Abstract
Background:
Although there are two HPV vaccines have been used to prevent cervical cancer, the cost limits their application in developing countries. The aim of this study was to evaluate the potential value of plant-based HPV16L1 and LTB proteins as a high-efficiency, low-cost and easy-to-use HPV16L1 oral vaccine.
Results:
Transgenic plant-derived HPV16L1 and LTB were identified, which display potent immunogenicity and biologic activity. Higher levels of specific IgG and IgA levels of HPV16L1 were induced when mice were immunized with L1 combined with LTB by the oral route. The stimulation index (SI) of spleen cells from the L1/LTB-immunized group was significantly higher than that in the L1-immunized group (p < 0.05). The percentage of IFN-γ+/IL-4+ CD4+ T cells from the L1/LTB group was clearly increased compared with that in the L1 and control groups (p < 0.05).
Methods:
Plant-expressed HPV16L1 and LTB proteins were extracted from transgenic tobacco leaves, and their biologic characteristics and activity were examined with electron microscopy and GM1-binding assays respectively. Mice were immunized orally with either HPV16L1 or LTB alone or in combination. Induced mucosal and systemic immune responses were detected by ELISA, Hemagglutination inhibition (HAI), lymphocyte proliferation assays and flow cytometry analysis.
Conclusion:
Strong mucosal and systemic immune responses were induced by transgenic tobacco derived HPV16-L1 and LTB combined immunization. This study will lay the foundation for the development of a new type of vaccine to decrease HPV16 infections, which may lead to the prevention of cervical cancer.
Introduction
Cervical cancer is the most common genital cancer and is the major cancer-related cause of death among women in developing countries. Human papillomavirus (HPV), especially HPV16, has been accepted as a major cause of cervical cancer.Citation1 It has been proven that the recombinant HPV16 L1 protein, a major capsid protein of HPV, can self-assemble into VLPs (virus-like particles), which are capable of inducing a high titer of IgG, IgA and IgM.Citation2 It has been shown that HPV16L1 protein as VLPs induce antibody that can protect the host from virus infection and inhibit tumor formation.Citation3-Citation5
Two HPV vaccines, Gardasil and Cervarix, have been commercially available since 2006. Both vaccines have been shown to prevent precancerous lesions in the cervix. Gardasil can protect women from infection by four common HPV types (16, 18, 6 and 11), while Cervarix prevents two high-risk HPV type infections (16 and 18).Citation6 However, the cost limits the use of these two vaccines in developing countries, where most of patients with cervical cancer are living. Therefore, reducing vaccine cost is a new challenge in the field of HPV vaccines. In this regard, it has been reported that plant-expressed HPV VLPs were able to elicit humoral and cytotoxic T-cell activity in mice.Citation5,Citation7
E. coli heat labile enterotoxin (LT) is one of the powerful mucosal adjuvants used in animals. It consists of a homo-pentamer of cell-binding B subunits associated with a single toxic-active A subunit. The A subunit is responsible for toxicity, catalyzing ADP ribosylation of Gsa, increasing cyclic AMP (cAMP) levels and producing chloride efflux and fluid loss.Citation8 The nontoxic B subunit (LT-B) is also a potent immunogen, avoiding tolerance induction when administered mucosally and stimulating strong secretory and systemic antibody responses.Citation9-Citation13 Therefore, LT-B is widely used as an efficient mucosal adjuvant.
Recently, transgenic plants have been recognized as the most inexpensive and convenient bioreactor for preparing a variety of genetically engineered proteins.Citation14,Citation15 In this study, HPV16L1 and LT-B were expressed at high levels in transgenic tobacco plants. To demonstrate the immunogenicity of L1 and the efficiency of LT-B as an immune adjuvant, the recombinant L1 and LT-B proteins were used singly or in combination to immunize animals. This study will lay the foundation for the development of a new type of vaccine to decrease HPV16 infection, which may lead to the prevention of cervical cancer.
Results
Characteristics of HPV16L1 and LT-B expressed by transgenic plants
Both HPV16L1 and LT-B were highly expressed in transgenic tobacco plants as quantified by ELISA. The yield relative to total soluble cell protein reached 0.22–0.31% for L1 and 0.35–0.76% for LT-B. The yields for both L1 and LT-B were significantly increased relative to expression by pBI without SEKDEL sequences (data not shown). To demonstrate the assembly of HPV16L1, the L1 protein extracted from transgenic plants was observed by electron microscopy (EMS). EMS data showed that L1 expressed by tobacco plants formed hollow spherical particles 55 nm in diameter, indicating that the recombinant L1 protein is able to self-assemble (). LT-B expressed by plants also can bind with GM1, similar to rLT-B expressed by bacteria ().
Figure 1. Biologic characteristics of transgenic plant-expressed HPV16L1 and LT-B. (A) Extracted protein from HPV16L1-transformed plants shows 50~60 nm hollow spherical particles when observed by electron micrograph (50000x), which are consistent with HPV16 VLPs (arrows). (B) The capacity of plant-derived LT-B binding to GM1 ganglioside. (C) The histology of intestine mucous membrane after oral LT administration (HE20x). The intestinal villus appears normal in the LT-B group, but is heavily swollen and dropsical in the control group. (D) The weight of small intestine from mice after administration of LT orally. *p < 0.05 vs. control group.
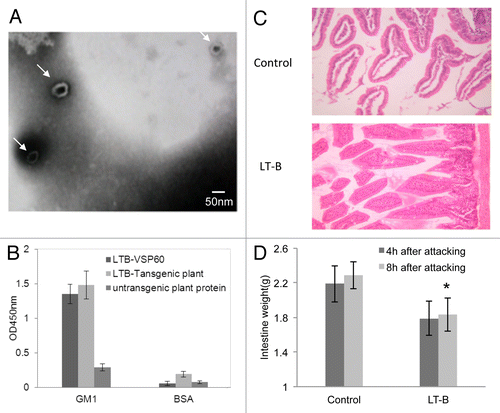
LT-B immunization reduces intestinal damage caused by LT STa challenge
Mice challenged with LT STa were apparently normal in both LT-B-immunized and unimmunized groups and did not display any illness symptoms such as diarrhea and shock, but with reduced activities. However, the intestine of all mice showed different degrees of swelling and effusion. Swelling was clearly more serious in mice from the unimmunized group. The intestinal wall was bright red with a diameter ~2–3mm, indicating that the mesenteric vasculature was markedly hyperemic. For the LT-B-immunized group, the intestinal wall diameter was ~1.5–2.5mm and reddish, while the mesenteric vasculature was filled but not hyperemia. The average weight of mice from the immunized group was significantly lower than that from the unimmunized group 8 h after challenge (). As shown in , the intestinal mucosa of mice from the unimmunized group was necrotic with edema, but there were no obvious lesions observed in the intestine of immunized mice. Taken together, LT-B immunization is able to neutralize the effect of LT STa challenge.
Specific antibodies are detected in HPV16L1-immunized mice
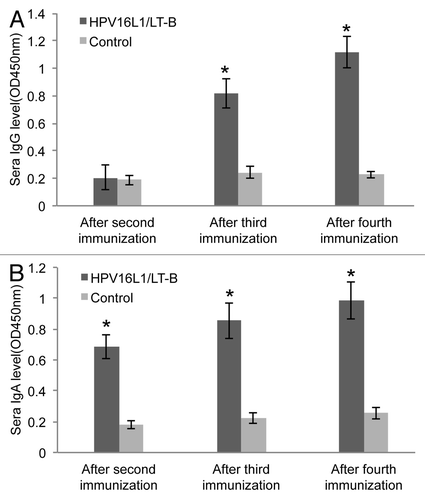
Serum IgG and IgA levels of mice from the groups immunized with HPV16L1 and HPV16L1/LT-B were determined by ELISA. L1-specific IgG and IgA were detected in sera after the second, third and fourth immunizations, but not in the non-transgenic plant protein-immunized group. Although the levels of L1-specific IgG in the L1 and L1/LT-B immunized groups () were not significantly different, the level of L1-specific IgA was increased markedly in both immunized groups, especially in the L1/LT-B group. Furthermore, the IgA level in the L1 group was significantly lower than that in the L1/LT-B group (). The dynamic outcome of the L1-specific IgG and IgA levels showed that the increased rate of IgA in the combined group was greater than that in the group immunized with L1 alone (data not shown), indicating that the combining L1 / LT-B elicits significantly higher IgA responses compared with L1 alone, meaning that LT-B is required for inducing mucosal IgA. The secretion of IgA in the intestine was detected 14 d after the last immunization. The specific IgA levels of both the L1 and the L1/LT-B immunized groups were higher than that in the control group, and the IgA level of the combined group was significantly higher than that in the L1 group when the intestinal fluid was tested at a 1:5 dilution ().
Figure 2. Specific IgG and IgA level of HPV16L1 in immunized mice. (A and B) HPV16L1 specific IgG (A) and IgA (B) levels in serum of immunized mice. (C) HPV16L1 specific IgA levels in intestine secretion. *p < 0.05 vs. control group, #p < 0.05 vs. HPV16L1 group. (D) Hemagglutination inhibition assay (HAI). The ratios shown below are dilutions of the sera polyclonal antibodies.
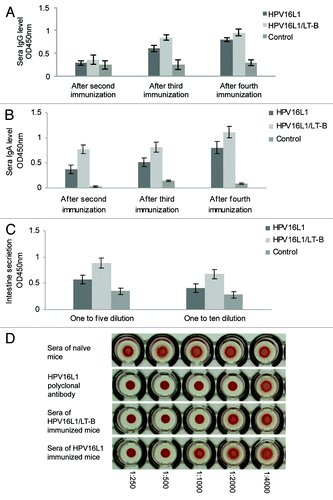
We have found that transgenic plants expressed HPV16L1 protein that causes murine erythrocyte agglutination, which can be specifically abolished by an anti-HPV16L1 monoclonal antibody.Citation16 To determine antibody titers in L1- and L1/LT-B-immunized mice, antiserum dilutions were evaluated for their ability to function in L1-induced hemagglutination inhibition (HAI). As shown in , the diluted sera were showed HAI activity to dilutions of < 1:1,000 for the L1/LT-B group and < 1:500 for the L1 group (), suggesting that the anti-L1 antibodies from immunized mice were functional with a high titer.
Specific antibody responses to LT-B in immunized mice
An ELISA was used to detect serum IgG and IgA levels of LT-B from mice immunized with L1/LT-B. In contrast to the nontransgenic plant protein immunized group, LT-B-specific IgG was produced after third immunization (). However, the LT-B-specific IgA level was increased significantly after the second immunization (). These results show that the mucosal immune response to LT-B is detected earlier than the humoral response.
Antigen-specific responses of spleen cells
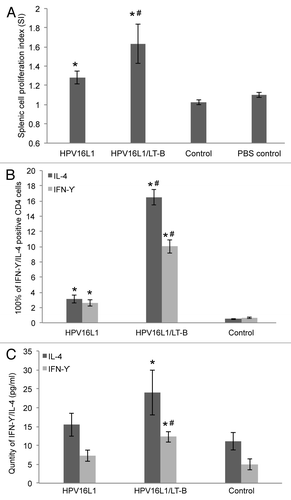
Two weeks after the fourth immunization, splenic cells were isolated and stimulated with L1 VLP protein to analyze cell proliferation. As shown in , splenic cells from both L1 and L1/LT-B groups responded to L1 VLP stimulation, and the stimulation index (SI) of L1/LT-B was significantly higher than that with L1 alone (p < 0.05). The percentage of IFN-γ/IL-4 positive CD4+ T cells was also detected by flow cytometry after stimulation with PMA. As shown in , the percentages of IFN-γ/IL-4 positive CD4+ T cells from both L1 and L1/LT-B groups were markedly increased compared with the control group. The positive cells of L1/LT-B were significantly higher than that with L1 alone (p < 0.05). As expected, the IFN-γ and IL-4 levels were higher in the splenic cell media as analyzed by ELISA (). These results demonstrate that LT-B is not only a mucosal adjuvant but also an effective adjuvant of systemic response.
Figure 4. Antigen-specific responses of splenic cells. (A) HPV16VLPs induced antigen-specific proliferative assay of splenocytes. (B) The frequency of IL-4+/IFN-γ+cells in CD4+splenocytes from immunized mice by flow cytometry analysis. (C) IL-4/IFN-γ level in supernatant of HPV16VLP stimulated splenic cells from immunized mice. *p < 0.05 vs. control group, #p < 0.05 vs. HPV16L1 group.
Discussion
Using plants as a biological reactor to produce vaccines and medicinal proteins has safety, expense and many other advantages.Citation17 Over the past several decades, people have successfully expressed more than 100 different antigens in tobacco, tomato, potato and other plants.Citation7,Citation18,Citation19 It has been proven that antigens expressed by plants are immunogenic and can induce the host to produce specific systemic and mucosal immune responses, many of which have been shown to protect the host from pathogenic microbes.
HPV16 virus mainly invades the ano-genital tract mucous of humans and causes infection in the urinary and reproductive areas. It is an important goal to stimulate the mucosa to produce an effective neutralizing antibody against HPV16 and protect the human from virus infection at the first line of defense.Citation20 It is well known that mucosal immunization and systemic immunity are different but related to each other. A systemic immunization approach usually stimulates the immune system to produce a specific antibody and cytotoxic T cells (CTL), but often cannot induce a mucosal immune response. The mucosal immune system is one of important parts of host immune system, and there are many immune cells within the mucosa that provide a strong immune protection barrier.
Mucosal vaccination not only can stimulate comprehensive immune responses including mucosal, humoral and cellular immune responses, but also has the potential advantage of convenience to users in terms of less pain, improved safety and lower cost. Studies have shown that the characteristics of mucosal immunity are part of mucosal tissue immune responses.
The present report suggests that plant-derived VLPs can induce mucosal IgA by the nasal or oral route. Previous studies indicated that VLPs can produce a higher titer of mucosal IgA antibodies only when VLPs are administered together with mucosal adjuvants (such as LT or CT)Citation21,Citation22; otherwise it can cause immune tolerance.
LT-B has been produced in different expression systems, such as bacteria, yeast or plants. Its use as an adjuvant in orally delivered vaccine formulations is well documented.Citation9-Citation13 However, our study is the first to induce mucosal immune responses against HPV infection by combining plant-expressed LT-B and HPV16L1. Clearly the combination was able to elicit L1-specific IgA at a remarkably higher level that that induced with L1 alone, which demonstrates that LT-B functions as an adjuvant in promoting L1-induced mucosal immune responses. These results also provide direct evidence that LT-B produced in transgenic tobacco plants is biologically active. Therefore, the use of plants for producing an immunogen for the oral route could be better accepted than needle injection. Also, not as much purification is needed for an oral vaccine, which decreases vaccine cost. Furthermore, an oral vaccine could elicit strong mucosal immune responses.
The L1-specific IgA level in the intestine fluid of the L1/LT-B group was significantly higher than that in the L1-alone group, which demonstrates that L1 vaccine could induce protective IgA in the intestinal mucosa when administered orally together with LT-B. Based on this, we speculate that specific protective antibodies also may be produced in the female reproductive tract, which is particularly important for preventing HPV infection.
Furthermore, splenic cell proliferation and the expression of IFN-γ and IL-4 in CD4+ T cells and spleen cell supernatants indicates that oral immunization with L1 could induce L1-specific cellular immune responses with LT-B functioning as an effective mucosal adjuvant. It is well known that both IFN-γ and IL-4 are representative of the cytokines in Th1 and Th2 type immune responses. LT-B may shift the Th1/Th2 balance to Th2 by promoting IL-4 secretion, which increases the IgA level.Citation23 Thus, it seems that LT-B as an effective mucosal adjuvant can play an important role in protection against HPV infection.
Recently, plants have emerged as alternative production systems for vaccines, since they may contribute to all of the critical features of effective vaccines. Plant systems do not harbor human or animal pathogens and, therefore, do not transmit such pathogens alone with the target subunit vaccine. The most important advantage of producing vaccines in edible parts of a plant is the potential to deliver them orally rather than intramuscularly,Citation24 thus providing a simple and easy means of administration to humans. Moreover, oral delivery stimulates mucosal immunity in the tissues lining the mouth, nose and esophagus that provide the first target of opportunity for pathogens to enter and infect the human body. In addition, production in plants reduces the overall cost of vaccination, which is often unaffordable in developing countries.
Our experimental results show that serum anti-L1 IgG and IgA can be detected after the second immunization. Along with time following immunization, the antibody level increases gradually, indicating that oral plant-derived L1 vaccine can produce effective mucosal and systemic immune responses, particularly with LT-B as adjuvant.
We use electron microscopy to analyze VLP assembly and use the HAI assay to detect the neutralization ability of HPV16 L1 extracts.Citation25 Papillomavirus pseudoviruses (PsV) may be useful to study the characteristics of plant-expressed HPV16 LI protein.Citation26 In a future study, we will find other methods like changing the expression system or modifying codons to improve the yield and purity of the expressed protein,Citation27 and use licensed HPV vaccine as the control.
In conclusion, we demonstrate that HPV16L1 expressed in transgenic tobacco plants display biological activity and immunogenicity and are able to induce the serum and mucosal antibodies, and to some extent strengthen cellular immune responses. Similarly LT-B protein expressed in transgenic tobacco plants function as an effective mucosal adjuvant. Therefore, this study lays the foundation for the development of new vaccines by using proteins expressed in transgenic plants to prevent HPV16 infection that leads to cervical cancer.
Materials and Methods
Animals
BALB/c female mice, 6–8 weeks of age, were housed in the animal experimental center in Medical College of Xi’an Jiaotong University. All animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee of the Xi'an Jiaotong University.
Plasmids construction, plant transformation and regeneration
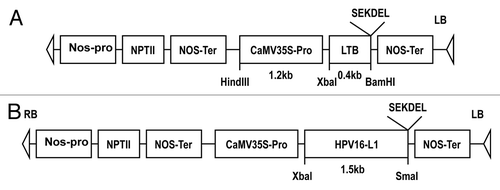
Plant transformation vector construction, tobacco plant transformation and regeneration were performed with minor modifications.Citation26 In brief, HPV16 L1 and LT-B genes with SEKDEL (Ser-Glu-Lys-Asp-Glu-Leu) sequences at the C-terminal were inserted into pBI121 binary vectors as shown in . The sequence SEKDEL has been shown to be a signal that leads to retention of at least two proteins in the endoplasmic reticulum of eukaryotic cells and to increase the yield of expressed protein.Citation28 The tobacco plants (Nicotiana tabacum L. cultivar Xanthi) were transformed using the leaf disc method by cocultivating with Agrobacterium tumefaciens LBA4404, which harbored the chimeric vector pBI-L1s or pBI-LTBs respectively. The regenerated transgenic tobacco plants of HPV16L1 and LT-B were verified as described before.Citation27,Citation29
HPV16L1 and LT-B proteins extraction
100 g of mature HPV16L1 and LT-B transgenic tobacco plants were mixed with 100 mL of plant protein extraction buffer, respectively, then ground rapidly in an organization homogenization device on ice. Protein extracts were separated by centrifugation at 12,000 rpm, 4°C for 20 min, then dissolved in 1/5 volume deionized water after freeze-drying, followed by dialysis with 0.9% NaCl (pH7.4) at 4°C for 16 h. The quantity of extracted proteins were monitored by BCA Protein Assay Kit and stored at -80°C.
ELISA quantification of HPV16 L1 and LT-B expression
Total soluble leaf protein from transgenic strains was quantified by the Bradford method. The expressions levels of HPV L1 and LT-B in transgenic tobacco leaves were determined by ELISA. Briefly, 96 well plates were coated with 100 μL plant protein extract or a certain quantity of purified baculovirus-produced HPV16 VLP or bacteria-expressed LT-B diluted serially in bicarbonate-buffered saline (pH 9.6) and kept at 4°C overnight. After blocking, plates were incubated with anti-HPV16 L1 monoclonal antibody, HRP-conjugated anti-mouse IgG and then with 1,3,6′,8′-tetramethyl-dianthrone (TMD) substrate solution. When reactions were complete, the absorbance at 450 nm was recorded with ELx800 absorbance microplate reader.
Electron microscopic analysis of HPV16 L1 extracts
The HPV16 L1 transgenic plant extract was dropped onto the carbon copper-coated grids, which were negatively stained with phosphotungstic acid and viewed using a H-600 transmission electron microscope.
ELISA analysis of the binding of LT-B with GM1
96-well plates were coated with GM1 (1 µg / well) in carbonate buffer overnight at 4°C. Diluted LT-B was added and incubated at 37°C for 2 h. After washing with PBS, rabbit anti-LT-B IgG was added and incubated at 37°C for 1 h. Bound antibody was probed with mouse anti-rabbit IgG (H+L) labeled with HRP, followed by incubated with TMB for 15–30 min, and read at A450. The LT-B VSP60Citation30 and the protein extraction from non-transgenic plant were used as positive and negative controls.
Experiment design and immune program
BALB/c female mice were random divided into five groups of 8 mice each. Before immunization, mice were fasted overnight while water was provided ad libitum.Citation31 Mice were immunized four times orally with 50 µg condensed plant extraction proteins consisting 0.38 µg HPV16L1 protein, 0.75 µg LT-B protein, or HPV16L1/LT-B mixture. PBS group was used as the negative control. The first immunization was at 0–2 d, the second at 8–10 d, the third at 29–31 d, and the fourth at 50–52 d. The animals were sacrificed two weeks after the last immunization.
Challenge assay of LT
Mice from LT-B group and PBS group were administered 75 μg LT STa [heat-labile enterotoxins (LT) and heat-stable enterotoxins (ST) antigens] (Sigma) orally two weeks after the last immunization. The activity of the mice was observed, and then mice were sacrificed, respectively, at 4 and 10 h post-challenge. The small intestine was examined, weighed and fixed with 10% formaldehyde to make pathological slides for hematoxylin and eosin (HE) staining.
ELISA for determining plant-derived L1- and LT-B-specific antibody
ELISA was used to quantify plant-derived HPV16L1- and LT-B-specific IgG and IgA levels in sera or intestinal secretions. 96-well plates were coated with insect cell-derived L1 or bacterial-derived LT-BCitation11,Citation32 at a concentration of 3 µg/well. Serum or intestinal secretion was incubated with coated proteins in 2-fold serial dilution at room temperature for 2 h. Bound antibody was probed with goat anti-mouse IgG (1:2,000, DAKO) or IgA (1:4,000, Southern Biotech Associates) labeled with HRP, followed by incubation with TMB for 15–30 min and read at A450.
Analysis of IFN-γ and IL-4 in spleen cell culture supernatants
Supernatant of cultured splenocytes in 96-well flat-bottom plates with or without HPV16L1 VLPs were harvested, and IFN-γ and IL-4 level were measured by sandwich ELISA. Anti-mouse-IFN-γ and -IL-4 antibodies were purchased from Jingmei Biotech Co. Ltd.
Hemagglutination inhibition (HAI) assay
The HAI assay has been described.Citation26 Briefly, an erythrocyte suspension was prepared from the fresh blood of C57BL/6 mice, then 100 c μL plant extract was plated on a 96-well U-bottomed plate and mixed with an equal volume of 1% (v/v) erythrocyte suspension which was pretreated with the sera of different immunized groups. For controls, the murine erythrocytes were mixed with sera from naïve mice or with rabbit anti-HPV16L1 polyclonal antibody, which was expressed in a baculovirus-infected insect system and purified in our laboratory.Citation32 After incubation, visualization and photography were done.
Lymphocyte proliferation assay
Mice were sacrificed 14 d after the last immunization. Splenocytes were isolated and resuspended in RPMI1640 medium supplemented with 10% fetal calf serum (Gibco), then 2 × 104 splenocytes were seeded in triplicate in 96-well flat-bottom plates and incubated for 3 d with 2 μg/mL HPV16 VLPs prepared in our laboratory.Citation32 0.5 mg/mL MTT was added to culture media and incubated at 37°C for 4 h. DMSO was added, and A570 was measured. The splenic cell proliferation index (SI) was calculated by the formula: SI = stimulated group OD value/control group OD value.
Flow cytometry
Five × 106 splenocytes of each group were cultured in complete culture medium supplemented with phorbol myristate acetate (PMA, 50 ng/mL) and ionomycin (1 μg/mL) for 4h at 37°C under a 5% CO2 atmosphere, and then cells were administered the secretion inhibitor Brefeldin A for 2 h. For analysis of IFN-γ and IL-4, 50 μL stimulated cells were incubated with FITC-labeled anti-mouse CD4 (BD Bioscience) at 4°C for 30 min, then cells were stained with phycoerythrin (PE)-labeled anti-mouse-IFN-γ or -IL-4 after fixation and permeabilization according to the manufacturer's instructions (eBioscience). Isotype controls were used to enable correct compensation and confirm antibody specificity. Stained cells were run on a Becton Dickinson FACS Calibur flow cytometer and data analyzed using Flow-Jo software (Tree Star, Inc.).
Data analysis
Data were processed using the SPSS17.0 software package for Windows. All results are expressed as mean ± SD (standard deviation). One-way analysis of variance (ANOVA) was employed to determine the difference among groups. Results were considered statistically significant if p < 0.05.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Reference
- McCance DJ. Human papillomaviruses and cervical cancer. J Med Microbiol 1998; 47:371 - 3; http://dx.doi.org/10.1099/00222615-47-5-371; PMID: 9879936
- Zhou J, Sun XY, Stenzel DJ, Frazer IH. Expression of vaccinia recombinant HPV 16 L1 and L2 ORF proteins in epithelial cells is sufficient for assembly of HPV virion-like particles. Virology 1991; 185:251 - 7; http://dx.doi.org/10.1016/0042-6822(91)90772-4; PMID: 1656586
- Revaz V, Benyacoub J, Kast WM, Schiller JT, De Grandi P, Nardelli-Haefliger D. Mucosal vaccination with a recombinant Salmonella typhimurium expressing human papillomavirus type 16 (HPV16) L1 virus-like particles (VLPs) or HPV16 VLPs purified from insect cells inhibits the growth of HPV16-expressing tumor cells in mice. Virology 2001; 279:354 - 60; http://dx.doi.org/10.1006/viro.2000.0717; PMID: 11145916
- Osen W, Jochmus I, Müller M, Gissmann L. Immunization against human papillomavirus infection and associated neoplasia. J Clin Virol 2000; 19:75 - 8; http://dx.doi.org/10.1016/S1386-6532(00)00090-1; PMID: 11091150
- Paz De la Rosa G, Monroy-García A, Mora-García MdeL, Peña CG, Hernández-Montes J, Weiss-Steider B, et al. An HPV 16 L1-based chimeric human papilloma virus-like particles containing a string of epitopes produced in plants is able to elicit humoral and cytotoxic T-cell activity in mice. Virol J 2009; 6:2 - 13; http://dx.doi.org/10.1186/1743-422X-6-2; PMID: 19126233
- De Vuyst H, Clifford GM, Nascimento MC, Madeleine MM, Franceschi S. Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: a meta-analysis. Int J Cancer 2009; 124:1626 - 36; http://dx.doi.org/10.1002/ijc.24116; PMID: 19115209
- Kohl TO, Hitzeroth II, Christensen ND, Rybicki EP. Expression of HPV-11 L1 protein in transgenic Arabidopsis thaliana and Nicotiana tabacum. BMC Biotechnol 2007; 7:56 - 70; http://dx.doi.org/10.1186/1472-6750-7-56; PMID: 17850660
- de Haan L, Hirst TR. Cholera toxin and related enterotoxins: a cell biological and immunological perspective. J Nat Toxins 2000; 9:281 - 97; PMID: 10994530
- Richards CM, Aman AT, Hirst TR, Hill TJ, Williams NA. Protective mucosal immunity to ocular herpes simplex virus type 1 infection in mice by using Escherichia coli heat-labile enterotoxin B subunit as an adjuvant. J Virol 2001; 75:1664 - 71; http://dx.doi.org/10.1128/JVI.75.4.1664-1671.2001; PMID: 11160664
- Williams NA, Hirst TR, Nashar TO. Immune modulation by the cholera-like enterotoxins: from adjuvant to therapeutic. Immunol Today 1999; 20:95 - 101; http://dx.doi.org/10.1016/S0167-5699(98)01397-8; PMID: 10098329
- Wang J, Li L, Zheng J, Yu J, Yang X, Geng YP, et al. Highly efficient expression, purification of recombinant LTB protein and its activity against mucosal immunoadjuvant by nasal immunization. Chin Med J (Engl) 2003; 116:1115 - 7; PMID: 12890398
- Norton EB, Lawson LB, Freytag LC, Clements JD. Characterization of a mutant Escherichia coli heat-labile toxin, LT(R192G/L211A), as a safe and effective oral adjuvant. Clin Vaccine Immunol 2011; 18:546 - 51; http://dx.doi.org/10.1128/CVI.00538-10; PMID: 21288994
- da Hora VP, Conceição FR, Dellagostin OA, Doolan DL. Non-toxic derivatives of LT as potent adjuvants. Vaccine 2011; 29:1538 - 44; http://dx.doi.org/10.1016/j.vaccine.2010.11.091; PMID: 21163247
- Zhao L, Hao D, Chen L, Lu Q, Zhang Y, Li Y, et al. Roles for a soybean RAV-like orthologue in shoot regeneration and photoperiodicity inferred from transgenic plants. J Exp Bot 2012; 63:3257 - 70; http://dx.doi.org/10.1093/jxb/ers056; PMID: 22389516
- Bahariah B, Parveez GK, Masani MY, Khalid N. Construction of phosphomannose isomerase (PMI) transformation vectors and evaluation of the effectiveness of vectors in tobacco (Nicotiana tabacum L). Bioinformation 2012; 8:151 - 7; http://dx.doi.org/10.6026/97320630008151; PMID: 22368388
- Zheng J, Yang X, Sun Y, Lai B, Wang Y. Stable high-level expression of truncated human papillomavirus type 16 L1 protein in Drosophila Schneider-2 cells. Acta Biochim Biophys Sin (Shanghai) 2008; 40:437 - 42; http://dx.doi.org/10.1111/j.1745-7270.2008.00417.x; PMID: 18465029
- Waheed MT, Gottschamel J, Hassan SW, Lössl AG. Plant-derived vaccines: An approach for affordable vaccines against cervical cancer. Hum Vaccin Immunother 2012; 8:8; PMID: 22327500
- Ma JK. Genes, greens, and vaccines. Nat Biotechnol 2000; 18:1141 - 2; http://dx.doi.org/10.1038/81113; PMID: 11062427
- Tiwari S, Verma PC, Singh PK, Tuli R. Plants as bioreactors for the production of vaccine antigens. Biotechnol Adv 2009; 27:449 - 67; http://dx.doi.org/10.1016/j.biotechadv.2009.03.006; PMID: 19356740
- Lowy DR, Kirnbauer R, Schiller JT. Genital human papillomavirus infection. Proc Natl Acad Sci USA 1994; 91:2436 - 40; http://dx.doi.org/10.1073/pnas.91.7.2436; PMID: 8146136
- Breitburd F, Coursaget P. Human papillomavirus vaccines. Semin Cancer Biol 1999; 9:431 - 44; http://dx.doi.org/10.1006/scbi.1999.0147; PMID: 10712890
- Holmgren J, Czerkinsky C, Eriksson K, Mharandi A. Mucosal immunisation and adjuvants: a brief overview of recent advances and challenges. Vaccine 2003; 21:Suppl 2 S89 - 95; http://dx.doi.org/10.1016/S0264-410X(03)00206-8; PMID: 12763689
- Plant A, Williams R, Jackson ME, Williams NA. The B subunit of Escherichia coli heat labile enterotoxin abrogates oral tolerance, promoting predominantly Th2-type immune responses. Eur J Immunol 2003; 33:3186 - 95; http://dx.doi.org/10.1002/eji.200324154; PMID: 14579287
- Streatfield SJ, Howard JA. Plant production systems for vaccines. Expert Rev Vaccines 2003; 2:763 - 75; http://dx.doi.org/10.1586/14760584.2.6.763; PMID: 14711360
- Liu HL, Li WS, Lei T, Zheng J, Zhang Z, Yan XF, et al. Expression of human papillomavirus type 16 L1 protein in transgenic tobacco plants. Acta Biochim Biophys Sin (Shanghai) 2005; 37:153 - 8; PMID: 15756416
- Buck CB, Pastrana DV, Lowy DR, Schiller JT. Generation of HPV pseudovirions using transfection and their use in neutralization assays. Methods Mol Med 2005; 119:445 - 62; PMID: 16350417
- Maclean J, Koekemoer M, Olivier AJ, Stewart D, Hitzeroth II, Rademacher T, et al. Optimization of human papillomavirus type 16 (HPV-16) L1 expression in plants: comparison of the suitability of different HPV-16 L1 gene variants and different cell-compartment localization. J Gen Virol 2007; 88:1460 - 9; http://dx.doi.org/10.1099/vir.0.82718-0; PMID: 17412974
- Zagouras P, Rose JK. Carboxy-terminal SEKDEL sequences retard but do not retain two secretory proteins in the endoplasmic reticulum. J Cell Biol 1989; 109:2633 - 40; http://dx.doi.org/10.1083/jcb.109.6.2633; PMID: 2592401
- Liu HL, Zhang Z, Li WS, Zheng J, Kong LH, Wang Y, Si LS. Expression of E. coli heat-lablie enterotoxin B subunit in transgenic tobacco plants. Journal of medical colleges of PLA 2005; 20:262 - 7
- Yili W, Lusheng S. Expression of recombinant LTB protein in Marine vibrio VSP60. Methods Mol Biol 2005; 308:43 - 9; PMID: 16082024
- Lauterslager TG, Florack DE, van der Wal TJ, Molthoff JW, Langeveld JP, Bosch D, et al. Oral immunisation of naive and primed animals with transgenic potato tubers expressing LT-B. Vaccine 2001; 19:2749 - 55; http://dx.doi.org/10.1016/S0264-410X(00)00513-2; PMID: 11257419
- Zheng J, Ma J, Yang XF, Liu HL, Cheng HW, Si LS, et al. Highly efficient and economical baculovirus expression system for preparing human papillomavirus type16 virus-like particle. Acta Biochim Biophys Sin (Shanghai) 2004; 36:548 - 52; http://dx.doi.org/10.1093/abbs/36.8.548; PMID: 15295647