Abstract
Seasonal influenza in healthy working-age adults accounts for a substantial part of the socioeconomic burden of this disease. Intanza® 9 μg (sanofi pasteur) is a microneedle-delivered intradermal trivalent inactivated influenza vaccine approved in 2009 for the prevention of seasonal influenza in adults 18 to 59 y of age. The microneedle system reliably and reproducibly delivers the vaccine to the dermis. Clinical studies show that Intanza 9 μg is as immunogenic and as well tolerated in working-age adults as a reference intramuscular trivalent inactivated vaccine. Local reactions to Intanza 9 μg, mainly erythema, are transient, mostly mild or moderate, and do not affect acceptability. Intanza 9 μg is considered satisfactory by at least 95% of both vaccinees and prescribers, especially because of the short needle and rapid administration. Because Intanza® 9 μg offers an alternative to intramuscular vaccines, it might help increase influenza vaccine coverage rates.
Burden of Seasonal Influenza in Adults 18 to 59 Years of Age
Seasonal influenza is a threat to public health, particularly in older adults (≥ 65 y), children up to 5 y of age, pregnant women and people with certain chronic diseases and conditions.Citation1 In the United States, influenza is estimated to cause 1.4 to 16.7 deaths per 100,000 persons.Citation2 Globally, influenza is estimated to cause three to five million cases of severe illness and 250,000 to 500,000 deaths each year.Citation3 Most deaths and hospitalizations due to influenza are in high-risk groups.Citation3-Citation7
Although influenza rarely causes hospitalization and death in healthy non-elderly adults, it produces significant morbidity in this group.Citation8 In non-elderly adults, common symptoms are initial fever and chills, which can be accompanied or followed by headache, sore throat, dry cough, nasal discharge, loss of appetite, hoarseness, chest congestion, fatigue, malaise and muscle, abdominal, chest and joint pain. Gastrointestinal complaints can also occur but are less common in this group than in children. Recovery can take up to one week,Citation9 and approximately 1 in 10 non-elderly adults age suffer from influenza-related complications, especially bronchitis, sinusitis, rhinitis and otitis media.Citation10 More serious but relatively rare complications include pneumonia, superinfections, exacerbation of asthma and cardiac, neurological and cerebrovascular complications.Citation8 Each episode of laboratory-confirmed influenza results in 1.5 to 4.9 working days lost,Citation11 and vaccine-preventable influenza causes as much as 740 work days lost per 1,000 person-years in working-age adults.Citation12 Overall, loss of productivity and missed work days caused by influenza are estimated to represent one-fifth to one-third of the annual economic loss from influenza.Citation13,Citation14 For example, in 2003, lost work and work productivity resulted in $17 billion of $84 billion in influenza-related costs in the US.Citation13
Influenza Vaccination Efficacy in Adults 18 to 59 Years of Age
Several studies have examined vaccine efficacy in working-age adults (18 to 59 y of age) within and outside the workplace. A systematic review in 2010 reported that vaccine efficacy (capacity of the vaccines to prevent influenza A or B and its complications) in healthy adults is around 75% when the vaccine and circulating strains are well-matched,Citation15 and meta-analysis of data from randomized trials estimated the efficacy of trivalent influenza vaccine (TIV) to be 59% (95% confidence interval, 51% to 67%) against PCR- or culture-confirmed influenza in adults 18 to 65 y of age.Citation16 Studies directly comparing the efficacy of TIV and live attenuated influenza vaccine in adults have generated conflicting results and do not demonstrate a clear advantage of one vaccine over the other.Citation17
In working-age adults in the US, influenza vaccination has been reported to reduce lost work days by 32% to 43% and visits to physicians’ offices by 42% to 44%.Citation18,Citation19 Another study in the US found that in working adults 50 to 64 y of age, vaccination reduces days with decreased ability to perform activities, work days lost and days working while ill with influenza-like illness by more than 60%.Citation20 In addition, the PRISMA study found that influenza vaccination prevented 78% of deaths, 87% of hospitalizations and 26% of general practitioner visits in high-risk adults between the ages of 18 and 64 y.Citation21 Cost-effectiveness modeling indicates that in adults under 50 y of age, vaccination is the most cost-effective means of preventing influenza and is more cost-effective than treatment post-infection.Citation22 Finally, because infected working-age adults might be able to transmit influenza to more vulnerable individuals, vaccination of adults 18 to 59 y may benefit other age or risk groups that they come into contact with.
Influenza Vaccination Coverage in Adults 18 to 59 Years of Age
Many countries now aim at increasing vaccine coverage not only in older adults, children and other at-risk groups but also in working-age adults. In 2010, the US Centers for Disease Control and Prevention Advisory Committee on Immunization Practices recommended universal influenza vaccination for all persons ≥ 6 mo of age,Citation23 and their objectives for 2020, stated in the Department of Health and Human Services “Healthy People 2020,” are to reach an influenza vaccination coverage of 80% in non-institutionalized working-age adults without high-risk conditions and 90% in all adults institutionalized or with high-risk conditions.Citation24 A universal influenza immunization program has also been in place in Ontario, Canada since 2010.Citation25 In other countries, there are currently no specific recommendations for influenza immunization coverage in healthy working-age adults, although in 2003, the World Health Organization recommended that coverage rates for all persons with underlying diseases reach 75% by 2010,Citation26 a target officially adopted in 2009 by the European Union for the 2014/2015 influenza season.Citation27
Despite expanded recommendations and the projected savings from them, influenza vaccination coverage remains below these targets. In European countries, influenza vaccination coverage during the 2007/2008 influenza season varied from 10% to 29% in the general population and 11% to 56% in non-elderly individuals with chronic illness.Citation28 In the United States, the most recently reported seasonal influenza vaccination coverage rates (2010/2011 influenza season) were 30% in adults 18 to 49 y, 46% in adults 50 to 64 y and 69% in adults 65 y and older.Citation29
The most common reasons for the low influenza vaccine uptake are mistaken assumptions by consumers, including perceived resistance to influenza and fear of contracting influenza from the vaccine, followed by not having previously considered vaccination and not having a recommendation from the family doctor to be vaccinated.Citation28,Citation30-Citation33 A survey of healthcare providers found that, in addition to these factors, consumers tend to be concerned by lack of insurance coverage and a fear of needles.Citation33 An Israeli study found fear of needles in 1 in 5 travelers visiting a travel health clinic, suggesting that fear of injections might interfere with the receipt of vaccines,Citation34 and a European survey found that 1 in 4 households would be encouraged to be vaccinated if there were other ways of receiving the vaccine than injection with a needle.Citation35 Surveys of vaccination behavior and acceptance in Australia, Argentina, Turkey and the Czech Republic in 2010–2011 found that not feeling at risk of catching the flu and not being encouraged by medical professionals to be vaccinated are the two main reasons for missing influenza vaccinations and that direct communication from physician is the most effective reminder to be vaccinated.Citation36,Citation37
Intradermal Vaccination
Until recently, seasonal influenza vaccines have been administered by intramuscular (IM) injection. Offering alternatives to IM injection might help improve vaccination coverage. Intradermal (ID) vaccination is one alternative. The immunogenicity of vaccines delivered via the ID route is thought to be enhanced by the high density of dermal dendritic cells in the skin and by efficient lymphatic drainage via the skin’s rich microvascular network.Citation38,Citation39 Current understanding of the cellular response to intradermal vaccination was summarized in a 2011 review by Combadiere and Liard.Citation40 Following injection into the skin, resident antigen-presenting cells, especially epidermal keratinocytes, Langerhans cells and dermal dendritic cells, capture and are activated by vaccine antigens, leading to their migration through the dermis and eventually to the draining lymph nodes. Recruited innate immune cells in the skin also capture the antigens or are activated via Toll-like receptors and nucleotide-binding domain leucine-rich repeat receptors. In addition, small vaccine components (< 400 nm) can be passively transported to the secondary lymphoid organs via the microvascular and lymphatic networks. In the draining lymph nodes, antigen-presenting cells present the antigens to CD4+ and CD8+ T cells and to B cells. This activates the T cells, leading to proliferation and differentiation into effector and then memory cells, and induces the clonal expansion and activation of B cells in germanative centers. Effector T and B cells also migrate to the vaccination zone where they eliminate vaccine antigens and differentiate into memory T and B cells. In summary, memory T and B cells generated in the draining lymph nodes and periphery following intradermal vaccination provide long-term immunity against the targeted antigen.
ID vaccination has been studied since the early 1930s,Citation41 and its use for the prevention of seasonal influenza was first described later the same decade.Citation42 The ID route is effective for a variety of antigens and has been most extensively studied for influenza, hepatitis B and rabies.Citation43 ID vaccination has recently attracted great interest because of technical advances that now make it easy and routine.
Several efforts have been made to develop ID influenza vaccines in the hope of improving immune responses in elderly adults or frail individuals or to provide an alternative to IM vaccination.Citation44 Until recently, ID injection had to be performed using the Mantoux method, in which the surface of the skin is stretched and the tip of a 27-gauge needle is inserted.Citation45 This method can give inconsistent results, is technically difficult to perform and can be painful for the vaccinee.Citation39 However, the development of safe, effective and reproducible microinjection systems have recently made routine ID administration feasible.Citation46
Intanza® 9 μg: An Intradermal Split-Virion TIV Delivered by Microinjection for Adults 18 to 59 y of Age
Intanza® 9 μg is an intradermal (ID) split-virion TIV delivered with the BD Soluvia™ microinjection system (Becton Dickinson),Citation47 an optimized single-use system. The microinjection system consists of a prefilled 0.5 ml glass syringe fitted with a 30 gauge, short-bevel microneedle that protrudes 1.5 mm from a depth-limiting tip ().Citation47 The microinjection needle inserts perpendicularly to the skin, which improves the reliability of ID vaccine delivery. The system also includes a shield that covers the needle after injection, avoiding accidental needle-stick injuries to healthcare workers and preventing needle reuse. Preclinical studies using pig dermis, a model for human skin, showed that, even with little or no previous experience by the vaccine administrator, the microinjection system delivered vaccine into the dermis 91% of the time.Citation47 Clinical evaluation showed that ID injection with the microinjection system is technically easy to perform, with nearly 100% of successful injections even for practitioners with no experience with the microinjection device.Citation47 An echographic study in adults indicated that the 1.5 mm microneedle in Intanza should deliver the vaccine into the dermis and not into the subdermal blood vessels or nerves, regardless of the subject’s sex, age, ethnicity or body mass index.Citation48
Figure 1. The Intanza 9 μg system. (A) Features of the Intanza 9 μg system. The enlarged area shows images of the microinjection needle and a regular IM needle. (B) ID injection with the Intanza 9 μg system.
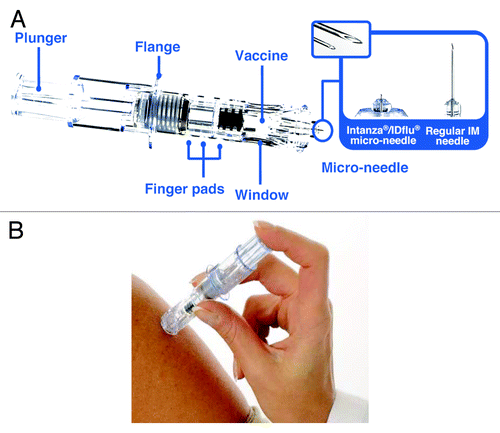
Intanza 9 μg contains A/H1N1, A/H3N2 and B strains at 9 μg hemagglutinin per strain and is manufactured with the same quality and using the same method (with the exception of a concentration step) as Vaxigrip® (sanofi pasteur), an IM split-virion TIV that has been used for more than 45 y and has an established record of safety and efficacy at preventing seasonal influenza.Citation12,Citation49 In 2009, the 9 μg dosage of Intanza (also marketed under the name IDflu®) was approved in Europe for adults 18 to 59 y of age and the 15-μg dosage was approved for adults 60 y of age and older.Citation50,Citation51 Fluzone® ID (sanofi pasteur), approved in the US in 2011 for adults 18 to 65 y of age, is another ID split-virion TIV containing 9 μg hemagglutinin per strain and delivered with the Soluvia microinjection system.Citation52,Citation53 However, Fluzone ID is tested and licensed separately and is not considered equivalent to Intanza. Therefore, the remainder of this review discusses only data for Intanza 9 μg. Reviews of Fluzone ID and Intanza 15 μg have been previously published.Citation50,Citation52
Immunogenicity of Intanza 9 μg in Adults 18 to 59 y of Age
The immunogenicity of Intanza 9 μg in adults 18 to 59 y of age has been assessed in two phase II and one phase III randomized, multicenter clinical trials. The type of study, number of subjects, clinical trial registry number and dates the studies were performed are summarized in and the immunogenicity results for these trials are shown in . These studies compared Intanza 9 μg to an IM reference, which contains 15 μg HA of each of the three influenza strains. Immunogenicity in all of these clinical trials was measured by hemagglutinination inhibition (HI) assay. The European Medicines Agency Committee for Proprietary Medicinal Products (CPMP) requires that vaccines used in adults 18 to 59 y induce seroconversion [post-vaccination HI titer ≥ 40 in pre-vaccination seronegative subjects or a significant (≥ 4-fold) increase in HI titer] in more than 40% of vaccinees, a post-vaccination vs. pre-vaccination geometric mean titer ratio > 2.5 and a post-vaccination HI titer ≥ 40 (i.e., seroprotective) in at least 70% of vaccinees.Citation54
Table 1. Clinical studies in which Intanza 9 μg was evaluated in adults 18 to 59 y of age
Table 2. Immunogenicity of Intanza 9 μg and the IM reference vaccine (Vaxigrip) in adults 18 to 59 y of age
A 3 y phase II trial (NCT00703651) compared the immunogenicity of the TIV administered using the microinjection system or administered IM (i.e., Vaxigrip) in adults 18 to 59 y of age.Citation55 Subjects received the final version of Intanza 9 μg only during the third year of the study. The third-year results showed that Intanza 9 μg induced a non-inferior immune response compared with the IM reference vaccine and met the CPMP criteria for all three viral strains ().Citation54 These findings were confirmed in a second phase II trial (NCT00258934).Citation56 This second phase II study also showed that one year after vaccination, antibody titers with Intanza 9 μg remained above baseline and were similar to those obtained with the IM reference.
Arnou et al. recently reported results from a phase III study of Intanza 9 μg in 2,225 adults 18 to 59 y (NCT00383539) ().Citation57 The results confirmed that Intanza 9 μg induces similar immunogenicity as Vaxigrip and that it meets CPMP criteria for all three vaccine strains. The study also showed equivalent immunogenicity of three lots of the ID vaccine.
Safety and Tolerability of Intanza 9 μg in Adults 18 to 59 y of Age
The two phase II studies and the recent phase III study examined the safety and tolerability of Intanza 9 μg in adults 18 to 59 y of age.Citation55-Citation57 No treatment-related serious adverse events were reported in any of these trials and similar rates of solicited systemic reactions (), severe systemic reactions and unsolicited adverse reactions were reported for the ID 9 μg vaccine and the IM reference. One exception was myalgia, which was reported to be more frequent with the IM vaccine in the second phase IICitation56 and in the phase III study.Citation57 The most common solicited systemic reaction was headache, which occurred in 14% to 33% of subjects in all trials. Severe systemic reactions were rare, occurring in less than 3% of vaccinees with either the ID or the IM vaccine.
Table 3. Percentage of clinical trial subjects 18 to 59 y of age with solicited reactions within 7 d of vaccination with Intanza 9 μg and the IM reference vaccine (Vaxigrip)
In the safety database used for approval of Intanza 9 μg in Europe, injection-site reactions occurred in 92.7% of 2,384 adults 18 to 59 y of age.Citation58 In the individual clinical trials, injection site pain was reported by 38% to 43% of the subjects vaccinated with Intanza 9 μg and was generally mild (). This is similar to the frequency with the IM reference (37% to 48%).Citation55-Citation57 Other injection site reactions, including erythema, swelling, induration and pruritus, also generally mild, were more frequent with Intanza 9 μg than with the IM reference. This is not surprising because the inflammatory process induced by ID vaccination occurs just below the skin surface and therefore can be easily seen and felt, whereas with IM vaccination, the inflammatory process begins deep in the muscle. Finally, all injection site reactions to Intanza 9 μg peaked one day after vaccination and resolved within 3 to 4 d without treatment. Collectively, the clinical trial data show that Intanza 9 μg is well tolerated and that it has a similar systemic safety profile as the IM reference.
Approval and indications for Intanza 9 μg
Intanza 9 μg was approved for the prevention of seasonal influenza in all adults 18 to 59 y of age by the European Medicines Agency in 2009.Citation58 It is now available in more than 40 countries in the northern and southern hemispheres. Intanza 9 μg comes as a 0.1 ml suspension in a prefilled, single-use microinjection syringe. The Intanza unit can be stored at 2°C to 8°C protected from light for up to 1 y.Citation59 Contraindications to Intanza 9 μg include hypersensitivity to the vaccine ingredients and fever or acute infection at the time of vaccination. Intanza 9 μg can be given at the same time as other vaccines by using separate limbs.
Acceptability Studies
During the phase III study of Intanza 9 μg, a validated, self-administered questionnaire was used to help determine whether injection site reactions affect vaccine acceptability.Citation60 This questionnaire, called VAPI © for Vaccinees’ Perception of Injection, was developed based on interviews with vaccinees to assess acceptance of influenza vaccination by the ID and IM routes.Citation61 Of 1679 vaccinees receiving Intanza 9 μg, 97% considered injection site pain and other local reactions to be totally or very acceptable and 94% were not bothered or were only a little bothered by the vaccination.Citation60 Moreover, 96% considered the vaccine to be totally or very acceptable. Also, 98% reported that their sleep and arm movements were not at all or only a little bothered. Overall perception of Intanza 9 μg was good, with 96% reporting to be satisfied or very satisfied with the injection system and 95% willing to be revaccinated.
Results were very similar in post-marketing surveys. In Australia and Argentina during the 2010 southern hemisphere influenza season, 98% of vaccinees were satisfied or very satisfied with Intanza 9 μg, and 95% reported they preferred to receive the same vaccination next year.Citation36 Vaccinees reported that the main reasons for satisfaction were that the injection was considered minimally painful and that the vaccination was quickly administered. In addition, 85% of prescribers were satisfied or very satisfied with Intanza 9 μg. A second survey reported the opinions of adults ≥ 18 y of age in the Czech Republic and adults 18 to 59 y of age in Turkey during the 2010/2011 northern hemisphere influenza. It found that 96% of vaccinees were satisfied or very satisfied with Intanza and 94% would prefer to receive the same vaccination next year.Citation37 The study also found that 95% of vaccine prescribers were satisfied or very satisfied with the intradermal vaccine and 83% preferred intradermal over intramuscular vaccination.
A survey in France and Germany performed in 2009 examined the willingness of physicians to prescribe and individuals to be vaccinated with Intanza.Citation62 In both countries, the majority of physicians (87% in France and 94% in Germany) found Intanza 9 μg appealing, and most (73% and 61%, respectively) reported that they preferred to prescribe Intanza 9 μg over conventional IM vaccination. Intanza was also considered favorably by the general public; 90% in both countries found Intanza appealing, and most vaccinated for influenza during the previous influenza season (75% in Germany and 71% in France) reported preferring to receive Intanza rather than the conventional IM vaccine the following year. Overall, both physicians and the general public considered the thin, short needle as the most important benefit of Intanza. Collectively, the results of the surveys and the phase III questionnaire show that ID vaccination for seasonal influenza using Intanza 9 μg is well accepted both by adult vaccinees and prescribers.
Conclusions and Future Directions
Intanza 9 μg, approved first in 2009 in the European Union for adults 18 to 59 y of age, has equivalent immunogenicity as the IM reference vaccine, Vaxigrip. Except for an increase in certain injection-site reactions with Intanza 9 μg, tolerability is similar. Local reactions to Intanza 9 μg are transient, mostly mild or moderate, and do not affect acceptability. Intanza 9 μg is well accepted by both vaccinees and prescribers, especially because of benefits inherent to the microinjection system. Because Intanza 9 μg offers an alternative to IM injection, it might help increase influenza vaccine coverage rates, although further studies are needed to confirm this possibility and to assess consumer preference. Furthermore, the effectiveness of Intanza 9 µg in preventing influenza in working-age adults has not been reported and remains to be confirmed.
| Abbreviations: | ||
| CPMP | = | Committee for Proprietary Medicinal Products |
| HI | = | hemagglutinination inhibition |
| ID | = | intradermal |
| IM | = | intramuscular |
| TIV | = | trivalent influenza vaccine |
Acknowledgments
The authors thank Dr. Phillip Leventhal (4Clinics) for medical writing assistance. Medical writing assistance was paid for by sanofi pasteur.
References
- Monto AS. Seasonal influenza and vaccination coverage. Vaccine 2010; 28:Suppl 4 D33 - 44; http://dx.doi.org/10.1016/j.vaccine.2010.08.027; PMID: 20713259
- Centers for Disease Control and Prevention (CDC). Estimates of deaths associated with seasonal influenza --- United States, 1976-2007. MMWR Morb Mortal Wkly Rep 2010; 59:1057 - 62; PMID: 20798667
- World Health Organization. Influenza (seasonal). Geneva: World Health Organization Media Centre, 2009.
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289:179 - 86; http://dx.doi.org/10.1001/jama.289.2.179; PMID: 12517228
- Glezen WP, Greenberg SB, Atmar RL, Piedra PA, Couch RB. Impact of respiratory virus infections on persons with chronic underlying conditions. JAMA 2000; 283:499 - 505; http://dx.doi.org/10.1001/jama.283.4.499; PMID: 10659876
- Barker WH. Excess pneumonia and influenza associated hospitalization during influenza epidemics in the United States, 1970-78. Am J Public Health 1986; 76:761 - 5; http://dx.doi.org/10.2105/AJPH.76.7.761; PMID: 3717461
- Barker WH, Mullooly JP. Impact of epidemic type A influenza in a defined adult population. Am J Epidemiol 1980; 112:798 - 811; PMID: 7457471
- Cox NJ, Subbarao K. Influenza. Lancet 1999; 354:1277 - 82; http://dx.doi.org/10.1016/S0140-6736(99)01241-6; PMID: 10520648
- Treanor JJ, Hayden FG, Vrooman PS, Barbarash R, Bettis R, Riff D, et al, US Oral Neuraminidase Study Group. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. JAMA 2000; 283:1016 - 24; http://dx.doi.org/10.1001/jama.283.8.1016; PMID: 10697061
- Roche Pharmaceuticals. Easing the burden: the challenge of managing influenza. Am J Manag Care 2000; 6:Suppl S276 - 81; PMID: 10977474
- Keech M, Beardsworth P. The impact of influenza on working days lost: a review of the literature. Pharmacoeconomics 2008; 26:911 - 24; http://dx.doi.org/10.2165/00019053-200826110-00004; PMID: 18850761
- Arnoux S, Weinberger C, Gessner BD. Vaccine-preventable influenza disease burden from clinical trials of Vaxigrip - an inactivated split virion influenza vaccine - supports wider vaccine use. Vaccine 2007; 25:7720 - 31; http://dx.doi.org/10.1016/j.vaccine.2007.08.063; PMID: 17920168
- Molinari NA, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine 2007; 25:5086 - 96; http://dx.doi.org/10.1016/j.vaccine.2007.03.046; PMID: 17544181
- Xue Y, Kristiansen IS, de Blasio BF. Modeling the cost of influenza: the impact of missing costs of unreported complications and sick leave. BMC Public Health 2010; 10:724; http://dx.doi.org/10.1186/1471-2458-10-724; PMID: 21106057
- Jefferson T, Di Pietrantonj C, Rivetti A, Bawazeer GA, Al-Ansary LA, Ferroni E. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev 2010; CD001269; PMID: 20614424
- Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:36 - 44; http://dx.doi.org/10.1016/S1473-3099(11)70295-X; PMID: 22032844
- Tosh PK, Jacobson RM, Poland GA. Influenza vaccines: from surveillance through production to protection. Mayo Clin Proc 2010; 85:257 - 73; http://dx.doi.org/10.4065/mcp.2009.0615; PMID: 20118381
- Bridges CB, Thompson WW, Meltzer MI, Reeve GR, Talamonti WJ, Cox NJ, et al. Effectiveness and cost-benefit of influenza vaccination of healthy working adults: A randomized controlled trial. JAMA 2000; 284:1655 - 63; http://dx.doi.org/10.1001/jama.284.13.1655; PMID: 11015795
- Nichol KL, Lind A, Margolis KL, Murdoch M, McFadden R, Hauge M, et al. The effectiveness of vaccination against influenza in healthy, working adults. N Engl J Med 1995; 333:889 - 93; http://dx.doi.org/10.1056/NEJM199510053331401; PMID: 7666874
- Nichol KL, D’Heilly SJ, Greenberg ME, Ehlinger E. Burden of influenza-like illness and effectiveness of influenza vaccination among working adults aged 50-64 years. Clin Infect Dis 2009; 48:292 - 8; http://dx.doi.org/10.1086/595842; PMID: 19115970
- Hak E, Buskens E, van Essen GA, de Bakker DH, Grobbee DE, Tacken MA, et al. Clinical effectiveness of influenza vaccination in persons younger than 65 years with high-risk medical conditions: the PRISMA study. Arch Intern Med 2005; 165:274 - 80; http://dx.doi.org/10.1001/archinte.165.3.274; PMID: 15710789
- Rothberg MB, Rose DN. Vaccination versus treatment of influenza in working adults: a cost-effectiveness analysis. Am J Med 2005; 118:68 - 77; http://dx.doi.org/10.1016/j.amjmed.2004.03.044; PMID: 15639212
- Fiore AE, Uyeki TM, Broder K, Finelli L, Euler GL, Singleton JA, et al, Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep 2010; 59:RR-8 1 - 62; PMID: 20689501
- Centers for Disease Control. Healthy People 2020 Summary of Objectives. 2011.
- Schabas RE. Mass influenza vaccination in Ontario: a sensible move. CMAJ 2001; 164:36 - 7; PMID: 11202665
- World Health Organization. Resolution of the World Health Assembly WHA 56.19. Prevention and control of influenza pandemics and annual epidemics. 56th World Health Assembly. Geneva, 2003.
- The Council of the European Union. Council recommendation of 22 December 2009 on seasonal influenza vaccination. Official Journal of the European Union 2009; L348:71 - 2
- Blank PR, Schwenkglenks M, Szucs TD. Vaccination coverage rates in eleven European countries during two consecutive influenza seasons. J Infect 2009; 58:446 - 58; http://dx.doi.org/10.1016/j.jinf.2009.04.001; PMID: 19446340
- Centers for Disease Control and Prevention (CDC). Interim results: state-specific influenza vaccination coverage--United States, August 2010-February 2011. MMWR Morb Mortal Wkly Rep 2011; 60:737 - 43; PMID: 21659982
- Kroneman MW, van Essen GA. Stagnating influenza vaccine coverage rates among high-risk groups in Poland and Sweden in 2003/4 and 2004/5. Euro Surveill 2007; 12:E1 - 2; PMID: 17991383
- Müller D, Szucs TD. Influenza vaccination coverage rates in 5 European countries: a population-based cross-sectional analysis of the seasons 02/03, 03/04 and 04/05. Infection 2007; 35:308 - 19; http://dx.doi.org/10.1007/s15010-007-6218-5; PMID: 17885730
- Madjid M, Alfred A, Sahai A, Conyers JL, Casscells SW. Factors contributing to suboptimal vaccination against influenza: results of a nationwide telephone survey of persons with cardiovascular disease. Tex Heart Inst J 2009; 36:546 - 52; PMID: 20069079
- Johnson DR, Nichol KL, Lipczynski K. Barriers to adult immunization. Am J Med 2008; 121:Suppl 2 S28 - 35; http://dx.doi.org/10.1016/j.amjmed.2008.05.005; PMID: 18589065
- Nir Y, Paz A, Sabo E, Potasman I. Fear of injections in young adults: prevalence and associations. Am J Trop Med Hyg 2003; 68:341 - 4; PMID: 12685642
- Blank PR, Schwenkglenks M, Szucs TD. Influenza vaccination coverage rates in five European countries during season 2006/07 and trends over six consecutive seasons. BMC Public Health 2008; 8:272; http://dx.doi.org/10.1186/1471-2458-8-272; PMID: 18673545
- Eizenberg P, Booy R, Naser N, Mason G, Stamboulian D, Weber F. Acceptance of Intanza® 9 μg intradermal influenza vaccine in routine clinical practice in Australia and Argentina. Adv Ther 2011; 28:640 - 9; http://dx.doi.org/10.1007/s12325-011-0042-0; PMID: 21751080
- Prymula R, Usluer G, Altinel S, Sichova R, Weber F. Acceptance and opinions of Intanza/IDflu intradermal influenza vaccine in the Czech Republic and Turkey. Adv Ther 2012; 29:41 - 52; http://dx.doi.org/10.1007/s12325-011-0090-5; PMID: 22228256
- Durando P, Iudici R, Alicino C, Alberti M, de Florentis D, Ansaldi F, et al. Adjuvants and alternative routes of administration towards the development of the ideal influenza vaccine. Hum Vaccin 2011; 7:Suppl 29 - 40; http://dx.doi.org/10.4161/hv.7.0.14560; PMID: 21245655
- Lambert PH, Laurent PE. Intradermal vaccine delivery: will new delivery systems transform vaccine administration?. Vaccine 2008; 26:3197 - 208; http://dx.doi.org/10.1016/j.vaccine.2008.03.095; PMID: 18486285
- Combadiere B, Liard C. Transcutaneous and intradermal vaccination. Hum Vaccin 2011; 7:811 - 27; http://dx.doi.org/10.4161/hv.7.8.16274; PMID: 21817854
- Tuft L. Active immunization against typhoid fever with particular reference to an intradermal method. J Lab Clin Med 1931; 16:552
- Francis T, Magill TP. The antibody response of human subjects vaccinated with the virus of human influenza. J Exp Med 1937; 65:251 - 9; http://dx.doi.org/10.1084/jem.65.2.251; PMID: 19870599
- Nicolas JF, Guy B. Intradermal, epidermal and transcutaneous vaccination: from immunology to clinical practice. Expert Rev Vaccines 2008; 7:1201 - 14; http://dx.doi.org/10.1586/14760584.7.8.1201; PMID: 18844594
- Ansaldi F, Durando P, Icardi G. Intradermal influenza vaccine and new devices: a promising chance for vaccine improvement. Expert Opin Biol Ther 2011; 11:415 - 27; http://dx.doi.org/10.1517/14712598.2011.557658; PMID: 21299438
- Brewer TF. Preventing tuberculosis with bacillus Calmette-Guérin vaccine: a meta-analysis of the literature. Clin Infect Dis 2000; 31:Suppl 3 S64 - 7; http://dx.doi.org/10.1086/314072; PMID: 11010824
- Prausnitz MR, Mikszta JA, Cormier M, Andrianov AK. Microneedle-based vaccines. Curr Top Microbiol Immunol 2009; 333:369 - 93; http://dx.doi.org/10.1007/978-3-540-92165-3_18; PMID: 19768415
- Laurent PE, Bonnet S, Alchas P, Regolini P, Mikszta JA, Pettis R, et al. Evaluation of the clinical performance of a new intradermal vaccine administration technique and associated delivery system. Vaccine 2007; 25:8833 - 42; http://dx.doi.org/10.1016/j.vaccine.2007.10.020; PMID: 18023942
- Laurent A, Mistretta F, Bottigioli D, Dahel K, Goujon C, Nicolas JF, et al. Echographic measurement of skin thickness in adults by high frequency ultrasound to assess the appropriate microneedle length for intradermal delivery of vaccines. Vaccine 2007; 25:6423 - 30; http://dx.doi.org/10.1016/j.vaccine.2007.05.046; PMID: 17640778
- Delore V, Salamand C, Marsh G, Arnoux S, Pepin S, Saliou P. Long-term clinical trial safety experience with the inactivated split influenza vaccine, Vaxigrip. Vaccine 2006; 24:1586 - 92; http://dx.doi.org/10.1016/j.vaccine.2005.10.008; PMID: 16271424
- Atmar RL, Patel SM, Keitel WA. Intanza(®): a new intradermal vaccine for seasonal influenza. Expert Rev Vaccines 2010; 9:1399 - 409; http://dx.doi.org/10.1586/erv.10.134; PMID: 21105776
- Duggan ST, Plosker GL. Intanza 15 microg intradermal seasonal influenza vaccine: in older adults (aged >or=60 years). Drugs Aging 2010; 27:597 - 605; http://dx.doi.org/10.2165/11203880-000000000-00000; PMID: 20583853
- Ansaldi F, de Florentiis D, Durando P, Icardi G. Fluzone(®) Intradermal vaccine: a promising new chance to increase the acceptability of influenza vaccination in adults. Expert Rev Vaccines 2012; 11:17 - 25; http://dx.doi.org/10.1586/erv.11.154; PMID: 22149703
- Frenck RW Jr., Belshe R, Brady RC, Winokur PL, Campbell JD, Treanor J, et al. Comparison of the immunogenicity and safety of a split-virion, inactivated, trivalent influenza vaccine (Fluzone®) administered by intradermal and intramuscular route in healthy adults. Vaccine 2011; 29:5666 - 74; http://dx.doi.org/10.1016/j.vaccine.2011.06.010; PMID: 21699951
- Committee for Proprietary Medicinal Products. Note for guidance on harmonization of requirements for influenza vaccines (CPMP/BWP/214/96). London: European Agency for the Evaluation of Medicinal Products, 1997.
- Beran J, Ambrozaitis A, Laiskonis A, Mickuviene N, Bacart P, Calozet Y, et al. Intradermal influenza vaccination of healthy adults using a new microinjection system: a 3-year randomised controlled safety and immunogenicity trial. BMC Med 2009; 7:13; http://dx.doi.org/10.1186/1741-7015-7-13; PMID: 19341446
- Leroux-Roels I, Vets E, Freese R, Seiberling M, Weber F, Salamand C, et al. Seasonal influenza vaccine delivered by intradermal microinjection: A randomised controlled safety and immunogenicity trial in adults. Vaccine 2008; 26:6614 - 9; http://dx.doi.org/10.1016/j.vaccine.2008.09.078; PMID: 18930093
- Arnou R, Eavis P, Pardo JR, Ambrozaitis A, Kazek MP, Weber F. Immunogenicity, large scale safety and lot consistency of an intradermal influenza vaccine in adults aged 18-60 years: Randomized, controlled, phase III trial. Hum Vaccin 2010; 6:346 - 54; http://dx.doi.org/10.4161/hv.6.4.10961; PMID: 20372053
- European Medicines Agency. Assessment report for Intanza®. London: European Medicines Agency, 2009.
- European Medicines Agency. Intanza 9 μg summary of product characteristics. 2011.
- Reygrobellet C, Viala-Danten M, Meunier J, Weber F, Nguyen VH. Perception and acceptance of intradermal influenza vaccination: Patient reported outcomes from phase 3 clinical trials. Hum Vaccin 2010; 6:336 - 45; http://dx.doi.org/10.4161/hv.6.4.10753; PMID: 20372083
- Chevat C, Viala-Danten M, Dias-Barbosa C, Nguyen VH. Development and psychometric validation of a self-administered questionnaire assessing the acceptance of influenza vaccination: the Vaccinees’ Perception of Injection (VAPI) questionnaire. Health Qual Life Outcomes 2009; 7:21; http://dx.doi.org/10.1186/1477-7525-7-21; PMID: 19261173
- Arnou R, Frank M, Hagel T, Prébet A. Willingness to vaccinate or get vaccinated with an intradermal seasonal influenza vaccine: a survey of general practitioners and the general public in France and Germany. Adv Ther 2011; 28:555 - 65; http://dx.doi.org/10.1007/s12325-011-0035-z; PMID: 21626269