics of clinical research in the Third World. N Engl J Med 1997; 337:847 - 9; http://dx.doi.org/10.1056/NEJM199709183371209; PMID: 9295243
- Angell M. Investigators’ responsibilities for human subjects in developing countries. N Engl J Med 2000; 342:967 - 9; http://dx.doi.org/10.1056/NEJM200003303421309; PMID: 10738056
- Siegfried N, Clarke M, Volmink J. Randomised controlled trials in Africa of HIV and AIDS: descriptive study and spatial distribution. BMJ 2005; 331:742; http://dx.doi.org/10.1136/bmj.331.7519.742; PMID: 16195291
- Naranbhai V, Karim QA. HIV prevention and treatment research in sub-Saharan Africa: where are the adolescents?. AIDS 2006; 20:1090 - 1; http://dx.doi.org/10.1097/01.aids.0000222094.82529.20; PMID: 16603874
- Lutje V, Gerritsen A, Siegfried N, Garner P. Registering and analyzing malaria clinical trials in Africa: the PACT registry initiative. In: 5th MIM Pan-African Malaria Conference; 2-6 November 2009; Nairobi, Kenya; 2-6 November 2009.
- Abrams A, Siegfried N. The Pan African Clinical Trials Registry: year one data analysis of the only African member of the World Health Organization Network of Primary Registries. J Evid Based Med 2010; 3:195 - 200; http://dx.doi.org/10.1111/j.1756-5391.2010.01099.x; PMID: 21349070
- Harrison A, Cleland J, Gouws E, Frohlich J. Early sexual debut among young men in rural South Africa: heightened vulnerability to sexual risk?. Sex Transm Infect 2005; 81:259 - 61; http://dx.doi.org/10.1136/sti.2004.011486; PMID: 15923298
- Jaspan HB, Cunningham CK, Tucker TJ, Wright PF, Self SG, Sheets RL, et al, HIV Vaccine Adolescent Trials Working Group. Inclusion of adolescents in preventive HIV vaccine trials: public health policy and research design at a crossroads. J Acquir Immune Defic Syndr 2008; 47:86 - 92; http://dx.doi.org/10.1097/QAI.0b013e31815d2f27; PMID: 17984759
- Jaspan HB, Soka NF, Mathews C, Flisher AJ, Mark D, Middelkoop K, et al. A qualitative assessment of perspectives on the inclusion of adolescents in HIV vaccine trials in South Africa. Int J STD AIDS 2010; 21:172 - 6; http://dx.doi.org/10.1258/ijsa.2009.008484; PMID: 20215620
- Abrams A, Siegfried N, Geldenhuys H. Adolescent experiences in a vaccine trial: a pilot study. S Afr Med J 2011; 101:884 - 6; PMID: 22273030
- Mahomed H, Shea J, Kafaar F, Hawkridge T, Hanekom WA, Hussey GD. Are adolescents ready for tuberculosis vaccine trials?. Vaccine 2008; 26:4725 - 30; http://dx.doi.org/10.1016/j.vaccine.2008.06.088; PMID: 18620015
- Valente TW, Zogg JB, Christensen S, Richardson J, Kovacs A, Operskalski E. Using social networks to recruit an HIV vaccine preparedness cohort. J Acquir Immune Defic Syndr 2009; 52:514 - 23; http://dx.doi.org/10.1097/QAI.0b013e3181acff91; PMID: 19584741
- Lema VM. Therapeutic misconception and clinical trials in sub-Saharan Africa: a review. East Afr Med J 2009; 86:291 - 9; PMID: 20358792