Abstract
The safety and immunogenicity of a new candidate tuberculosis (TB) vaccine, FP85A was evaluated alone and in heterologous prime-boost regimes with another candidate TB vaccine, MVA85A. This was an open label, non-controlled, non-randomized Phase I clinical trial. Healthy previously BCG-vaccinated adult subjects were enrolled sequentially into three groups and vaccinated with FP85A alone, or both FP85A and MVA85A, with a four week interval between vaccinations. Passive and active data on adverse events were collected. Immunogenicity was evaluated by Enzyme Linked Immunospot (ELISpot), flow cytometry and Enzyme Linked Immunosorbent assay (ELISA). Most adverse events were mild and there were no vaccine-related serious adverse events. FP85A vaccination did not enhance antigen 85A-specific cellular immunity. When MVA85A vaccination was preceded by FP85A vaccination, cellular immune responses were lower compared with when MVA85A vaccination was the first immunisation. MVA85A vaccination, but not FP85A vaccination, induced anti-MVA IgG antibodies. Both MVA85A and FP85A vaccinations induced anti-FP9 IgG antibodies. In conclusion, FP85A vaccination was well tolerated but did not induce antigen-specific cellular immune responses. We hypothesize that FP85A induced anti-FP9 IgG antibodies with cross-reactivity for MVA85A, which may have mediated inhibition of the immune response to subsequent MVA85A. ClinicalTrials.gov identification number: NCT00653770
Introduction
There were 8.8 million new cases and almost 1.5 million deaths from tuberculosis (TB) in 2010.Citation1 While global TB incidence, death rates and prevalence are falling, new strategies are required if the Stop TB partnership targets are to be achieved.Citation1 Developing a new vaccine is one key strategy. The existing TB vaccine, Mycobacterium bovis Bacille Calmette Guèrin (BCG) is cost-effective in preventing severe disease in childhood, but prevention of adult pulmonary disease is inconsistent.Citation2,Citation3 Additionally, BCG is contraindicated in people infected with HIV due to the risk of disseminated BCG disease.Citation4 Our approach is to develop a new vaccine regime to boost BCG, retaining BCG’s effectiveness in infants, while improving protection against adult pulmonary disease.
Antigen-specific T cell responses are a central requirement of vaccine-induced protection against TB. CD4+ T cells are essential, but not sufficient, for protective immunity against Mycobacterium tuberculosis (M.tb) and CD8+ T cells are also important.Citation5 Recombinant viral vectors, such as poxviruses, are a particularly effective way of boosting pre-existing T cell responses, when used in heterologous prime-boost strategies. Clinical trials of candidate malaria vaccines suggest improved boosting of antigen specific CD8+ T cells following vaccination with two heterologous recombinant poxvirus vectors.Citation6 We have developed two non-replicating recombinant poxvirus-vectored candidate vaccines, Modified Vaccinia virus Ankara (MVA) and Fowlpox virus (FP9), each encoding mycobacterial antigen 85A (85A) and named MVA85A and FP85A respectively. MVA85A has been evaluated in several clinical trials since 2002 and induces a high frequency of CD4+ T cells and modest CD8+ T cell responses in healthy and HIV and M.tb -infected human subjects in the UK and Africa.Citation7-Citation16 FP85A has not previously been evaluated in human subjects. Vaccinating guinea pigs sequentially with BCG, MVA85A and FP85A enhanced protection against M.tb-challenge compared with vaccination with BCG alone.Citation17
Here, we present the results of the first clinical trial evaluating the safety and immunogenicity of FP85A vaccination of BCG-vaccinated healthy human subjects in heterologous prime-boost regimes with MVA85A. Primary outcomes were passively and actively collected adverse event (AE) data of vaccine safety. Secondary outcomes were the cellular immunogenicity (magnitude of antigen-specific T cell responses) of vaccinations, evaluated by ex-vivo interferon gamma (IFNγ) Enzyme Linked Immunospot (ELISpot) assay. In addition, cryopreserved samples were stored for further exploratory immunology assays, including analysis of soluble cytokines within the first week after vaccination.
Results
Participant flow and recruitment
Between July 2007 and January 2009, 44 subjects were screened and 31 healthy adults enrolled (). All participants completed follow up by January 2010. Recruitment ended before the planned sample size of 36 subjects had been enrolled, as it was not possible to extend the expiry date for FP85A.
Baseline data
There were 12 subjects each in Group 1 and Group 2 and seven subjects in Group 3 (). More females than males were enrolled. Group 1 had a lower proportion of male subjects than Groups 2 and 3. The ages of subjects and pre-vaccination mycobacterial exposure were comparable between groups ().
Table 1. Baseline data. The number of subjects within each group and their relative genders, ages and continents of birth are shown
Safety evaluation
Group 1
Following vaccination with FP85A, all subjects developed a local reaction comprising erythema and induration, followed by scaling (dry, peeling skin) (). Most subjects also reported mild vaccine-site tenderness and pruritus. Moderate feverish symptoms, associated with a body temperature of 37.7⁰C, were recorded on the day of FP85A vaccination by one subject. All other systemic AEs after FP85A vaccination in Group 1 were mild.
Table 2. Adverse events. The numbers of subjects within each group reporting each adverse event and the days of onset of each adverse event are shown. Where three or more subjects in a group reported a particular adverse event, the median day of onset is shown, with range in parentheses. The median diameter of erythema and induration measured by investigators at day two (time of peak local reaction size) is shown, with range in parentheses
One serious AE (SAE) occurred in a subject in Group 1 (FP85A) during the time course of the trial, but was not related to vaccination. This SAE consisted of a day case hospital admission 11 months after vaccination for arthroscopy, following a knee injury sustained six months after vaccination.
Group 2
All subjects developed a local reaction (erythema and induration) following vaccination with MVA85A, consistent with previous trials.Citation11,Citation15,Citation16 Most subjects also experienced mild vaccine-site tenderness, pruritus and scaling (). All systemic AEs after MVA85A vaccination in Group 2 were mild.
The proportions of subjects experiencing local reactions after FP85A in Group 2 and the diameters of local reactions were similar to those in Group 1 (). Symptoms of feverishness (in the absence of a documented fever) were reported by two subjects on the day of vaccination with FP85A (four weeks after MVA85A). All other systemic AEs after FP85A vaccination in Group 2 were mild. There were no SAEs in Group 2.
Group 3
Local reactions after FP85A vaccination were as described above for Groups 1 and 2 (). All systemic AEs after FP85A vaccination in Group 3 were mild.
The peak (day 2) diameters of erythema and induration after MVA85A vaccination were larger in Group 3, when MVA85A was the second poxvirus vaccination, compared with Group 2, when MVA85A was the first poxvirus vaccination (). There was one episode of moderate sleep disturbance four days after MVA85A vaccination. All other systemic AEs after MVA85A vaccination in Group 3 were mild and there were no SAEs.
Vaccine immunogenicity determined by ex-vivo IFNγ ELISpot
The kinetics and magnitude of the antigen-specific T cell responses to stimulation with 85A peptides following FP85A and MVA85A vaccination were assessed by ex-vivo IFNγ ELISpot assay.
After FP85A vaccination in Group 1, 85A responses did not increase compared with baseline levels at screening (, ).
Figure 2. IFNγ ELISpot responses to 85A and soluble serum cytokines. (A) Longitudinal IFNγ ELISpot responses to the single 85A peptide pool. Each dot represents an individual subjects’ response and median responses are connected by lines. Group 1 = FP85A vaccination week 0; Group 2 = MVA85A vaccination week 0, FP85A vaccination week four; Group 3 = FP85A vaccination week 0, MVA85A vaccination week four. No increases in responses to antigen 85A were seen after FP85A vaccination in Group 1. MVA85A vaccination induced strong IFNγ T cell responses to antigen 85A in Group 2, which were maintained throughout the 52 week follow up, but were not boosted by subsequent FP85A vaccination at week four. There were no responses after FP85A vaccination in Group 3, but subsequent MVA85A vaccination at week four induced moderate IFNγ T cell responses to antigen 85A. (B) Proportion of subjects with detectable soluble serum cytokines. The bars show the proportion of subjects within each group, in whose serum, any cytokines were detectable. Group 1 = FP85A vaccination week 0; Group 2 = MVA85A vaccination week 0, FP85A vaccination week four; Group 3 = FP85A vaccination week 0, MVA85A vaccination week four; days = days since enrolment. Serum IFNγ and TNFα were detected in no more than one subject’s serum at any one time point. IL-8 was detected in all Group 2 subjects’ serum by day seven (one week after MVA85A vaccination).
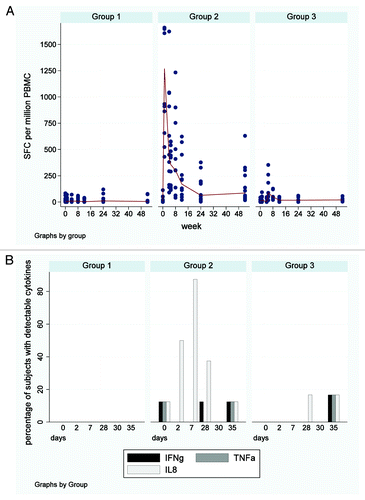
Table 3. Statistics for IFNγ ELISpot responses to antigen 85A. Cellular IFNγ secretion by PBMC in response to antigen 85A stimulation was evaluated using ELISpot assays to determine the number of spot forming cells (SFC). Post-vaccination responses were compared with baseline pre-vaccination responses using a paired analysis () and responses between groups were also compared ().
In Group 2, MVA85A vaccination expanded 85A-specific IFNγ-secreting T cells, which peaked at week one and were still maintained above baseline at week 52 (, ). The magnitude of responses was comparable to those reported in previously published trials of MVA85A in BCG-vaccinated healthy adults (data not shown).Citation15,Citation16 After subsequent FP85A vaccination at week four, responses were not boosted, but continued to fall from the peak at week one.
In Group 3, 85A responses peaked at week five, one week after MVA85A vaccination. Responses declined to pre-vaccination levels after week eight (). The magnitude of responses to MVA85A in Group 3, when MVA85A was preceded by FP85A, was significantly lower compared with responses in Group 2 ().
Evaluation of soluble Th1 cytokines in the first week after vaccination
In Group 1, serum IFNγ, tumor necrosis factor α (TNFα) and interleukin 8 (IL-8) were not detected either before or within seven days of FP85A vaccination (). In Group 2, IL-8 was detected in all subjects’ serum samples seven days after MVA85A vaccination and remained detectable in three subjects’ serum samples at week four (day 28). One week after FP85A vaccination, IL-8 was only detectable in one subjects’ serum. In Group 3, none of the cytokines were detectable one week after FP85A vaccination. IL-8 was detectable on the day of MVA85A vaccination and all three cytokines were detected in one subject’s serum sample one week after MVA85A vaccination.
Anti-vector studies
Responses to CD4+and CD8+T cell epitopes in Vaccinia and MVA
Responses to Vaccinia CD4+ and CD8+ T cell epitopes were detectable in some, but not all subjects, both before and after immunisation in all three groups (, ). The magnitude of all anti-vector responses in all groups were considerably lower than antigen 85A responses using cryopreserved peripheral blood mononuclear cells (PBMC, (data not shown).
Figure 3. IFNγ ELISpot responses to Vaccinia CD4+ and CD8+ T cell epitopes. (A) Longitudinal IFNγ ELISpot responses to known Vaccinia CD4 and CD8 epitopes Each dot represents an individual subjects’ response and median responses are connected by lines. Group 1 = FP85A vaccination week 0; Group 2 = MVA85A vaccination week 0, FP85A vaccination week four; Group 3 = FP85A vaccination week 0, MVA85A vaccination week four. Minimal, transient increases in Vaccinia CD8+ responses compared with baseline were observed after FP85A vaccination in Group 1. Transient responses to Vaccinia CD4+ and CD8+ epitopes were observed after MVA85A, but not FP85A vaccination in Group 2. In Group 3, responses to Vaccinia epitopes did not significantly increase after either FP85A or MVA85A vaccinations. (B) Correlation between IFNγ ELISpot responses to Vaccinia CD4+ and CD8+ at the time of MVA85A vaccination and IFNγ ELISpot responses to antigen 85A one week after MVA85A vaccination. Each dot represents an individual subjects’ responses. Pre-vaccination responses were from the day of MVA85A vaccination (Group 2 = week 0; Group 3 = week four). Post-vaccination responses were from samples taken one week after MVA5A vaccination (Group 2 = week one; Group 3 = week five). Circles = responses to Vaccinia CD4+ epitopes; diamonds = responses to Vaccinia CD8+ epitopes. No relationship between pre-vaccination anti-vector responses (y axes) and post-vaccination T cell responses (x axes) for MVA85A vaccination was found.
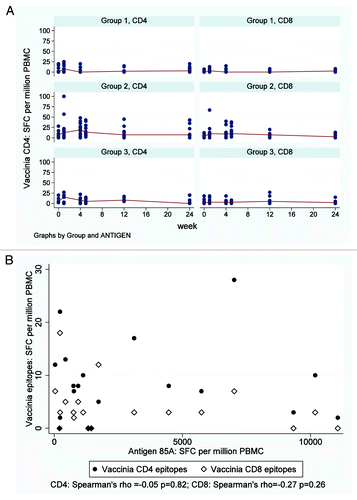
Table 4. Anti-vector IFNγ ELISpot and ELISA statistics. Cellular IFNγ secretion by PBMC in response to stimulation with Vaccinia CD4+ and CD8+ epitopes was evaluated using ELISpot assays to determine the number of spot forming cells (SFC) (). Serum IgG responses to FP9 and MVA were evaluated by ELISA (). Post-vaccination responses were compared with baseline pre-vaccination responses using a paired analysis
In Group 1, responses to the CD4+ and CD8+epitopes increased transiently one week after FP85A vaccination ().
In Group 2, responses to both epitopes increased after MVA85A vaccination and were significantly higher than baseline at week four, the time of subsequent FP85A vaccination (). FP85A vaccination did not boost responses to the CD4+ or CD8+ epitopes.
In Group 3, following the transiently increased responses after FP85A vaccination at week one, responses were similar to screening levels before MVA85A vaccination at week four, and were not boosted by MVA85A vaccination ().
There were no correlations between ELISpot responses to the CD4+ or CD8+ epitopes before MVA85A vaccination and 85A-specific ELISpot responses one week after MVA85A vaccination ().
Insert and Vector-specific IgG levels pre and post-vaccination
IgG responses to r85A
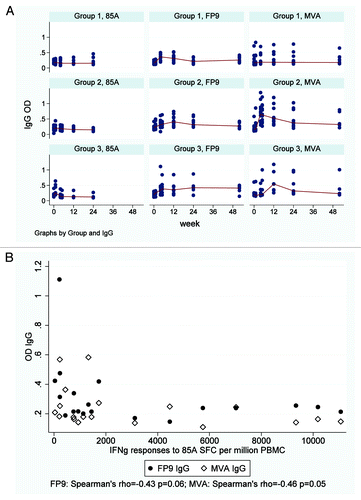
A small, transient increase in IgG responses to recombinant 85A (r85A) was observed after FP85A vaccination in Groups 1 and 3 but not after MVA85A vaccination in Group 2 (Fig. 4A). Following the subsequent vaccinations in Group 2 (FP85A) and Group 3 (MVA85A), anti-r85AIgG levels did not increase significantly.
IgG responses to FP9
Anti-FP9 IgG levels increased after vaccination with FP85A in Group 1 (, ). In Group 2, anti-FP9 IgG levels increased after MVA85A vaccination and were boosted by FP85A vaccination at week four (, ). Anti-FP9 IgG levels after FP85A vaccination in Group 3 were not boosted by subsequent MVA85A vaccination (, ). There was a trend toward a negative correlation between pre-MVA85A anti-FP9 IgG levels and post-MVA85A IFNγ ELISpot responses to single pool 85A in Groups 2 and 3 ().
Figure 4. Serum IgG ELISA responses to r85A, MVA and FP9. (A) Longitudinal r85A IgG, FP9 IgG and MVA IgG responses detected by ELISA Each dot represents an individual subjects’ response and median responses are connected by lines. Group 1 = FP85A vaccination week 0; Group 2 = MVA85A vaccination week 0, FP85A vaccination week four; Group 3 = FP85A vaccination week 0, MVA85A vaccination week four. Anti-vector antibody responses were generally stronger than antigen-specific anti-85A IgG responses. In Group 1, FP85A vaccination induced an FP9 IgG response, but no MVA IgG antibodies. In Group 2, MVA85A vaccination induced FP9 IgG and MVA IgG responses. Subsequent FP85A vaccination boosted the FP9 IgG response but did not boost the MVA IgG response. In Group 3, MVA85A vaccination induced an MVA IgG response, but did not boost the FP9 IgG response to prior FP85A vaccination. (B) Correlation between FP9 IgG and MVA IgG levels detectable by ELISA at the time of MVA85A vaccination and IFNγ ELISpot responses to antigen 85A one week after MVA85A vaccination. Each dot represents an individual subjects’ responses. Pre-vaccination responses were from the day of MVA85A vaccination (Group 2 = week 0; Group 3 = week four). Post-vaccination responses were from samples taken after MVA5A vaccination (Group 2 = week four; Group 3 = week 12). Circles = FP9 IgG levels; diamonds = MVA IgG levels. There were trends toward negative correlations between pre-MVA85A FP9 and MVA IgG levels and post-MVA85A IFNγ ELISpot responses to single pool 85A in Groups 2 and 3.
IgG Responses to MVA
Anti-MVA IgG levels peaked four weeks after MVA85A vaccination in Group 2 and were not boosted by subsequent FP85A vaccination, but remained above baseline throughout follow-up (; ). Anti-MVA IgG levels did not increase significantly after FP85A vaccination but did increase after subsequent MVA85A vaccination in Group 3, peaking at week 12 and remaining above baseline until week 24 (). At the time of MVA85A vaccination, anti-MVA IgG levels were similar between Groups 2 and 3. There was a trend toward a negative correlation between pre-vaccination anti-MVA IgG levels and post-MVA85A vaccination IFNγ ELISpot responses to single pool 85A ().
Discussion
This clinical trial provides further evidence for the safety of recombinant FP9 and MVA vectored vaccines in a healthy adult population. FP85A and MVA85A vaccines were well tolerated in all regimes. The frequencies of local and systemic AEs were comparable to previous clinical trials evaluating MVA85A vaccination and FP9 and MVA-vectored candidate malaria vaccines.Citation11,Citation15,Citation16,Citation18,Citation19 Peak local reactions were larger in diameter in the FP85A-MVA85A regime in Group 3, compared with when MVA85A was the first vaccination. However, as previously discussed, this was not associated with increased frequency or severity of other local or systemic AEs; local reaction sizes were comparable by one week and the group size was small, so the significance is uncertain.Citation20 All other local and systemic AEs in these subjects were mild and AEs were otherwise comparable between groups, as observed in previous clinical trials of FP9 and MVA vectored candidate malaria vaccines.Citation19
MVA85A, but not FP85A vaccination induced strong 85A-specific cellular immunity. FP85A vaccination did not boost the responses to prior MVA85A vaccination (Group 2) and responses to MVA85A vaccination were inhibited by prior FP85A vaccination (Group 3). The same trend was observed in analysis of soluble cytokines, with IL-8 detected after MVA85A vaccination in Group 2 but not in Group 3.
Recombinant FP9-vectored vaccines induce weaker immune responses than recombinant MVA vaccines and MVA85A elicits unusually high responses compared with other recombinant MVA vaccines.Citation11,Citation13,Citation15,Citation16,Citation21-Citation23 In malaria vaccine clinical trials with a number of different antigen inserts, an increased IFNγ response compared with baseline was seen in FP9-MVA regimes with a similar interval between vaccinations with different viral vectors.Citation6,Citation18 Given the strong immune responses to MVA85A vaccination, we would expect at least modest antigen-specific immune responses following vaccination with FP85A.
Identity polymerase chain reaction (PCR) and sequencing assays had confirmed the presence of the 85A insert within the FP9 vector and no wild type FP9 was present. The clinical grade FP85A vaccine also passed annual murine potency assays, involving evaluation for antigen-specific cellular immune responses, which were lower for FP85A than MVA85A (data not shown). The antigen insert was therefore both present within the recombinant vector and recognizable by the adaptive immune system. In the clinical trial, FP85A induced local and systemic reactions typical of poxviruses, providing additional evidence that the viral vector was immunologically active. Positive and negative controls excluded technical problems with the assays and results were reproduced using frozen samples.
Serum was evaluated for the presence of the Th1 cytokines IFNγ and TNFα. The chemokine IL-8 was also measured because microarray analysis has previously demonstrated IL-8 to be one of the genes induced by MVA-infection of a cell.Citation24 We speculate that IL-8 may be one of the mediators involved in directing the magnitude of the antigen-specific response to MVA85A. IL-8 is released by macrophages in response to M.tb components, is chemotactic to neutrophils and thought to be important in granuloma formation and protection against disease.Citation25,Citation26 It would be interesting to evaluate further the role of IL-8 in early innate and adaptive cellular immune responses to MVA85A vaccination.
We used cryopreserved PBMC to investigate the inhibitory effect of prior vaccination with FP85A on the antigen-specific response to MVA85A vaccination. CD4+ and CD8+ T cell responses were detected upon stimulation of PBMC with Vaccinia epitopes following MVA85A vaccination in Group 2, but not in Group 3. No cell-mediated responses to Vaccinia epitopes were detected following FP85A vaccination. We therefore examined the serum IgG responses to MVA and FP9. Anti-MVA IgG antibodies were detected following MVA85A vaccination, but not after FP85A vaccination. Anti-FP9 IgG levels increased after MVA85A vaccination as well as after FP85A vaccination, suggesting anti-FP9 IgG is cross-reactive for MVA85A.
In conclusion, FP85A vaccination was safe and well tolerated in healthy adults. However, unlike MVA85A vaccination, FP85A vaccination did not increase 85A-specific immune responses. FP85A vaccination inhibited the antigen-specific and vector-specific cellular responses to subsequent MVA85A vaccination. We speculate that anti-FP9 IgG antibodies which are cross-reactive with MVA85A may be one factor mediating the inhibition of antigen-specific cellular immune responses to vaccination with MVA85A.
Materials and Methods
Study design
This was an open label, non-randomized, Phase I safety and immunogenicity clinical trial in healthy, previously BCG-vaccinated, adult subjects.
Participants
Subjects were recruited from the Oxford region in the UK. Inclusion criteria were healthy adults; aged 18–50; BCG-vaccinated; seronegative for HIV, hepatitis B and hepatitis C viruses; no clinically significant abnormalities in hematology (full blood count), or biochemistry (sodium, potassium, creatinine, urea, albumin, bilirubin, Alkaline Phosphatase and Alanine aminotransferase) tests. Exclusion criteria were evidence of latent M.tb infection (LTBI) by Mantoux reaction (diameter greater than 15mm) or IFNγ ELISpot responses to M.tb-specific antigens ESAT-6 or CFP-10. Mantoux tests were performed by clinically qualified investigators according to national guidelines.Citation27 Females entering the study were required to have a negative pregnancy test and plans for reliable contraception for the duration of inclusion.
Ethics
This study was approved by the Medicines and Healthcare products Regulatory Agency (MHRA) and conducted in accordance with the Declaration of Helsinki. Ethical approval was obtained from the Gene Therapy Advisory Committee (GTAC) and Site Specific Assessment performed by the Oxfordshire Research Ethics Committee (OxRecA). Written informed consent was obtained from all subjects prior to participation.
Interventions
Interventions were two candidate TB vaccines, FP85A and MVA85A. FP85A is a recombinant FP9 vector encoding antigen 85A. FP9 is a fully sequenced, live, highly attenuated form of a European strain of Fowlpox virus, derived by multiple passages of the wild-type Fowlpox virus in avian cells.Citation28 FP85A was constructed using an established protocol.Citation29 The 85A DNA sequence (derived from M.tb H37Rv) was ligated into the unique SmaI cloning site of the Fowlpox shuttle vector pEFL29, placing gene expression under the control of the Vaccinia virus P7.5 promoter. Recombinant viruses were prepared by in vitro recombination of the shuttle vector encoding 85A with FP9 in primary cultures of chicken embryo fibroblasts (CEFs) and selected by repeated plaque purification in CEF monolayers. The MVA85A vaccine was constructed as previously described.Citation30
Clinical grade MVA85A and FP85A vaccines were produced under Good Manufacturing Practice conditions by IDT Biologika GmbH (Dessau-Rosslau, Germany).
All vaccine doses were 5 × 107 plaque forming units (pfu) administered by intradermal injection into the deltoid area of the arm. The volumes of vaccine administered were 70µl (FP85A) or 135µl (MVA85A). In Group 1, the vaccine was administered into the opposite arm compared with BCG. In Groups 2 and 3, where two vaccines were administered with a four week interval, vaccines were injected into opposite arms.
Sample size
The planned sample size was 36 subjects, with 12 subjects in each group, aiming to detect frequently occurring AEs. Sample size calculations were performed using Stata 9 and 12 subjects per group gave a 90% power to detect a 40% difference in immune responses between two groups.
Enrolment
Subjects were allocated into three groups sequentially, in order of enrolment. Subjects in Group 1 were vaccinated with FP85A at enrolment. An interim safety analysis of FP85A vaccination was performed before enrolling subjects into Groups 2 and 3. Subjects in Group 2 were vaccinated with MVA85A at enrolment and FP85A at week four. Subjects in Group 3 were vaccinated with FP85A at enrolment and MVA85A at week four. Subjects were followed up regularly for one year following enrolment.
Safety analysis
Daily diary cards recording local and systemic AEs, local reaction sizes and body temperature were completed by subjects for seven days following each vaccination. Blood samples for hematology and biochemistry analysis were taken at screening and weeks one and 12 for all groups and additionally at week four for Groups 2 and 3. Solicited and unsolicited AEs were recorded by investigators in case report forms at each follow up appointment. The criteria for assigning AE severity and causality have been described previously.Citation20 All AEs deemed possibly, probably or definitely related to vaccination have been reported. The transverse diameters of erythema and induration (palpable hardening of skin) were measured by clinically qualified and trained investigators two and seven days after each vaccination and four, eight, 12, 24 and 52 weeks after enrolment.
Immunological assays
Blood samples for exploratory immunology analyses were taken at screening and two days after vaccination and weeks one, four, eight, 12, 24 and 52 for all groups and additionally at week five for Groups 2 and 3. At each time point except day two, 50ml lithium-heparinized blood and five ml serum sample were taken. A maximum of 20ml blood was taken two days after vaccination. PBMC were extracted from lithium heparinized blood as previously described.Citation11
ELISpot assays
The principal readout for evaluating vaccine-induced cellular immunogenicity was by ex-vivo IFNγ ELISpot assay using fresh PBMC as previously described.Citation8,Citation11 The antigens used were seven pools of antigen 85A peptides; a single pool of all 66 85A peptides; r85A; purified protein derivative (PPD) as described.Citation8,Citation11 For detection of LTBI at screening, wells were plated with ESAT-6 and CFP-10 peptides as described.Citation11
Anti-vector IFNγ ELISpot was performed using frozen PBMCs, stored in liquid nitrogen. Cells were flash thawed at 37°C, resuspended in R10 and centrifuged at 1400rpm for seven minutes. All samples had a viability of greater than 95%. Cells were rested overnight at 37°C, 5% CO2in R10 containing 10U/ml of Benzonase (Novagen) at 1x106 PBMC/ml. They were then washed and plated according to the ELISpot protocol.
Anti-vector IFNγ responses were mapped to CD4+ (27 peptides) and CD8+ (36 peptides) T cell epitopes present in Vaccinia and MVA (Table S1). Peptides were synthesized according to the sequences obtained from published literature.Citation31-Citation37 As these assays were performed on frozen cells, all samples were also re-tested with the 85A single 66-peptide pool. All ELISpot assays included unstimulated cells as a negative control and 10μg/ml Staphylococcal enterotoxin B (SEB, Sigma) as a positive control.
Detection of soluble cytokines
Serum samples from enrolled subjects were evaluated for the presence of soluble cytokines IFNγ, TNFα and IL-8. Frozen serum samples from screening and days two and seven were thawed at room temperature. To each FlowCytomix reaction, 25 µL of serum was added, and the assay performed according to the manufacturer’s instructions (FlowCytomix Basic kit and Simplex kits for IL-8, TNFα and IFNγ, Bender MedSystems). The cytokine-bound beads were detected on a Beckman Coulter CyAN flow cytometer and the results analyzed using the Bender MedSystems Flow Cytomix Pro 2.3 software.
IgG enzyme linked immunosorbent assay (ELISA)
IgG was measured in serum samples, tested in duplicate. NUNC Immuno Plates (Fisher) were coated with r85A (5μg/ml); FP9 (5x105 pfu/well); or MVA (5x105 pfu/well) in 0.05M carbonate-bicarbonate buffer and incubated overnight at 4°C. Plates were washed in PBS/Tween20 and blocked with 1% Casein in PBS (Fisher Scientific) for one hour, before the addition of serum, diluted 1:50 (r85A plates) or 1:100 (viral plates) in Casein. Plates containing serum were incubated for one hour and washed five times with PBS/Tween. Goat anti-human IgG alkaline phosphatase secondary antibody (Sigma) was added and plates incubated for one hour, and washed five times. Plates were developed by adding 50µl of Diethanolamine buffer (Fisher) with 4-Nitrophenyl Phosphate tablet (Sigma) according to manufacturer’s recommendation and read at 13 min (r85A plates) or seven minutes (viral plates), timed from the beginning of the addition of developing buffer.
Statistical analysis
Post-vaccination responses to each stimulating antigen within each regime were compared with pre-vaccination (baseline) responses using the Wilcoxon signed-rank test (Stata Statistical Software, Release 9.0, 2005). Non-parametric tests were used as the data were not normally distributed.
The overall magnitude of vaccine-induced IFNγ T cell ELISpot responses was summarized using the area under the curve (AUC) for each stimulating antigen and regime after subtracting pre-vaccination responses (Stata). AUC responses were compared between groups using the Mann Whitney U test. Where differences in AUC between groups were detected, peak (week one) and plateau (week 52) responses were compared, using the Mann-Whitney U test.
Post-vaccination anti-vector cellular and humoral responses within each group were compared with pre-vaccination responses using the Wilcoxon signed-rank test. The relationship between pre-vaccination anti-vector cellular and antibody responses and vaccine-induced cellular immune responses was evaluated by calculating rank correlation coefficient (Spearman’s rho, Stata).
| Abbreviations: | ||
| AE | = | adverse event |
| AUC | = | area under the curve |
| BCG | = | Mycobacterium bovis Bacille Calmette Guèrin |
| ELISA | = | Enzyme Linked Immunosorbent assay |
| ELISpot | = | Enzyme Linked Immunospot |
| FP85A, candidate TB vaccine | = | FP9 expressing antigen 85A |
| FP9 | = | attenuated fowlpox virus |
| IFNγ | = | interferon gamma |
| IL-8 | = | interleukin 8 |
| LTBI | = | latent M.tb infection |
| M.tb | = | Mycobacterium tuberculosis |
| MVA | = | Modified Vaccinia Virus Ankara |
| MVA85A, candidate TB vaccine | = | MVA expressing antigen 85A |
| pfu | = | plaque forming units |
| SAE | = | serious adverse event |
| SFC | = | spot forming cells |
| TB | = | Tuberculosis |
| TNFα | = | tumor necrosis factor alpha |
Additional material
Download Zip (41 KB)Acknowledgments
We are grateful to all the trial participants. Oxford University was the sponsor for these clinical trials. HMcS is a Wellcome Trust Senior Clinical Research Fellow. AVSH is a Wellcome Trust Principal Fellow. HMcS, SCG and AVSH are Jenner Institute Investigators.
Disclosure of Potential Conflicts of Interest
A.A.P., S.G., A.V.S.H. and H.M.S. are named inventors on a composition of matter patent for MVA85A, and are shareholders in a Joint Venture formed for the further development of this vaccine.
Financial Disclosure
This trial was funded by charitable grants from Europe Aid; TBVAC (EU 6th Framework Programme); The Oxford Biomedical Research Centre and the Wellcome Trust.
References
- Global Tuberculosis Control. WHO report 2011. Geneva: World Health Organisation, 2011.
- Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet 2006; 367:1173 - 80; http://dx.doi.org/10.1016/S0140-6736(06)68507-3; PMID: 16616560
- Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV, et al. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA 1994; 271:698 - 702; http://dx.doi.org/10.1001/jama.1994.03510330076038; PMID: 8309034
- Revised BCG vaccination guidelines for infants at risk for HIV infection. Wkly Epidemiol Rec 2007; 82:193 - 6; PMID: 17526121
- Flynn JL. Immunology of tuberculosis and implications in vaccine development. Tuberculosis (Edinb) 2004; 84:93 - 101; http://dx.doi.org/10.1016/j.tube.2003.08.010; PMID: 14670350
- Vuola JM, Keating S, Webster DP, Berthoud T, Dunachie S, Gilbert SC, et al. Differential immunogenicity of various heterologous prime-boost vaccine regimens using DNA and viral vectors in healthy volunteers. J Immunol 2005; 174:449 - 55; PMID: 15611270
- Scriba TJ, Tameris M, Smit E, van der Merwe L, Hughes EJ, Kadira B, et al. A phase IIa trial of the new tuberculosis vaccine, MVA85A, in HIV- and/or Mycobacterium tuberculosis-infected adults. Am J Respir Crit Care Med 2012; 185:769 - 78; http://dx.doi.org/10.1164/rccm.201108-1548OC; PMID: 22281831
- Minassian AM, Rowland R, Beveridge NE, Poulton ID, Satti I, Harris S, et al. A Phase I study evaluating the safety and immunogenicity of MVA85A, a candidate TB vaccine, in HIV-infected adults. BMJ Open 2011; 1:e000223; http://dx.doi.org/10.1136/bmjopen-2011-000223; PMID: 22102640
- Ota MO, Odutola AA, Owiafe PK, Donkor S, Owolabi OA, Brittain NJ, et al. Immunogenicity of the tuberculosis vaccine MVA85A is reduced by coadministration with EPI vaccines in a randomized controlled trial in Gambian infants. Sci Transl Med 2011; 3:88ra56; http://dx.doi.org/10.1126/scitranslmed.3002461; PMID: 21697532
- Scriba TJ, Tameris M, Mansoor N, Smit E, van der Merwe L, Mauff K, et al. Dose-finding study of the novel tuberculosis vaccine, MVA85A, in healthy BCG-vaccinated infants. J Infect Dis 2011; 203:1832 - 43; http://dx.doi.org/10.1093/infdis/jir195; PMID: 21606542
- Sander CR, Pathan AA, Beveridge NE, Poulton I, Minassian A, Alder N, et al. Safety and immunogenicity of a new tuberculosis vaccine, MVA85A, in Mycobacterium tuberculosis-infected individuals. Am J Respir Crit Care Med 2009; 179:724 - 33; http://dx.doi.org/10.1164/rccm.200809-1486OC; PMID: 19151191
- Brookes RH, Hill PC, Owiafe PK, Ibanga HB, Jeffries DJ, Donkor SA, et al. Safety and immunogenicity of the candidate tuberculosis vaccine MVA85A in West Africa. PLoS One 2008; 3:e2921; http://dx.doi.org/10.1371/journal.pone.0002921; PMID: 18698342
- Hawkridge T, Scriba TJ, Gelderbloem S, Smit E, Tameris M, Moyo S, et al. Safety and immunogenicity of a new tuberculosis vaccine, MVA85A, in healthy adults in South Africa. J Infect Dis 2008; 198:544 - 52; http://dx.doi.org/10.1086/590185; PMID: 18582195
- Beveridge NE, Price DA, Casazza JP, Pathan AA, Sander CR, Asher TE, et al. Immunisation with BCG and recombinant MVA85A induces long-lasting, polyfunctional Mycobacterium tuberculosis-specific CD4+ memory T lymphocyte populations. Eur J Immunol 2007; 37:3089 - 100; http://dx.doi.org/10.1002/eji.200737504; PMID: 17948267
- Pathan AA, Sander CR, Fletcher HA, Poulton I, Alder NC, Beveridge NE, et al. Boosting BCG with recombinant modified vaccinia ankara expressing antigen 85A: different boosting intervals and implications for efficacy trials. PLoS One 2007; 2:e1052; http://dx.doi.org/10.1371/journal.pone.0001052; PMID: 17957238
- McShane H, Pathan AA, Sander CR, Keating SM, Gilbert SC, Huygen K, et al. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat Med 2004; 10:1240 - 4; http://dx.doi.org/10.1038/nm1128; PMID: 15502839
- Williams A, Goonetilleke NP, McShane H, Clark SO, Hatch G, Gilbert SC, et al. Boosting with poxviruses enhances Mycobacterium bovis BCG efficacy against tuberculosis in guinea pigs. Infect Immun 2005; 73:3814 - 6; http://dx.doi.org/10.1128/IAI.73.6.3814-3816.2005; PMID: 15908420
- Walther M, Woodruff J, Edele F, Jeffries D, Tongren JE, King E, et al. Innate immune responses to human malaria: heterogeneous cytokine responses to blood-stage Plasmodium falciparum correlate with parasitological and clinical outcomes. J Immunol 2006; 177:5736 - 45; PMID: 17015763
- Webster DP, Dunachie S, McConkey S, Poulton I, Moore AC, Walther M, et al. Safety of recombinant fowlpox strain FP9 and modified vaccinia virus Ankara vaccines against liver-stage P. falciparum malaria in non-immune volunteers. Vaccine 2006; 24:3026 - 34; http://dx.doi.org/10.1016/j.vaccine.2005.10.058; PMID: 16488059
- Rowland R, Brittain N, Poulton ID, Minassian AM, Sander C, Porter DW, et al. A review of the tolerability of the candidate TB vaccine, MVA85A compared with BCG and Yellow Fever vaccines, and correlation between MVA85A vaccine reactogenicity and cellular immunogenicity. Trials in Vaccinology 2012; 1:27 - 35; http://dx.doi.org/10.1016/j.trivac.2012.07.001
- Keefer MC, Frey SE, Elizaga M, Metch B, De Rosa SC, Barroso PF, et al, NIAID HIV Vaccine Trials Network. A phase I trial of preventive HIV vaccination with heterologous poxviral-vectors containing matching HIV-1 inserts in healthy HIV-uninfected subjects. Vaccine 2011; 29:1948 - 58; http://dx.doi.org/10.1016/j.vaccine.2010.12.104; PMID: 21216311
- Porter DW, Thompson FM, Berthoud TK, Hutchings CL, Andrews L, Biswas S, et al. A human Phase I/IIa malaria challenge trial of a polyprotein malaria vaccine. Vaccine 2011; 29:7514 - 22; http://dx.doi.org/10.1016/j.vaccine.2011.03.083; PMID: 21501642
- Currier JR, Ngauy V, de Souza MS, Ratto-Kim S, Cox JH, Polonis VR, et al. Phase I safety and immunogenicity evaluation of MVA-CMDR, a multigenic, recombinant modified vaccinia Ankara-HIV-1 vaccine candidate. PLoS One 2010; 5:e13983; http://dx.doi.org/10.1371/journal.pone.0013983; PMID: 21085591
- Guerra S, López-Fernández LA, Conde R, Pascual-Montano A, Harshman K, Esteban M. Microarray analysis reveals characteristic changes of host cell gene expression in response to attenuated modified vaccinia virus Ankara infection of human HeLa cells. J Virol 2004; 78:5820 - 34; http://dx.doi.org/10.1128/JVI.78.11.5820-5834.2004; PMID: 15140980
- Sawant KV, Cho H, Lyons M, Ly LH, McMurray DN. Guinea pig neutrophil-macrophage interactions during infection with Mycobacterium tuberculosis. Microbes Infect 2010; 12:828 - 37; http://dx.doi.org/10.1016/j.micinf.2010.05.009; PMID: 20685396
- Sterling TR, Dorman SE, Chaisson RE, Ding L, Hackman J, Moore K, et al. Human immunodeficiency virus-seronegative adults with extrapulmonary tuberculosis have abnormal innate immune responses. Clin Infect Dis 2001; 33:976 - 82; http://dx.doi.org/10.1086/322670; PMID: 11528568
- Tuberculosis. Green Book: Department of Health, 2011:403-7.
- Laidlaw SM, Skinner MA. Comparison of the genome sequence of FP9, an attenuated, tissue culture-adapted European strain of Fowlpox virus, with those of virulent American and European viruses. J Gen Virol 2004; 85:305 - 22; http://dx.doi.org/10.1099/vir.0.19568-0; PMID: 14769888
- Qingzhong Y, Barrett T, Brown TD, Cook JK, Green P, Skinner MA, et al. Protection against turkey rhinotracheitis pneumovirus (TRTV) induced by a fowlpox virus recombinant expressing the TRTV fusion glycoprotein (F). Vaccine 1994; 12:569 - 73; http://dx.doi.org/10.1016/0264-410X(94)90319-0; PMID: 8036832
- McShane H, Behboudi S, Goonetilleke N, Brookes R, Hill AV. Protective immunity against Mycobacterium tuberculosis induced by dendritic cells pulsed with both CD8(+)- and CD4(+)-T-cell epitopes from antigen 85A. Infect Immun 2002; 70:1623 - 6; http://dx.doi.org/10.1128/IAI.70.3.1623-1626.2002; PMID: 11854254
- Howles S, Guimarães-Walker A, Yang H, Hancock G, di Gleria K, Tarragona-Fiol T, et al. Vaccination with a modified vaccinia virus Ankara (MVA)-vectored HIV-1 immunogen induces modest vector-specific T cell responses in human subjects. Vaccine 2010; 28:7306 - 12; http://dx.doi.org/10.1016/j.vaccine.2010.08.077; PMID: 20816902
- Meyer VS, Kastenmuller W, Gasteiger G, Franz-Wachtel M, Lamkemeyer T, Rammensee HG, et al. Long-term immunity against actual poxviral HLA ligands as identified by differential stable isotope labeling. J Immunol 2008; 181:6371 - 83; PMID: 18941228
- Strug I, Calvo-Calle JM, Green KM, Cruz J, Ennis FA, Evans JE, et al. Vaccinia peptides eluted from HLA-DR1 isolated from virus-infected cells are recognized by CD4+ T cells from a vaccinated donor. J Proteome Res 2008; 7:2703 - 11; http://dx.doi.org/10.1021/pr700780x; PMID: 18507432
- Calvo-Calle JM, Strug I, Nastke MD, Baker SP, Stern LJ. Human CD4+ T cell epitopes from vaccinia virus induced by vaccination or infection. PLoS Pathog 2007; 3:1511 - 29; http://dx.doi.org/10.1371/journal.ppat.0030144; PMID: 17937498
- Terajima M, Cruz J, Leporati AM, Demkowicz WE Jr., Kennedy JS, Ennis FA. Identification of vaccinia CD8+ T-cell epitopes conserved among vaccinia and variola viruses restricted by common MHC class I molecules, HLA-A2 or HLA-B7. Hum Immunol 2006; 67:512 - 20; http://dx.doi.org/10.1016/j.humimm.2005.12.004; PMID: 16829305
- Oseroff C, Kos F, Bui HH, Peters B, Pasquetto V, Glenn J, et al. HLA class I-restricted responses to vaccinia recognize a broad array of proteins mainly involved in virulence and viral gene regulation. Proc Natl Acad Sci U S A 2005; 102:13980 - 5; http://dx.doi.org/10.1073/pnas.0506768102; PMID: 16172378
- Drexler I, Staib C, Kastenmuller W, Stevanović S, Schmidt B, Lemonnier FA, et al. Identification of vaccinia virus epitope-specific HLA-A*0201-restricted T cells and comparative analysis of smallpox vaccines. Proc Natl Acad Sci U S A 2003; 100:217 - 22; http://dx.doi.org/10.1073/pnas.262668999; PMID: 12518065