Abstract
Combined therapy using chemotherapeutic drugs and immunotherapeutics offers some promise for treating patients with cancer. In this study, we evaluated whether cisplatin delivered by intratumoral (IT)-electroporation (EP) might enhance antitumor activity against established B16 melanoma and whether further addition of intramuscular (IM)-EP of IL-12 cDNA to IT-EP of cisplatin might augment antitumor therapeutic activity, with a focus on the underlining antitumor mechanism(s). When tumor (7 mm)-bearing animals were treated locally with cisplatin by IT-EP, they showed tumor growth inhibition significantly more than those without IT-EP. Moreover, IL-12 cDNA delivered by IM-EP was also able to inhibit tumor growth significantly more than control vector delivery. This tumor growth inhibition was mediated by NK cells, but not CD4+ T or CD8+ T cells, as determined by immune cell subset depletion and IFN-γ induction. Moreover, concurrent therapy using IT-EP of cisplatin plus IM-EP of IL-12 cDNA displayed antitumor therapeutic synergy. This therapeutic synergy appeared to be mediated by increased sensitivity of cisplatin-treated tumors to NK cell-mediated tumor killing. Taken together, these data support that cisplatin delivery by IT-EP plus IL-12 gene delivery by IM-EP are more effective at inducing antitumor therapeutic responses through increased sensitivity of cisplatin-treated tumors to NK cell-mediated tumor killing. This combined approach might have some implication for treating melanoma in patients.
Introduction
Melanoma is a malignant tumor of melanocyte, which is found mainly in skin. The incidence of this malignancy has been increasing yearly and the prognosis for metastatic melanoma is still poor. Furthermore, therapy for this disease is complicated by higher rates of metastasis and its resistance to many chemotherapeutic drugs.Citation1,Citation2 Despite this, numerous preclinical and clinical studies have shown that immune-based therapy might be beneficial for treating melanoma. For example, tumor infiltrating lymphocytes (TILs) expanded ex vivo and then delivered into patients with melanoma are effective for removing tumor masses in the patients.Citation3,Citation4
IL-12 is a heterodimeric cytokine consisting of two chains, p35 and p40. This cytokine is mainly produced by activated antigen-presenting cells including macrophages, dendritic cells, and B cells.Citation5,Citation6 IL-12 promotes natural killer (NK) cell activity and enhances CTL maturation.Citation7 Furthermore, IL-12 induces interferon (IFN)-γ production by NK cells.Citation5 Numerous studies have reported that IL-12 has a beneficial role in inducing antitumor effects in vivo. For instance, direct administration of IL-12 proteins or cDNA expressing IL-12 can affect tumor progression and metastasis in animal models.Citation8-Citation10 Similarly, intratumoral (IT) injection of adenovirus expressing IL-12 has been demonstrated to be effective for suppression of tumor growth in various animal models.Citation11-Citation14 More recently, IT-electroporation (EP) delivery of IL-12 cDNA resulted in the induction of CTLs specific for Trp2180–188 epitopes, which were responsible for systemic antitumor therapeutic activity.Citation15 On the other hand, intramuscular (IM) injection of DNA vaccines coding for a whole Trp2 proteins has been known to induce Ag-specific CTL responses and antitumor protection from B16 melanoma.Citation16,Citation17 In terms of B16 antigens, moreover, intravenous delivery of Trp2 peptides+poly (I:C)+anti-CD40 Abs were effective at achieving a dramatic level of B16 melanoma regression.Citation18 Our unpublished data also showed that concurrent application of Trp2 peptides+CpG-ODN+anti-4.1BB Abs resulted in regressing large-established melanoma through more induction of Ag-specific CTL responses and their infiltration into the tumor sites. These numerous studies suggest that Trp2 antigenic proteins, which are involved in the synthesis of melanin, might be a potential target for immune-based therapy against melanoma.
Chemotherapy acts against tumor cells by direct induction of apoptosis. In addition, some chemotherapeutic drugs are known to suppress myeloid-derived suppressor cells, thus enhancing antitumor immune responses.Citation19 In addition, chemotherapy can alter the biochemical nature of tumor cells, leading to their increased susceptibility to CTL-mediated tumor cell killing.Citation20,Citation21 In melanoma, chemotherapy induces IT expression of chemokines in favor of immune cell infiltration and tumor control.Citation22 In delivery of chemotherapeutic drugs, EP has proven to be very effective by increasing cell membrane permeability and intracellular access.Citation23 Here we investigate whether a combined use of IT-EP of cisplatin plus IM-EP of IL-12 cDNA might lead to enhanced antitumor therapeutic responses to large-established melanoma, with a special focus on the antitumor therapeutic mechanism(s).
Results
Local delivery of cisplatin using IT-EP controls melanoma more effectively than that without IT-EP
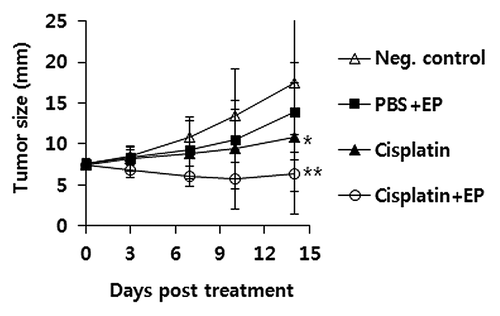
To test whether IT-EP of cisplatin was more effective at controlling melanoma, tumor (7 mm)-bearing animals were treated with cisplatin by IT-EP vs. IT delivery without EP. For this study, a cisplatin dose of 2.5 mg/kg was chosen as this dose was previously observed to be both effective at decreasing tumor growth and not lethal to animals in the TC-1 tumor model.Citation20 As seen in , IT delivery of cisplatin without EP resulted in suppressing tumor growth over the time points, as compared with non-treated control. However, IT-EP delivery of cisplatin showed tumor growth suppression significantly more than IT delivery of cisplatin without EP. Thus, this study shows that IT delivery of cisplatin using EP can make tumor cells more susceptible to cisplatin-mediated tumor cell killing, possibly by increasing more uptake of the chemotherapeutic drug, cisplatin into melanoma cells.
Figure 1. IT-EP of cisplatin results in more inhibition of melanoma growth. Groups of mice (n = 5/group) received 5 × 105 B16 cells s.c. per mouse on their right flank. When the tumor size reached 7 mm in diameter, the animals were injected i.t. with cisplatin at 2.5 mg/kg, followed by EP. Tumor sizes were measured at various time points throughout the experiment. Values and bars represent mean tumor size and the SD, respectively. *p < 0.05 compared with neg. control, **p < 0.05 compared with cisplatin.
IL-12 cDNA delivered by IM-EP controls melanoma more effectively than negative controls while a further addition to Trp2 DNA vaccines has no significant effect on tumor growth inhibition
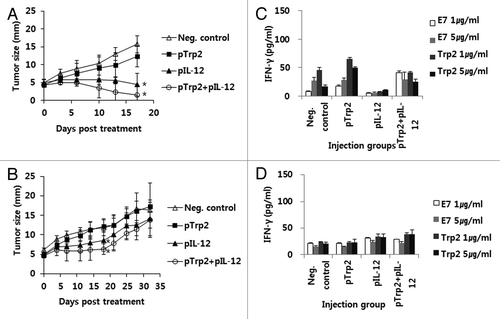
We previously reported that IT-EP of IL-12 cDNA was effective at regressing large-established melanoma through induction of Trp2180–188-specific CD8+ CTL responses.Citation15 In the current study, we investigated whether IM-EP of IL-12 cDNA in combination with DNA vaccines coding for a whole Trp2 protein could also induce tumor cell killing in a manner similar to IT-EP of IL-12. In this study, we also injected animals thrice at 0, 4 and 11 d as this injection protocol was found to be more effective at controlling established tumor in the TC-1 tumor model.Citation25 We first delivered tumor (4 mm)-bearing mice with 50 and 30 μg of IL-12 cDNA by IM-EP, but the tested animals were dead after IL-12 treatment possibly due to the side effects of IL-12 (data not shown). We next tested 10 μg of IL-12 cDNA along with 50 μg of Trp2 DNA vaccines for their antitumor therapeutic activity as well as their tolerability. As shown in , tumor (4 mm)-bearing mice treated by IM-EP with IL-12 cDNA in the presence or absence of Trp2 DNA vaccines showed a more dramatic level of tumor growth suppression over the time points, as compared with those treated with pcDNA3. However, addition of Trp2 DNA vaccines to IL-12 cDNA resulted in insignificant effects on overall tumor growth inhibition. However, IL-12 cDNA at the tested dose made half of the tested mice dead within 3 weeks post-treatment. We next decreased the IL-12 dose to 5 μg per mouse. As seen in , tumor (4 mm)-bearing mice treated by IM-EP with 5 μg of IL-12 cDNA in the presence or absence of Trp2 DNA vaccines displayed more tumor growth suppression over the time points, as compared with those treated without IL-12. At this dose, moreover, all tested animals showed no death over the time points. Thus, these studies suggest that IL-12 cDNA delivered at 5 μg per mouse by IM-EP is tolerable and can induce antitumor therapeutic activity to a significant level and that addition of Trp2 DNA vaccines might have no dramatic effect on tumor growth suppression.
Figure 2. Antitumor therapeutic and Trp2-specific IFN-γ responses by IM-EP of Trp2 DNA vaccines and IL-12 cDNA. (A and B) Groups of mice (n = 5/group) were injected s.c. with 5 x 105 B16 cells/mouse. When tumor sizes reached 4 mm, the animals received IM-EP of Trp2 DNA vaccines (50 μg/mouse) and IL-12 cDNA at the dose of 10 μg (A) and 5 μg (B) per mouse at 0, 4 and 11 d. Tumor sizes were measured at various time points throughout the experiment. Values represent mean tumor size and the SD (C) Similar experiments in except that tumor-bearing mice were sacrificed at 16 d and the spleen cells were stimulated in vitro with 1 or 5 μg/ml of B16 Trp2 peptides and control E7 peptides. The cell supernatants were used for measuring IFN-γ using ELISA. Values and bars represent mean IFN-γ amounts and the SD, respectively. (D) Naïve mice were immunized with Trp2 DNA vaccines (50 μg/mouse) and IL-12 cDNA (5 μg/mouse) by IM-EP at 0 and 1 weeks. At 2 weeks, animals were sacrificed and then spleen cells were stimulated in vitro with 1 or 5 μg/ml of B16 Trp2 peptides and control E7 peptides. The cell supernatants were used for measuring IFN-γ using ELISA. Values and bars represent mean IFN-γ amounts and the SD, respectively. *p < 0.05 compared with neg. control.
IM-EP of IL-12 cDNA induces NK cells for tumor growth inhibition
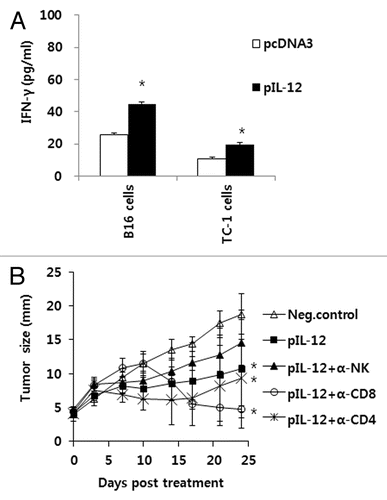
We next determined the antitumor therapeutic mechanism(s) mediated by IM-EP of IL-12 cDNA. For this, we treated tumor-bearing mice with IL-12 cDNA (5 μg/mouse) and Trp2 DNA vaccines (50 μg/mouse) by IM-EP and then tested the induction level of Trp2-specific IFN-γ responses after stimulation of spleen cells in vitro with Trp2 peptides. As shown in , all tested groups failed to induce the production of IFN-γ upon stimulation with Trp2 peptides. A similar finding was observed when naïve mice were immunized with Trp2 DNA vaccines plus IL-12 cDNA and then tested (). These data suggest that Trp2 DNA vaccines even in the presence of IL-12 cDNA are unable to induce Ag-specific CD8+ T cell responses and that IM-EP of IL-12 cDNA might not utilize Trp2-specific CD8+ T cells for tumor control. Taken together, these data suggest that IL-12 cDNA delivered by IM-EP can induce antitumor activity in a manner completely different from that by IT-EP. To further test whether IM-EP of IL-12 cDNA might lead to induction of immune cells specific for B16 tumor cells vs control TC-1 cells, we treated tumor-bearing mice with IL-12 cDNA and then spleen cells were stimulated in vitro with B16 and control TC-1 tumor cells. shows the production level of IFN-γ. Immune cells from tumor-bearing animals under IM-EP of IL-12 responded equally to both B16 cells and control TC-1 cells by increasing IFN-γ production, compared with those receiving control vector (pcDNA3), suggesting that IM-EP of IL-12 cDNA might induce IFN-γ production in a tumor antigen-independent fashion. Based upon this finding, we speculated that IM-EP of IL-12 cDNA might activate NK cells, which might be responsible for tumor growth inhibition. To test this hypothesis, B16 melanoma-bearing animals were treated with IL-12 cDNA by IM-EP and then depleted of CD4+ T cells, CD8+ T cells, and NK cells. As shown in , NK cell depletion eliminated the antitumor effect of IM-EP of IL-12 cDNA. On the other hand, depletion of CD4+ and CD8+ T cells following after IM-EP of IL-12 cDNA had no deleterious effect. Therefore, these results suggest that NK cells are the main effector cells responsible for tumor control by IM-EP of IL-12 cDNA.
Figure 3. Antitumor effector mechanisms by IM-EP of IL-12 cDNA. (A) Groups of mice (n = 5/group) were injected s.c. with 5 x 105 B16 cells/mouse. When tumor sizes reached 4 mm, the animals received IM-EP of IL-12 cDNA at the dose of 5 μg per mouse at 0, 4 and 11 d. At 16 d, the mice were sacrificed and spleen cells were stimulated in vitro for 3 d with mitomycin C-treated B16 and control TC-1 cells. The cell supernatants were used for measuring IFN-γ using ELISA. (B) Groups of mice (n = 5/group) were injected s.c. with 5 x 105 B16 cells/mouse. When tumor sizes reached 4 mm, the animals received IM-EP of IL-12 cDNA (5 μg/mouse) at 0, 4 and 11 d. Some mice were depleted of CD4+ T cells, CD8+ T cells and NK cells using antibodies, as described in “Materials and Methods.” Tumor sizes were measured as described in . *p < 0.05 compared with pcDNA3 or neg. control.
Combined therapy using IT-EP of cisplatin and IM-EP of IL-12 cDNA controls melanoma more effectively than either therapy alone
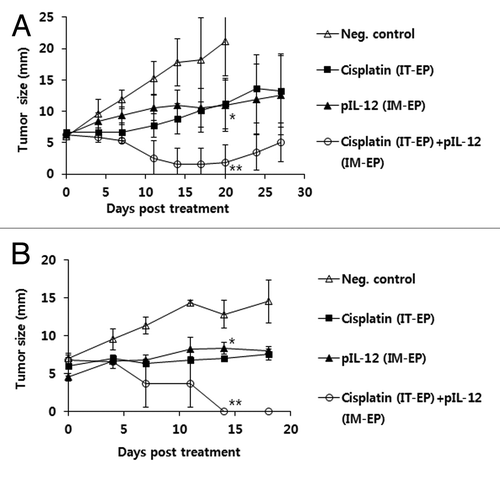
As we observed that IT-EP of cisplatin was more effective at inhibiting melanoma growth than IT delivery without EP and that IM-EP of IL-12 cDNA was also effective at inhibiting melanoma growth by activating NK cells, we next tested whether these two therapy modalities together might treat melanoma better. As shown in , combined therapy showed melanoma growth suppression over the time points significantly more than either one alone. However, single therapy also showed tumor growth suppression significantly more than negative controls as we previously observed. Thus, these data clearly show that combined therapy using IT-EP of cisplatin and IM-EP of IL-12 cDNA might be more effective for treating melanoma in this model.
Figure 4. Combined antitumor therapeutic effects of IT-EP of cisplatin plus IM-EP of IL-12 cDNA. (A) Groups of mice (n = 5/group) were challenged s.c. with 5 × 105 B16 cells/mouse. When tumor sizes reached 7 mm, the animals were injected once with cisplatin at 2.5 mg/kg by IT-EP. Some groups of mice received IM-EP of IL-12 cDNA at 5 μg per mouse at 0, 4 and 11 d. Tumor sizes were measured at various time points throughout the experiment. Values and bars represent mean tumor size and the SD, respectively. (B) Similar experiments in . *p < 0.05 compared with neg. control, **p < 0.05 compared with either cisplatin or pIL-12.
Synergistic antitumor activity is mediated by increased sensitivity of cisplatin-treated melanoma to NK cell-mediated tumor killing
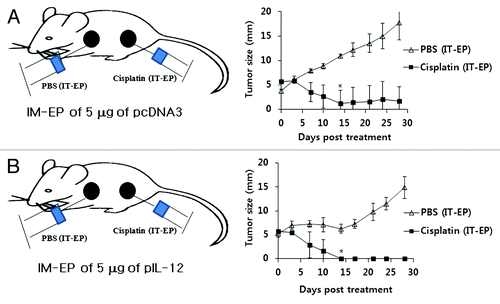
It was possible that some of cisplatin-treated tumor cells might be apoptotic and that IL-12 proteins resulting from IM-EP of IL-12 cDNA might stimulate antigen presenting cells to cross-prime and cross-present the apoptotic tumor-derived antigens for induction of Ag-specific CD8+ T cell responses. To test this hypothesis, we evaluated the level of Trp2-specific CTL lytic activity in tumor-bearing mice under concurrent therapy using IT-EP of cisplatin and IM-EP of IL-12 cDNA. As seen in , all tested mice failed to display Trp2-specific CTL lytic activity in vivo, suggesting that NK cells but not CD8+ T cells are mainly associated with this synergistic tumor control. We next hypothesized that cisplatin-treated tumor cells might be more sensitive to NK cell-mediated killing. This increased susceptibility might be responsible for antitumor therapeutic synergy. To test this hypothesis, we first formed two tumor masses located distantly on the abdominal part and then evaluated the regression status of tumors upon local treatment with cisplatin (right tumor) and PBS (left tumor) by IT-EP while injecting IL-12 cDNA by IM-EP. shows some differences in tumor size of animals under no IL-12 injection between cisplatin and PBS treatments. However, tumors of animals under IM-EP of IL-12 cDNA regressed more dramatically by cisplatin treatment, as opposed to PBS treatment (). Moreover, the growth of PBS-treated tumor was also retarded in an early stage of treatment. Thus, these data show that NK cells can remove cisplatin-treated tumors more effectively than non-treated tumors in vivo. Thus, this finding supports the notion that antitumor therapeutic synergy is mediated by increased sensitivity of cisplatin-treated tumors to NK-mediated killing in this model.
Figure 5. Evaluation of Trp2-specific CTL lytic activity by IT-EP of cisplatin plus IM-EP of IL-12 cDNA. Groups of mice (n = 5/group) were challenged s.c. with 5 × 105 B16 cells/mouse. When tumor sizes reached 6 mm, animals were injected once with cisplatin (2.5 mg/kg) by IT-EP and at the same time treated with 5 μg of IL-12 cDNA per mouse by IM-EP at 0, 4 and 11 d. At 16 d post-treatment, Trp2 peptide-pulsed (CFSE high) and un-pulsed (CFSE low) spleen cells were injected i.v. into the treated mice, as described in “Materials and Methods.” Next day, the mice were sacrificed and spleen cells were used for FACS analysis to measure the levels of CFSE labeled cells of each subset. M1, un-pulsed CFSE low population; M2, Trp2-pulsed CFSE high population.
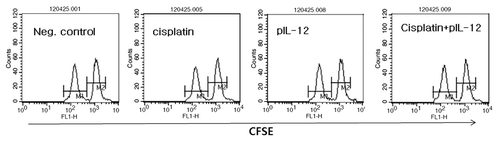
Figure 6. Mechanism(s) for antitumor therapeutic synergy by IT-EP of cisplatin plus IM-EP of IL-12 cDNA. Groups of mice (n = 5/group) were challenged s.c. into the right and left flanks with 5 × 105 B16 cells/mouse. When tumor size reached 5–6 mm on each flank, the left tumor was treated once with PBS by IT-EP while the right one with cisplatin (2.5 mg/kg) by IT-EP. These mice were concurrently treated with 5 μg of pcDNA3 (A) and IL-12 cDNA (B) by IM-EP at 0, 4 and 11 d. Tumor sizes were measured over time. *p < 0.05 compared with PBS.
Discussion
In this study, we observed that IL-12 cDNA treatment by IM-EP was able to inhibit the growth of established B16 tumors. In this case, however, we failed to see any significant role of Trp2180–188-specific CD8+ CTLs for antitumor therapeutic activity. Recently, our group reported that IL-12 cDNA delivered by IT-EP induced Trp2180–188-specific CD8+ CTL for eradicating B16 melanoma.Citation15 In this observation, however, IM-EP of IL-12 cDNA appeared to activate NK cells for B16 melanoma control, as determined by immune cell subset depletion. The involvement of NK cells in tumor growth inhibition by IM-EP of IL-12 cDNA was confirmed by tumor antigen-independent IFN-γ induction patterns we observed in the present study and further by our subsequent finding that concurrent therapy using IT-EP of cisplatin plus IM-EP of IL-12 cDNA failed to induce Trp2-specific CTL responses in vivo. These data are directly in line with the previous report that NK cells are mainly responsible for tumor control by IM-EP of IL-12 cDNA.Citation26 Taken together, these data support the notion that IL-12 cDNA delivered by IM-EP activates NK cells, thereby suppressing melanoma growth.
It is noteworthy here that Trp2 DNA vaccines even in the presence of IL-12 cDNA failed to induce Ag-specific IFN-γ responses in vitro and Ag-specific CTL lytic activity in vivo. In our study, moreover, Trp2 DNA vaccines were unable to induce antitumor therapeutic activity against B16 melanoma. Contrary to this, Trp2 DNA vaccines have been reported to induce Trp2-specific CTL responses and antitumor protection from B16 melanoma cell challenges.Citation16,Citation17 The reason for this apparently conflicting finding between ours and others is not clearly known. However, our subsequent western blot assay and DNA sequencing analysis confirmed that Trp2 DNA vaccines tested here expressed a right size (69 kDa) of Trp2 proteins and possessed a right DNA sequence including the Trp2 epitope sequence (data not shown).
In chemotherapy for treating melanoma, cisplatin has been tested here. It is a drug which inhibits DNA replication. Previously, IT-EP of chemotherapeutic agents has been reported to be more effective for tumor control through increasing cell membrane permeability and intracellular access of the drugs.Citation23 In this study, we also found that cisplatin delivered by IT-EP worked significantly better at suppressing melanoma growth, as compared with IT injection of cisplatin without EP. These data clearly show that local delivery of a chemotherapeutic drug into tumors by EP might be a more attractive method to treat tumors, in particular melanoma usually found on the skin of patients. In our observation, moreover, concurrent therapy of melanoma-bearing mice with cisplatin by IT-EP plus IL-12 cDNA by IM-EP resulted in antitumor therapeutic synergy against large established tumor, as compared with either one alone. This result suggests that cisplatin-based chemotherapy in combination with IL-12 gene therapy can elicit dramatically better tumor control.
We further evaluated the mechanism(s) as to how antitumor therapeutic synergy was mediated by cisplatin plus IL-12 plasmids. We first speculated that cisplatin-treated tumor cells might be taken up by antigen presenting cells, resulting in elicitation of tumor Ag-specific CTL effector cell activities. This hypothesis was somewhat reasonable as cisplatin-treated animals were treated concurrently with IL-12 cDNA. This is based on the previous report that cisplatin can cause tumor cell apoptosis,Citation27 which can engage in antigen presentation.Citation28,Citation29 However, this model of an induction in antigen-presenting capability by B16 tumor cell apoptosis does not fit the extent of the induction of Ag-specific CTL lytic activity in vivo. For instance, CTL lytic activities were not detectable in any of tumor-bearing animals treated with cisplatin plus IL-12 cDNA. We next thought that cisplatin-treated tumor cells could be rendered more susceptible to NK cell-mediated killing. This increased sensitivity might be responsible for antitumor therapeutic synergy. This hypothesis is supported by our subsequent observation that cisplatin-treated tumors were more sensitive to NK cell-mediated killing, as compared with non-treated tumors. This data clearly suggests that increased tumor sensitivity to NK cell-mediated killing might be the possible mechanisms for antitumor therapeutic synergy we observed here. This is in line with the previous finding that cytotoxic drugs, such as 5-FU, dacarbazine or cisplatin, can sensitize tumor cells to CTL-mediated apoptosis in vitro and in vivo.Citation20,Citation30 Therefore, these data support the notion that antitumor therapeutic synergy of cisplatin (by IT-EP) plus IL-12 cDNA (by IM-EP) is mediated mainly by increased sensitivity of cisplatin-exposed tumors to NK cell-mediated killing.
It has been broadly reported that the therapeutic use of IL-12 can lead to serious toxic adverse effects, leading in many instances to death.Citation31 In the present studies, 5μg of IL-12 plasmid delivered by IM-EP had no apparent toxic effects or lethality. Nevertheless, we have observed that more than 10 μg of IL-12 plasmid delivered by IM-EP resulted in a gradual loss of body weights and subsequent death in most tested animals. Contrary to this, we previously found that 50 μg of IL-12 plasmid delivered by IT-EP was tolerable and safe for melanoma control.Citation15 Thus, it is likely that the levels or the duration of expression of IL-12 by IM-EP might be increased as compared with IT-EP, making it cautious to use therapeutically.
In conclusion, we observed an antitumor therapeutic synergy between cisplatin (by IT-EP) and IL-12 cDNA (by IM-EP) in animals with large established B16 melanoma. IL-12 treatment by IM-EP appeared to activate NK cells for melanoma control. Furthermore, increased sensitivity of cisplatin-treated tumor to NK-mediated tumor killing seemed to be mainly responsible for antitumor therapeutic synergy. Thus, this combined approach might have some implication for treating melanoma in patients.
Materials and Methods
Mice and tumors
Female 6 week-old C57BL/6 mice were purchased from Daehan Biolink Co., Chungbuk, Korea. Their care was performed under the guidelines of the Institutional Animal Care and Use Committee-approved protocols. B16 BL6 cells (C57BL/6 background) were purchased from the Korean Cell Line Bank (Seoul, Korea), and grown in complete DMEM medium (10% FBS, 1% L-glutamine, 1% penicillin/streptomycin). The tumor cells were washed two times with phosphate-buffered saline (PBS) and injected into mice.
Reagents and treatment
For IT-EP of cisplatin, mice were injected intratumorally (i.t.) with 100 μl of cisplatin at a dose of 2.5 mg/kg using a 31-gauge needle (BD, Franklin Lakes, NJ), followed by EP at 0.2 V for 4 sec using Cellectra® of VGX International Inc./Inovio in accordance with the manufacturer’s protocol. Cisplatin (0.5 mg/ml) was purchased from Ildong Pharmaceuticals Co. (Seoul, Korea). For IM-EP, mice were injected intramuscularly (i.m.) with 50 μg of pCMV-Trp2 and/or 5 to 10 μg of IL-12 plasmid DNAs (2.5 μg of pIL-12 p35 + 2.5 μg of pIL-12 p40 for 5 μg of pIL-12; 5 μg of pIL-12 p35 + 5 μg of pIL-12 p40 for 10 μg of pIL-12)Citation15 in a final volume of 50 μl in PBS using a 31-gauge needle (BD), followed by electroporation. pCMV-Trp2 encoding a human Trp2 protein was a kind gift of Dr. A. Lladser (Fundacion Cienciapara la Vida). Anti-Trp2 Abs were purchased from Bioss Inc. Plasmid DNA was produced in bacteria and purified by endotoxin-free Qiagen kits according to the manufacturer’s protocol (Qiagen). Trp2 peptides (SVYDFFVWL) and human papillomavirus (HPV) 16 E7 control peptides (RAHYNIVTF) were purchased from Peptron.
In vivo depletion of CD4+ T cells, CD8+ T cells or NK cells
Depletion studies were performed as previously described.Citation20,Citation24 For in vivo cell depletion, anti-CD4 (clone GK1.5), anti-CD8 (clone 2.43) and anti-NK (clone PK 136) antibodies were administered intraperitoneally (i.p.) on days –3, 0 and 3 of IM-EP of IL-12 cDNA. Antibody treatment resulted in more than 98% depletion of specific CD4+ and CD8+ T cell, and NK cell subsets of representative animals over 3 weeks period.
IFN-γ assay
A 1 ml aliquot containing 6 x 106 splenocytes was added to wells of 24 well plates containing 1 and/or 5 μg of Trp2 peptides and HPV16 E7 CTL peptides as a control peptide. In one case, splenocytes were also added to each well containing 2 × 106 B16 and control TC-1 cells previously treated for 3 h with mitomycin C. After 3 days incubation at 37°C in 5% CO2, cell supernatants were secured and then used for detecting levels of IFN-γ using commercial cytokine kits (BD) by adding the extracellular fluids to the IFN-γ-specific ELISA plates.
In vivo CTL lytic activity assay
One fraction of naïve mouse-derived splenocytes was pulsed with 5 μg of Trp2180–188 in cRPMI for 60 min at 37°C while the other one was left un-pulsed. To generate peptide-pulsed cells with high CFSE, the peptide-pulsed splenocytes were incubated with 20 μM CFSE in RPMI (2.5% FBS) for 15 min. However, the un-pulsed cells were incubated with 2.5 μM CFSE in RPMI (2.5% FBS) for 15 min to generate peptide-un-pulsed cells with low CFSE. The cells were then washed three times with PBS to remove unbound CFSE. Finally, an equal number of pulsed and un-pulsed cells (a total of 2 × 107 cells/0.4 ml/mouse) were injected intravenously (i.v.) into mice tested. After 18 h, the mice were sacrificed and then splenocytes were collected. After lysis of red blood cells, the spleen cells were analyzed for CFSE staining using a flow cytometer (BD). %lysis was calculated as 100 x [1-(runprimed/rprimed)]. r (ratio) was calculated as %CFSElow/%CFSEhigh.
Tumor cell challenge
For antitumor therapeutic studies, 5 × 105 B16 cells were injected subcutaneously (s.c.) into the flank of C57BL/6 mice. When tumors were approximately 4–7 mm in mean tumor size, animals were treated by IM-EP with 50 μg of pCMV-Trp2 plus 5 to 10 μg of IL-12 cDNA per mouse. Animals were also treated with cisplatin (2.5 mg/kg) by IT-EP. It took about 9–14 d for tumor to reach the size of 4–7 mm. Mice were monitored twice per week for tumor growth. Tumor size was measured in mm using a caliper, and was recorded as mean diameter [longest surface length (a) and width (b), (a+b)/2]. Mice were euthanized when tumor size reached more than 20 mm in mean diameter.
Statistical analysis
Statistical analysis was done by one-way ANOVA using the SPSS 17.0 software program. Values of experimental groups were compared with values of control group. The p values < 0.05 were considered significant.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0008060).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Li Y, McClay EF. Systemic chemotherapy for the treatment of metastatic melanoma. Semin Oncol 2002; 29:413 - 26; http://dx.doi.org/10.1053/sonc.2002.35237; PMID: 12407507
- Bajetta E, Del Vecchio M, Bernard-Marty C, Vitali M, Buzzoni R, Rixe O, et al. Metastatic melanoma: chemotherapy. Semin Oncol 2002; 29:427 - 45; http://dx.doi.org/10.1053/sonc.2002.35238; PMID: 12407508
- Rosenberg SA, Spiess P, Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science 1986; 233:1318 - 21; http://dx.doi.org/10.1126/science.3489291; PMID: 3489291
- Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer 2003; 3:666 - 75; http://dx.doi.org/10.1038/nrc1167; PMID: 12951585
- Kobayashi M, Fitz L, Ryan M, Hewick RM, Clark SC, Chan S, et al. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med 1989; 170:827 - 45; http://dx.doi.org/10.1084/jem.170.3.827; PMID: 2504877
- Robertson MJ, Soiffer RJ, Wolf SF, Manley TJ, Donahue C, Young D, et al. Response of human natural killer (NK) cells to NK cell stimulatory factor (NKSF): cytolytic activity and proliferation of NK cells are differentially regulated by NKSF. J Exp Med 1992; 175:779 - 88; http://dx.doi.org/10.1084/jem.175.3.779; PMID: 1346796
- Germann T, Gately MK, Schoenhaut DS, Lohoff M, Mattner F, Fischer S, et al. Interleukin-12/T cell stimulating factor, a cytokine with multiple effects on T helper type 1 (Th1) but not on Th2 cells. Eur J Immunol 1993; 23:1762 - 70; http://dx.doi.org/10.1002/eji.1830230805; PMID: 8102100
- Rakhmilevich AL, Turner J, Ford MJ, McCabe D, Sun WH, Sondel PM, et al. Gene gun-mediated skin transfection with interleukin 12 gene results in regression of established primary and metastatic murine tumors. Proc Natl Acad Sci U S A 1996; 93:6291 - 6; http://dx.doi.org/10.1073/pnas.93.13.6291; PMID: 8692808
- Yu WG, Ogawa M, Mu J, Umehara K, Tsujimura T, Fujiwara H, et al. IL-12-induced tumor regression correlates with in situ activity of IFN-gamma produced by tumor-infiltrating cells and its secondary induction of anti-tumor pathways. J Leukoc Biol 1997; 62:450 - 7; PMID: 9335314
- Dias S, Thomas H, Balkwill F. Multiple molecular and cellular changes associated with tumour stasis and regression during IL-12 therapy of a murine breast cancer model. Int J Cancer 1998; 75:151 - 7; http://dx.doi.org/10.1002/(SICI)1097-0215(19980105)75:1<151::AID-IJC23>3.0.CO;2-I; PMID: 9426704
- Gambotto A, Tüting T, McVey DL, Kovesdi I, Tahara H, Lotze MT, et al. Induction of antitumor immunity by direct intratumoral injection of a recombinant adenovirus vector expressing interleukin-12. Cancer Gene Ther 1999; 6:45 - 53; http://dx.doi.org/10.1038/sj.cgt.7700013; PMID: 10078963
- Mazzolini G, Qian C, Xie X, Sun Y, Lasarte JJ, Drozdzik M, et al. Regression of colon cancer and induction of antitumor immunity by intratumoral injection of adenovirus expressing interleukin-12. Cancer Gene Ther 1999; 6:514 - 22; http://dx.doi.org/10.1038/sj.cgt.7700072; PMID: 10608348
- Narvaiza I, Mazzolini G, Barajas M, Duarte M, Zaratiegui M, Qian C, et al. Intratumoral coinjection of two adenoviruses, one encoding the chemokine IFN-gamma-inducible protein-10 and another encoding IL-12, results in marked antitumoral synergy. J Immunol 2000; 164:3112 - 22; PMID: 10706701
- Ahn WS, Bae SM, Kim TY, Kim TG, Lee JM, Namkoong SE, et al. A therapy modality using recombinant IL-12 adenovirus plus E7 protein in a human papillomavirus 16 E6/E7-associated cervical cancer animal model. Hum Gene Ther 2003; 14:1389 - 99; http://dx.doi.org/10.1089/104303403769211619; PMID: 14577920
- Sin JI, Park JB, Lee IH, Park D, Choi YS, Choe J, et al. Intratumoral electroporation of IL-12 cDNA eradicates established melanomas by Trp2180-188-specific CD8+ CTLs in a perforin/granzyme-mediated and IFN-γ-dependent manner: application of Trp2180-188 peptides. Cancer Immunol Immunother 2012; 61:1671 - 82; http://dx.doi.org/10.1007/s00262-012-1214-8; PMID: 22382361
- Bronte V, Apolloni E, Ronca R, Zamboni P, Overwijk WW, Surman DR, et al. Genetic vaccination with “self” tyrosinase-related protein 2 causes melanoma eradication but not vitiligo. Cancer Res 2000; 60:253 - 8; PMID: 10667570
- Lladser A, Mougiakakos D, Tufvesson H, Ligtenberg MA, Quest AF, Kiessling R, et al. DAI (DLM-1/ZBP1) as a genetic adjuvant for DNA vaccines that promotes effective antitumor CTL immunity. Mol Ther 2011; 19:594 - 601; http://dx.doi.org/10.1038/mt.2010.268; PMID: 21157438
- Cho HI, Celis E. Optimized peptide vaccines eliciting extensive CD8 T-cell responses with therapeutic antitumor effects. Cancer Res 2009; 69:9012 - 9; http://dx.doi.org/10.1158/0008-5472.CAN-09-2019; PMID: 19903852
- Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res 2005; 11:6713 - 21; http://dx.doi.org/10.1158/1078-0432.CCR-05-0883; PMID: 16166452
- Bae SH, Park YJ, Park JB, Choi YS, Kim MS, Sin JI. Therapeutic synergy of human papillomavirus E7 subunit vaccines plus cisplatin in an animal tumor model: causal involvement of increased sensitivity of cisplatin-treated tumors to CTL-mediated killing in therapeutic synergy. Clin Cancer Res 2007; 13:341 - 9; http://dx.doi.org/10.1158/1078-0432.CCR-06-1838; PMID: 17200373
- Ramakrishnan R, Assudani D, Nagaraj S, Hunter T, Cho HI, Antonia S, et al. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J Clin Invest 2010; 120:1111 - 24; http://dx.doi.org/10.1172/JCI40269; PMID: 20234093
- Hong M, Puaux AL, Huang C, Loumagne L, Tow C, Mackay C, et al. Chemotherapy induces intratumoral expression of chemokines in cutaneous melanoma, favoring T-cell infiltration and tumor control. Cancer Res 2011; 71:6997 - 7009; http://dx.doi.org/10.1158/0008-5472.CAN-11-1466; PMID: 21948969
- Jaroszeski MJ, Gilbert R, Perrott R, Heller R. Enhanced effects of multiple treatment electrochemotherapy. Melanoma Res 1996; 6:427 - 33; http://dx.doi.org/10.1097/00008390-199612000-00004; PMID: 9013480
- Ye GW, Park JB, Park YJ, Choi YS, Sin JI. Increased sensitivity of radiated murine cervical cancer tumors to E7 subunit vaccine-driven CTL-mediated killing induces synergistic anti-tumor activity. Mol Ther 2007; 15:1564 - 70; http://dx.doi.org/10.1038/sj.mt.6300149; PMID: 17505485
- Lee IH, Park JB, Cheong M, Choi YS, Park D, Sin JI. Antitumor therapeutic and antimetastatic activity of electroporation-delivered human papillomavirus 16 E7 DNA vaccines: a possible mechanism for enhanced tumor control. DNA Cell Biol 2011; 30:975 - 85; http://dx.doi.org/10.1089/dna.2011.1266; PMID: 21649506
- Li S, Zhang L, Torrero M, Cannon M, Barret R. Administration route- and immune cell activation-dependent tumor eradication by IL12 electrotransfer. Mol Ther 2005; 12:942 - 9; http://dx.doi.org/10.1016/j.ymthe.2005.03.037; PMID: 15953768
- Kim JH, Ajaz M, Lokshin A, Lee YJ. Role of antiapoptotic proteins in tumor necrosis factor-related apoptosis-inducing ligand and cisplatin-augmented apoptosis. Clin Cancer Res 2003; 9:3134 - 41; PMID: 12912965
- Keane MM, Ettenberg SA, Nau MM, Russell EK, Lipkowitz S. Chemotherapy augments TRAIL-induced apoptosis in breast cell lines. Cancer Res 1999; 59:734 - 41; PMID: 9973225
- Zisman A, Ng CP, Pantuck AJ, Bonavida B, Belldegrun AS. Actinomycin D and gemcitabine synergistically sensitize androgen-independent prostate cancer cells to Apo2L/TRAIL-mediated apoptosis. J Immunother 2001; 24:459 - 71; http://dx.doi.org/10.1097/00002371-200111000-00003; PMID: 11759069
- Yang S, Haluska FG. Treatment of melanoma with 5-fluorouracil or dacarbazine in vitro sensitizes cells to antigen-specific CTL lysis through perforin/granzyme- and Fas-mediated pathways. J Immunol 2004; 172:4599 - 608; PMID: 15034078
- Leonard JP, Sherman ML, Fisher GL, Buchanan LJ, Larsen G, Atkins MB, et al. Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-gamma production. Blood 1997; 90:2541 - 8; PMID: 9326219