Abstract
Objective:
To evaluate the immunogenicity and safety of a pentavalent rotavirus vaccine (PRV) in Indian infants.
Study Design:
Open-label, single-arm multicentric study.
Setting:
Hospital facilities (out patients)
Subjects:
One hundred and ten (110) healthy Indian infants were enrolled between the ages of 6 weeks and 12 weeks.
Intervention:
Three doses of oral pentavalent rotavirus vaccine (PRV) were administered with an interval of 4 to 10 weeks (28 to 70 days).
Main Outcome Measures:
Immunogenicity of PRV was based on the proportion of infants exhibiting a >3-fold rise in serum anti rotavirus IgA antibodies (from pre dose 1 to 14 days post dose 3). Safety was evaluated for 14 days after each dose.
Results:
Of the 110 infants enrolled, 83% exhibited at least a 3-fold rise (seroconversion) in serum anti rotavirus IgA antibodies. There were no clinically significant adverse events reported.
Conclusions:
A 3-dose regimen of PRV was found to be immunogenic and well tolerated in healthy Indian infants.
Clinical trials registration:
ClinicalTrials.gov; NCT00496054
Introduction
Rotavirus is the leading cause of severe diarrhea-related illness and death in infants and young children below 5 y of age worldwide The rates of rotavirus illness among children in industrialized and less-developed countries are similar, indicating that clean water supplies and good hygiene have little effect on virus transmission; therefore, further improvements in water or hygiene are unlikely to have a substantial impact on disease prevention. It was observed that in USA, the rate of hospitalizations for gastroenteritis in young children declined only 16% during 1979–1995, despite the widespread availability of oral rehydration solutions and recommendations by experts, including the American Academy of Pediatrics (AAP) and the Centers for Disease Control and Prevention (CDC), for the use of oral rehydration solutions in the treatment of dehydrating gastroenteritis.Citation1-Citation3
Rotavirus accounts for approximately 527,000 deaths per year worldwide and 29% of all deaths due to diarrhea among children under 5 y of age.Citation4,Citation5Annually in India, rotavirus diarrhea causes an estimated 122,000–153,000 deaths, 457,000–884,000 hospitalizations, and 2 million outpatient visits in children less than 5 y of age. Each year, India spends a total of Rs 2.0–3.4 billion (US$ 41–72 million) in direct and indirect medical costs for the treatment of rotavirus.Citation6
The 5 most prevalent rotavirus genotype / serotype combinations, which account for more than 90% of cases of human rotavirus disease worldwide, are G1P1A[8], G2P1B[4], G3P1A[8], G4P1A[8], and G9P1A[8]. Within the Indian Rotavirus Strain Surveillance Network, rotavirus was detected in approximately 39% of all patients admitted for diarrhea and in whom rotavirus testing was performed. The most common types of strains were G2P[4] (26%), G1P[8] (22%), and G9P[8] (9%).[7] In India, rotavirus detection rates were greatest among children aged 6–23 mo (37% for age group 6–11 mo, 39% for ages 12–23 mo) . The detection rate for children less than age 6 mo was 13%.Citation7
India alone is estimated to account for approximately one-quarter of the global deaths from rotavirus. In 2007, an estimated 20% of all deaths in children < 5 y of age were due to diarrhea.Citation6,Citation7 The World Health Organizaiton (WHO) recommends that rotavirus vaccine for infants should be included in all national immunization programs. In countries where diarrheal deaths account for ≥ 10% of mortality among children aged < 5 y, the introduction of the vaccine is strongly recommended.Citation8 Thus, low socioeconomic developing countries like India with a huge burden of disease are likely to benefit the most from rota virus vaccine introductions.
RotaTeq™ (PRV or RV5, Merck and Co., Inc.,Whitehouse Station) is a pentavalent (G1, G2, G3, G4 and P1A[8]) human-bovine (WC3) reassortant vaccine. PRV has shown to be effective and well tolerated in randomized clinical trials [including the (REST) Rotavirus Efficacy and Safety Trial] and in post licensure surveillance in the US, and other parts of world. Recent studies in the US and Nicaragua showed that PRV is effective in protecting against rotavirus related hospitalizations.Citation9-Citation11 It is important to evaluate the immunogenicity and safety of PRV among healthy infants in India.
Results
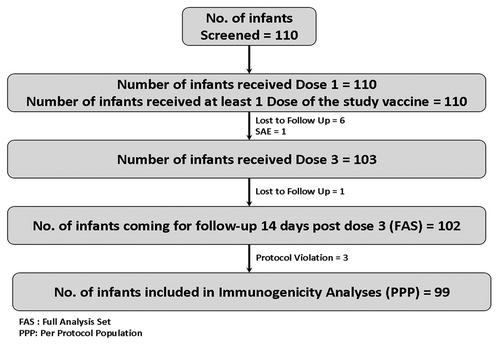
Of the total 110 infants enrolled in this study from May 2008 to August 2008, 103 (94%) received 3 doses, and 102 (93%) were followed for safety for 14 d post dose 3. A total of 99 infants were included in per-protocol population (PPP) analyses (). All the three protocol deviations were because of less than specified interval of 28 d between two study visits. These three subjects were kept out of the per protocol population for immunogenicity analysis. Out of 110 infants enrolled in the study, 63 (57%) were males. The mean age of the infants at the time of entry was 59 d.
Immunogenicity
Among the per protocol population (99 infants) included in the immunogenicity analyses in this clinical trial, 82 (83%) exhibited a ≥ 3 fold rise from pre dose 1 to post dose 3 in serum anti rotavirus IgA response. All the three protocol deviations were because of less than specified interval of 28 d between two study visits. The percentage of participants in the immunogenicity analyses in this clinical trial who exhibited ≥ 3 fold rise from pre dose 1 to post dose 3 for G1, G2, G3, G4 and P[1] Serum Neutralizing Antibodies(SNA) in the per protocol population were 37.37%, 14.14%, 29.29%, 35.35% and 29.29% respectively.
We also analyzed the data in the infants who had serum anti rotavirus IgA ≤ 20 IU/mL at baseline. There were 80 such infants and the seroconversion rate (exhibited a ≥ 3‐fold rise from pre dose 1 to post dose 3 in serum anti rotavirus IgA response) was 89% in these infants. 3-fold rise in the serum neutralizing antibodies against rotavirus serotypes G1, G2, G3, G4 and P[1] levels after 3 doses of vaccination is shown in .
Figure 2. ≥ 3 fold rise in the serum neutralizing antibodies against rotavirus serotypes G1, G2, G3, G4 and P[1] in the infants who had serum anti rotavirus IgA ≤ 20 IU/mL at baseline. 95% CI.
![Figure 2. ≥ 3 fold rise in the serum neutralizing antibodies against rotavirus serotypes G1, G2, G3, G4 and P[1] in the infants who had serum anti rotavirus IgA ≤ 20 IU/mL at baseline. 95% CI.](/cms/asset/a252da27-f94e-4b00-a22c-a280097f4ca4/khvi_a_10922341_f0002.gif)
Among the infants that had ≤ 20 IU/mL serum anti rota virus IgA at baseline, there were 80 infants that had received OPV concomitantly on the same day as PRV. We observed that the seroconversion of serum anti rotavirus IgA in infants receiving OPV concomitantly with PRV on the same day was 81%.
Geometric Mean Titers (GMTs) for serum anti‐rotavirus IgA response were calculated for per protocol group. shows the GMTs for serum anti‐rotavirus IgA response at pre dose 1 and post dose 3 for per protocol group.
Table 1. GMTs for serum anti-rotavirus Iga at pre-dose 1 and post dose 3 (per protocol group)
Safety
Of the 110 infants enrolled in the study, 104 (95%) were included in the safety analysis. At each visit, the subject’s parent/guardian was given a Vaccine Report Card (VRC). They were explained about the AE like diarrhea, fever etc and were instructed to record daily for 7 d the subject’s temperature, the number of episodes of vomiting and/or diarrhea, and behavioral symptoms.
A total of 47 (45%) infants reported at least one adverse event (AE) during the study. The most frequent adverse events reported were diarrhea 30 (29%); vomiting 29 (28%), and pyrexia 19 (18%). In this trial we did not test the stool samples for subjects with diarrhea for wild type Rota virus.
There was one Serious Adverse Event (SAE) reported. This was a cases of meningitis diagnosed on his visit for dose 2, treated with antibiotics and later discharged in a stable condition. The investigators assessed this serious adverse event as not related to the study vaccine.
There were no cases of intussusceptions seen in the recipients of the vaccine and there were no deaths reported in the study population.
Discussion
This study showed that among healthy Indian infants, 83% had at least a 3 fold-rise in serum anti rotavirus IgA after receiving 3 doses of PRV. In a posthoc analysis, among those infants who received RotaTeq® and OPV concomitantly, there was little difference in the percentage of infants who seroconverted compared with those who didn't receive the vaccines together. In addition, the vaccine was well tolerated among this population. These data are quite encouraging and particularly important for developing countries like India, where there is a huge burden of disease due to rotavirus and there is little data available for rotavirus vaccines.
The immunogenicity of RotaTeq® across different populations and geographic regions was assessed in several Phase II and III studies. Till date an immune correlate of protection against rotavirus disease has not been established with any rotavirus vaccine, the serum anti rotavirus IgA responses are used by regulatory agencies, as well as WHO, around the globe as a means of predicting efficacy.Citation12 Higher serum anti rotavirus IgA seroconversion with 3 doses of PRV may be similar to increasing levels of protection seen with multiple natural rotavirus infections and may contribute to longer protection.
In a previous study involving 735 healthy infants in Latin America, PRV and OPV administered concomitantly did not interfere with the immune response to poliovirus types 1, 2 or 3 when compared with non-concomitant use.Citation12,Citation13 It is common practice that OPV is administered at the same time as rotavirus vaccines in countries such as India and these data will be useful for recommending bodies to make future decisions about the use of rotavirus vaccines.
In a similar study done in India by Narang et al. using the monovalent rota virus vaccine (RIX4411) they observed serum anti rotavirus IgA seroconversion rate of 58.3% one month after receiving the second dose. In this study OPV was not given concomitantly along with the monovalent rotavirus vaccine, but rather separated by a 2 week interval.Citation14
One of the major strengths of this study was that it looked at the sero conversion rates post vaccination in Indian infants without imposing any restrictions on the use of OPV vaccine or the Pulse polio program. The data were quite encouraging and will be useful in Indian context. One of the limitations of the study was that this study looked at seroconversion rates before and after giving three doses of RotaTeq and did not look at the efficacy of the vaccine in preventing Rota virus diarrhea in vaccinated infants.
Pentavalent Rotavirus vaccine (PRV) has been highly effective with consistent resulted in several clinical trials across different populations and geographic regions (US, Europe, Latin America, Taiwan and Korea).Citation12 Observational data from USA where PRV was introduced in 2006 showed vaccine effectiveness against severe rotavirus gastroenteritis to be 85–95%. Data from 2007–2008 rotavirus seasons in the US showed there was a 64% reduction in number of positive rotavirus tests than in the pre vaccination period. The decline was also noted in age groups other than those vaccinated providing evidence of a potential herd effect.Citation15 The burden of rotavirus gastroenteritis on children and their families could be substantially reduced by routine rotavirus vaccination of infants.
In a recent publication from Indian Academy of Pediatrics Committee on Immunization (IAPCOI), the IAPCOI acknowledged the morbidity and mortality burden of rotavirus and need for a rotavirus vaccine. They thought that such a vaccine would be most needed in the national immunization program as the disease consequences are the most serious in the underprivileged. According to them, given the minimal impact that water and sanitation measures have had on the burden of rotavirus in developing areas, there is wide agreement that effective vaccination represents the most promising prevention strategy against the disease.Citation16
Rota virus vaccines can have big impact in reducing deaths due to Rota virus infections in developing countries like India, however we need to better understand the role of the amount of gastric acid in the digestive tract, micronutrient malnutrition, interfering gut flora, diarrheal and immune system diseases.Citation17
In conclusion, this study demonstrated that the pentavalent rotavirus vaccine was immunogenic and well tolerated in healthy India infants. This is encouraging as WHO has encouraged routine use of rotavirus vaccines as an integral part of a comprehensive strategy to control diarrheal diseases in developing countries like India.
Methods
Study design
The study was an open label, single-arm study trial conducted from May 2008 to November 2008 at four centers in India (Pune, Mumbai, Jaipur and Delhi). A total of 110 healthy infants between ages of 6 to 12 weeks of age were enrolled to receive three 2 mL doses of PRV orally at visits 4 to 10 weeks apart. The interval between doses was a minimum of 4 weeks (28 d) to a maximum of 10 weeks (70 d). Infants were excluded if they had active gastrointestinal illness, congenital abdominal disorders, intussusception, and impairment of immunological function or prior administration of rotavirus vaccine. There were no restrictions on breast feeding or on administration of other routine pediatric vaccines, including oral polio vaccine (including additional doses that may have been administered during the Pulse Polio Program). We did not test the infants for infections with other enteric infections prior to enrollment.
The study protocol was approved by the ethics review committees of participating sites, and written informed consent was obtained from each participant’s parent or guardian before enrollment. This trial was performed according to the ethical principles that have their origin in the Declaration of Helsinki, Good Clinical Practice (GCP) guidelines issued by the Central Drugs Standard Control Organization, Ministry of Health, Government of India and applicable regulatory requirements.
The per protocol population (PPP) excluded subjects according to the exclusion criteria and the following: (1) Subjects who did not have 3 vaccinations at 3 separate visits scheduled at least 4 weeks (28 d) apart were excluded; (2) Subjects who did not have valid serology results at scheduled bleeds (due to missing bleed, bleed off schedule, lost sample, insufficient quantity of sera for assay, etc.) were excluded at that time point (but eligible for inclusion at earlier and later time points for GMTs); (3) For analyses involving both prevalues and postvalues, e.g., 3‐fold rise analyses, a subject shall be excluded if a Pre dose 1 or a Post dose 3 value is missing.
Evaluation of immunogenicity
Immune responses to vaccination were assessed by collecting serum samples before the first dose and approximately 14 d after the third dose for measurement of anti rotavirus IgA titers and neutralizing antibodies against the G1, G2, G3, G4 and P[8] serotypes. Seroconversion was defined as an increase in the antibody titer by a factor of 3 or more from baseline. Assays were validated and performed at Cincinnati Children’s Hospital Medical Center.
In this trial we found that 19 infants had serum anti rotavirus IgA ≥ 20 IU/mL at baseline. These infants were probably exposed to rotavirus prior to enrollment in the study. We also analyzed seroconversion data in the subset of infants who had serum anti rotavirus IgA ≤ 20 IU/mL at baseline.
Safety assessment
The parents of infants were instructed to record solicited adverse events for 7 d after each dose and unsolicited adverse events for 14 d after each dose in the vaccine report cards (VRC) provided at each visit. All serious adverse experiences, including any potential cases of intussusception, which occurred at any time during the study period were to be reported immediately.
Safety was assessed on the total number of adverse events, vaccine-related adverse events and serious adverse events in the 14 d post vaccination period after each dose.
Acknowledgments
We are indebted to the infants and their parents/guardians who participated in this study. We are also thankful to Michelle Goveia, MD for the expert review of this manuscript (Senior Medical Director at Merck and Co., Inc., Whitehouse Station). This study was sponsored by Merck and Co., Inc., Whitehouse Station.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Parashar UD, Gibson CJ, Bresee JS, Glass RI. Rotavirus and severe childhood diarrhea. Emerg Infect Dis 2006; 12:304 - 6; http://dx.doi.org/10.3201/eid1202.050006; PMID: 16494759
- American Academy of Pediatrics Committee on Infectious Diseases. Prevention of rotavirus disease: guidelines for use of rotavirus vaccine. Pediatrics 2007; 119:171 - 82; http://dx.doi.org/10.1542/peds.2006-3134; PMID: 17200286
- Parashar UD, Alexander JP, Glass RI, Advisory Committee on Immunization Practices (ACIP), Centers for Disease Control and Prevention (CDC). Prevention of rotavirus gastroenteritis among infants and children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55:RR-12 1 - 13; PMID: 16902398
- Parashar UD, Burton A, Lanata C, Boschi-Pinto C, Shibuya K, Steele D, et al. Global mortality associated with rotavirus disease among children in 2004. J Infect Dis 2009; 200:Suppl 1 S9 - 15; http://dx.doi.org/10.1086/605025; PMID: 19817620
- Steele AD, Patel M, Parashar UD, Victor JC, Aguado T, Neuzil KM. Rotavirus vaccines for infants in developing countries in Africa and Asia: considerations from a world health organization-sponsored consultation. J Infect Dis 2009; 200:Suppl 1 S63 - 9; http://dx.doi.org/10.1086/605042; PMID: 19817616
- Tate JE, Chitambar S, Esposito DH, Sarkar R, Gladstone B, Ramani S, et al. Disease and economic burden of rotavirus diarrhoea in India. Vaccine 2009; 27:Suppl 5 F18 - 24; http://dx.doi.org/10.1016/j.vaccine.2009.08.098; PMID: 19931713
- Kang G, Arora R, Chitambar SD, Deshpande J, Gupte MD, Kulkarni M, et al, Indian Rotavirus Strain Surveillance Network. Multicenter, hospital-based surveillance of rotavirus disease and strains among indian children aged <5 years. J Infect Dis 2009; 200:Suppl 1 S147 - 53; http://dx.doi.org/10.1086/605031; PMID: 19817593
- Rotavirus vaccines:an update. [English, French.] Wkly Epidemiol Rec 2009; 84:533 - 40; PMID: 20034143
- Curns AT, Steiner CA, Barrett M, Hunter K, Wilson E, Parashar UD. Reduction in acute gastroenteritis hospitalizations among US children after introduction of rotavirus vaccine: analysis of hospital discharge data from 18 US states. J Infect Dis 2010; 201:1617 - 24; http://dx.doi.org/10.1086/652403; PMID: 20402596
- Patel M, Pedreira C, De Oliveira LH, Tate J, Orozco M, Mercado J, et al. Association between pentavalent rotavirus vaccine and severe rotavirus diarrhea among children in Nicaragua. JAMA 2009; 301:2243 - 51; http://dx.doi.org/10.1001/jama.2009.756; PMID: 19491186
- Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, et al, Rotavirus Efficacy and Safety Trial (REST) Study Team. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med 2006; 354:23 - 33; http://dx.doi.org/10.1056/NEJMoa052664; PMID: 16394299
- Ciarlet M, Schödel F. Development of a rotavirus vaccine: clinical safety, immunogenicity, and efficacy of the pentavalent rotavirus vaccine, RotaTeq. Vaccine 2009; 27:Suppl 6 G72 - 81; http://dx.doi.org/10.1016/j.vaccine.2009.09.107; PMID: 20006144
- Ciarlet M, Sani-Grosso R, Yuan G et al. Safety and immunogenicity of concomitant use of the pentavalent rotavirus vaccine (RotaTeqTM) and oral poliovirus vaccine (OPV). Presented at: European Society for Pediatric Infectious Diseases (ESPID). Porto, Portugal, 2-4 May 2007.
- Narang A, Bose A, Pandit AN, Dutta P, Kang G, Bhattacharya SK, et al. Immunogenicity, reactogenicity and safety of human rotavirus vaccine (RIX4414) in Indian infants. Hum Vaccin 2009; 5:414 - 9; PMID: 19276664
- Centers for Disease Control and Prevention (CDC). Reduction in rotavirus after vaccine introduction--United States, 2000-2009. MMWR Morb Mortal Wkly Rep 2009; 58:1146 - 9; PMID: 19847149
- Link: http://www.iapcoi.com/pdf/13-%20ROTA%20VIRUS%20VACCINES.pdf as accessed on 6 July 2011.
- Zaman K, Dang DA, Victor JC, Shin S, Yunus M, Dallas MJ, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 376:615 - 23; http://dx.doi.org/10.1016/S0140-6736(10)60755-6; PMID: 20692031