Abstract
Hepatitis A virus (HAV) remains a public health concern worldwide contributing to significant morbidity in developed and developing countries. This cross-sectional database study estimated the overall HAV seroprevalence and the seroprevalence by gender, age, region and socioeconomic status in Mexico. Between January and October 2010, serum samples collected during the National Health and Nutrition survey (ENSANUT 2006) were obtained from subjects aged 1–95 y. Subjects’ gender, age, geographical region and socioeconomic status were extracted from the survey and compiled into a subset database by the Mexican National Institute of Public Health. Anti-HAV antibodies were measured using a chemiluminescent immunoassay. A total of 3658 subjects were included in the according-to-protocol cohort. Overall, the HAV seroprevalence was 84.2%. The HAV seroprevalence rates were similar between females (86.1%) and males (82.2%). The percentage of subjects seropositive for anti-HAV antibodies was highest in adults aged ≥ 20 y (96.9%), followed by adolescents aged 10–19 y (80.1%) and lowest in children aged 1–9 y (45.0%) (p < 0.0001). Regionally, the highest HAV seroprevalence rate was observed in the South (88.8%) followed by Central and Northern Mexico and Mexico City (p = 0.02). The HAV seroprevalence was similar between subjects of high socioeconomic (90.1%) status and of low socioeconomic status (86.6%). This study confirms the intermediate HAV endemicity in Mexico. Cost-effectiveness studies are necessary to evaluate the inclusion of an effective hepatitis A vaccine from a population-based perspective in addition to continuous efforts to improve hygiene and sanitation that have a substantial impact on the disease burden.
Introduction
Hepatitis A virus (HAV) has a global distribution and remains a major public health issue worldwide.Citation1,Citation2 Globally, approximately 1.5 million clinical cases of hepatitis A occur annually.Citation1 In 2005, 121 million infections due to HAV were reported with the majority being observed in the age groups 2–14 y and > 30 y.Citation2 However, the true burden of the disease is considered to be higher as these estimates do not account for asymptomatic HAV infection in children and substantial under-reporting of cases.Citation2,Citation3 Although the mortality rate due to HAV infection has decreased with timely identification and management (including improved intensive care) reducing the number of deaths,Citation4 it still contributes to significant morbidity in both developed and developing countries.Citation1,Citation2,Citation5,Citation6
The prevalence of HAV infection is strongly influenced by the prevailing socioeconomic and general hygiene conditions in any country.Citation6 Changes in HAV endemicity patterns are a result of improvements in hygienic and socioeconomic conditions, as seen particularly in developing countries.Citation2,Citation7-Citation10 Endemicity level for a country is defined by age-seroprevalence surveys establishing the proportion of each age group that acquired immunity to HAV which is demonstrated by the presence of IgG anti-HAV antibodies in serum.Citation11 Countries are identified on three levels of endemicity: high endemic countries are where almost all persons have been in contact with HAV in childhood; intermediate endemic countries are where a considerable proportion of adults are susceptible to HAV and hepatitis A poses a significant healthcare burden; and low endemic countries are characterized by infection in high-risk people.Citation1
Historically, Latin American countries have been regions highly endemic for HAV with the infections occurring primarily in those less than 10 y of age.Citation12 Studies conducted in Mexico from the late 1990s onwards suggested a shift in the HAV endemicity pattern from high to intermediate, as evident from the increase in the mean age of infection over the years.Citation13-Citation15 Furthermore, correlations were drawn between the diversity in HAV transmission patterns across different regions in Mexico to the difference in sanitary and social conditions prevailing across regions.Citation16 Since other countries in the region, such as Argentina and Panama have introduced a universal hepatitis A immunization program, the incidence has significantly declined.Citation17,Citation18
Safe and effective hepatitis A vaccines that confer long-term protection have been available since 1992;Citation4,Citation19 however, they are not included in the universal mass vaccination (UMV) program in Mexico. Although effective vaccines against hepatitis A are available, they are utilized only in the private sector, which includes < 3–5% of the Mexican population. For this reason and the evolving HAV epidemiology in Mexico, current seroprevalence data are necessary to evaluate existing HAV prevention strategies.Citation1,Citation20,Citation21 However, recent evidentiary support from Mexico to formulate future vaccination programs are limited.Citation11 Therefore, the present study assessed the overall seroprevalence of HAV infection in the Mexican population. The study also determined the relationship of HAV seroprevalence with gender, age, regions in Mexico and socioeconomic status. The nature of such a seroprevalence study is important, as validity of this data may constitute an argument in favor of recommending routine vaccination and prompt future cost-effectiveness studies for planning preventative strategies.Citation13
Results
Demographic characteristics
A total of 3984 subjects were enrolled in the study of which 3,658 (1,030 children, 1,719 adolescents and 905 adults) subjects were included in the according-to-protocol (ATP) cohort. The ATP cohort included subjects who met all eligibility criteria, complied with procedures detailed in the protocol and for whom adequate serum samples were available and accurately identified. Of the enrolled subjects, 326 were eliminated from the analyses due to inadequate volume of serum sample for analysis. Overall, the median age of subjects was 29 y (range: 1–95 y) and 51.1% were female. The demographic characteristics according to region are provided in .
Table 1. Demographic characteristics of the overall population
Seroprevalence of HAV
Overall, the majority (84.2%) of subjects were seropositive for anti-HAV antibodies. The HAV seroprevalence rates for females [86.1% (95% CI: 83.3–88.9)] and males [82.2% (95% CI: 78.1–86.2)] were similar (p = 0.10).
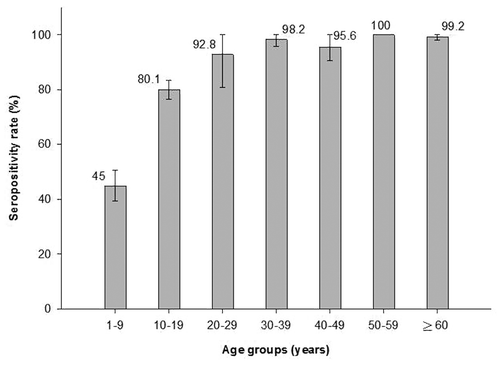
When analyzed according to age, HAV seroprevalence rates were observed to be highest in adults aged ≥ 20 y [96.9% (95% CI: 93.9–99.8)] and lowest in children aged 1–9 y [45.0% (95% CI: 39.4–50.5)] (p < 0.0001) (). HAV seroprevalence rates on a finer scale between ages 1 and 19 y are shown in . The HAV seroprevalence illustrates an increasing trend with advancing age .
Table 2. Seroprevalence of HAV (ATP cohort)
Table 3. Seroprevalence of HAV by age (1–19 y) (ATP cohort)
Figure 1. Seroprevalence of HAV by age group (ATP cohort). Seropositivity rate (%): Percentage of subjects who were seropositive (anti-HAV antibody concentrations ≥ 1 mIU/ml).
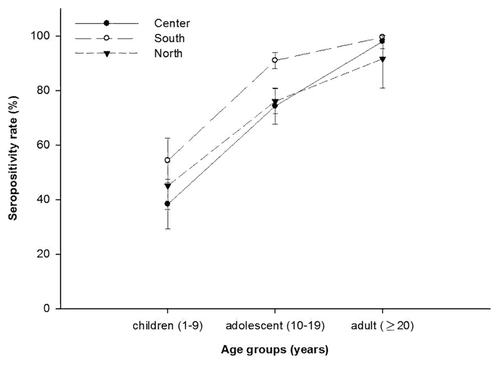
Among regions, HAV seroprevalence rates were highest in the South [88.8% (95% CI: 86.5–91.0)], followed by Central Mexico, Northern Mexico and Mexico City (p = 0.02) (). Further analysis by age groups across different regions showed that the highest seroprevalence across all three age groups was observed in Southern Mexico: children [54.2% (95% CI: 46.04–62.4)], adolescents [90.9% (95% CI: 87.9–93.8)] and adults [99.4% (95% CI: 98.7–100.0)] (). Across the three regions, the lowest HAV seroprevalence was observed among children while the highest seroprevalence was observed among the adolescent and adult groups. Further, seroprevalence rates were similar between urban (82.2%) and rural areas (86.9%).
Figure 2. Seroprevalence of HAV by age group and region (ATP cohort). Seropositivity rate (%): Percentage of subjects who were seropositive (anti-HAV antibody concentrations ≥ 1 mIU/ml).
The seroprevalence rates for HAV were similar for subjects of high socioeconomic status [90.1% (95% CI: 84.9–95.2)] and those of low socioeconomic status [86.6% (95% CI: 83.8–89.5)] ().
Discussion
This study presented the most recent data on the seroprevalence of HAV in the Mexican population to identify susceptible groups of the population in terms of socioeconomic status, age groups, gender and region, thereby allowing recommendations for the prevention of HAV infection. To our knowledge there are no recent HAV seroprevalence reports from Latin America. Presenting such data from Mexico (a major country in the region) is relevant in this aspect. Previous studies have shown that improved public health programs and sanitary conditions have had an impact on the epidemiological pattern of HAV endemicity in Latin American countries indicating a shift from high to intermediate endemicity.Citation14 The results from this study confirm the intermediate HAV endemicity and identify populations vulnerable to the disease in Mexico.
The assessment of the relationship between HAV seroprevalence and age showed that the anti-HAV seropositivity increased with age (p < 0.0001). The high seroprevalence for HAV among the older population is expected for any infectious disease that depends on environmental exposure for transmission.Citation22 These results are similar to a previous regional seroepidemiology study conducted across Latin America in the late 1990s where an increasing trend in the seroprevalence rates with advancing age was observed in Mexico.Citation13,Citation14 In this previous study, the seroprevalence rates were 40.5% observed in children aged 1–5 y, 68.6% in the 6–10 y age group, 88% in the 11–15 y age group, 92.9% in the 16–20 y age group, 96.5% in the 21–30 y age group and 97.8% in the 31–40 y age group.13.14 Another study conducted in Mexico surveyed serologic studies collected from patients attending a pediatric hospital between 1991 and 2005 and of 259 unvaccinated subjects (aged 1–16 y), 51% were seropositive for anti-HAV antibodies.Citation15 A previously conducted population-based survey study in Mexico that collected information from the National Health survey of 2000, has also indicated similar results in children (43.2%), adolescents (80.9%) and adults (98.4%).Citation16 The strength of the present study is that subjects of all ages were included and not limited to children and young adults.
This is in line with an intermediate endemicity pattern where a seroprevalence of 90% is not reached until early adulthood and a higher prevalence of HAV in older populations is explained by the fact that they have been previously exposed to HAV.Citation13 The high seropositivity observed among adolescents is an indication that the risk of early exposure to hepatitis A may have been greater than in recent times.Citation23 Adolescents represent a group at high risk of HAV infection as they are actively social and participate in activities such as travel to different regions (sometimes of high HAV endemicity) which increases the risk of HAV transmission.Citation23 It is important to consider the higher HAV seroprevalence observed in older populations while making future strategic recommendations as the severity of HAV infection is greater in older individuals.Citation11 The lower HAV seroprevalence seen in the younger population may be of concern due to the possibility of outbreaks occurring in this population and the potential risk of getting infected as they grow older and when the disease is more severe.Citation24 Further, asymptomatic children remain a common source of spreading the disease to older susceptible populations. Infection in children may result in serious consequences.
The findings of this study showed variations in HAV seroprevalences across regions of Mexico, the highest being from the Southern region of Mexico and the lowest seen among people living in Mexico City. This result is in line with a recent study conducted by Valdespino and his colleagues who reported higher rates of anti-HAV seropositivity in Southern Mexico followed by lower rates in Mexico City.Citation16 It is to be noted that Southern Mexico is associated with socioeconomic deprivation, poor health and a higher number of indigenous inhabitants, while urbanization is well noted with better socioeconomic conditions in Mexico City.Citation16 A statistically significant difference (p = 0.02) between the regions of Mexico was noted in the present study. This supports the fact that transmission patterns do indeed differ within regions of a country based on different hygiene and sanitary conditions, which subsequently determines the age of exposure to HAV.Citation25 From the study results, an increasing trend in HAV seroprevalence of age groups between regions in Mexico is noticed. Although, there are differences in the seroprevalence rates for HAV between the regions due to different hygiene and sanitation conditions, adolescents still remained the most susceptible groups to hepatitis A infection in all the regions of Mexico.
The results from the present study showed that seroprevalence rates were similar for people of high socioeconomic status and people of low socioeconomic status. It has been noted that substantial socioeconomic differences exist between Latin American countries and the regions within them.Citation26 Contrary to the present study results, Tanaka showed that higher seroprevalence rates for HAV were seen in people of low socioeconomic status than in those of high socioeconomic status (p < 0.001) which characterizes an intermediate endemicity pattern.Citation14 A point to be noted is that the number of subjects of low and middle socioeconomic status were reasonably higher than the number of subjects of high socioeconomic status enrolled in this study. While HAV incidence rates are generally higher in rural areas due to poor sanitary conditions, most rural people moving to urban areas may be immune to HAV, thereby reducing transmission of the virus through herd immunity.Citation27 However, we also acknowledge that migration of rural communities (especially indigenous people) to urban settings may also favor poor sanitary conditions, with the likelihood of the prevalence being high.Citation27 The contrasting results could also potentially be due to the definition of socioeconomic status in ENSANUT. An index of components was developed for different socioeconomic levels based on variables associated with household assets and electro-domestic items (from the survey) such as number of electronic devices (television set, radio, video recorder, telephones, computers), number of rooms (with/without bathrooms, kitchen or living rooms), availability of a refrigerator and washing machine and floor and rooftop construction materials.Citation28 The scores were stratified by tertiles in 3 categories: low, middle and high. Furthermore, ENSANUT explored the access to government programs aimed at improving conditions for people of low and middle socioeconomic status. These programs contributed in ways to reduce poverty through the development of basic capabilities thereby facilitating access to better socioeconomic conditions. Such programs have shown higher coverage among people of low socioeconomic status.Citation28
The common modes of HAV transmission occur via the fecal/oral route, person-to-person contact and ingestion of contaminated food and water.Citation1 Although the answer to such issues would be the maintenance of hygienic conditions, they are insufficient to contain the spread of HAV infection during outbreaks as they are slow to take effect and it takes a long time for changes to appear, as seen previously.Citation29,Citation30 In regions of intermediate endemicity, the primary mode of transmission is through person-to-person contact, often resulting in outbreaks. In such cases, the most effective means of controlling outbreaks and protecting the population is rapid vaccination. A review of outbreaks identified between 1994 and 2004 in the United States indicated that outbreaks declined significantly (p = 0.01) since the implementation of hepatitis A vaccination programs.Citation31 It is also to be noted that hepatitis A vaccination is more accessible to the private sector (than in the public sector), particularly among people of high socioeconomic status.Citation32 The higher HAV seroprevalence among people of high socioeconomic status may likely be due to vaccine-induced antibodies.
Cost-effectiveness studies from Argentina and the United States have indicated healthcare and economic benefits which have bolstered the decision to implement routine childhood vaccination against hepatitis A.Citation33,Citation34 Following initiation of universal childhood immunization with hepatitis A vaccine in countries like Argentina, the United States, Israel and Spain, it was suggested that the substantial decline in hepatitis A incidence rates across age groups and regions was due to herd immunity.Citation17,Citation35-Citation38 The findings from these studies imply that children are the basis for childhood immunization as they are the main carriers of HAV infection. A dynamic model for HAV transmission has recently been developed to quantify the impact of HAV universal childhood vaccination in Mexico which indicates that a 2-dose vaccination at 12 and 18 mo of age with 90% coverage might reduce the incidence of symptomatic HAV by 68–76% over 40 y.Citation39 With 70% and 90% vaccine coverage post-dose-1, the model projected 67–73% and 81–91% reduction in the incidence of all HAV infection (including asymptomatic) after 40 y of vaccination.Citation39 It has also been established that the HAV vaccine provides a long-term protection of at least 15 y.Citation19
While Tanaka has indicated that the strongest risk factor for HAV infection in Mexico was contaminated food and water,Citation14 and Valdespino suggested that the risk of infection in children younger than nine years of age may strongly be influenced by residence in southern states of Mexico and in rural communities, living in low-income families, and in households with limited access to sanitary facilities,Citation16 our study also addressed such risk factors but did not assess vaccination status for participants Despite these factors, implementation of 201hepatitis A vaccination is not routine and the limited use of vaccination in the private sector suggests low vaccination coverage for HAV in the region. Additionally, there are recommendations only to vaccinate against HAV in adolescents or during outbreaks in Mexico, but this is not routinely performed.Citation32,Citation40 As a result, at the time of the study, no vaccination school based programs were implemented and consequently low vaccination coverage in the adolescent age group has been previously reported by Tanaka.Citation41
In conclusion, this study provided the most recent evidence to confirm the shift in HAV seroprevalence from high to intermediate endemicity in Mexico, and the data obtained provides support for the development of cost-effectiveness studies to evaluate further vaccination policy in Mexico. Therefore, along with continuous efforts to improve hygiene and sanitary conditions, the eventual inclusion of an effective hepatitis A vaccine in the UMV may be an appropriate preventative strategy to protect the health of infants, adolescents and adults in Mexico.
Materials and Methods
Study design and subjects
This observational, cross-sectional database study was conducted in Mexico between January and October 2010 (NCT01160081) based on the data and serum samples from subjects who participated in the National Health and Nutrition Survey (ENSANUT 2006) which was conducted between October 2005 and May 2006. This survey aimed to collect nationwide baseline information on the prevalence of infectious and chronic diseases and their associated risk factors using a probabilistic multistage stratified cluster sampling design. A random selection was performed from each one of the 47,152 households visited to interview the following subjects: a child (< 10 y), an adolescent (10–19 y), and an adult (≥ 20 y). Blood samples were obtained from 30% of the randomly selected individuals. The ENSANUT survey was approved by the Ethics Commission from the Mexican National Institute of Public Health.
A sample size of 3984 subjects (aged 1–95 y) previously enrolled in ENSANUT 2006 who had already provided their consent was selected for this study by random simple sampling stratified by age in the serum bank. This sample size was expected to provide 80% statistical power assuming an overall seroprevalence ranging from 25% in children to 95% in adults. Information from structured qualitative questionnaires used in ENSANUT 2006 regarding subjects’ gender, age, geographical region and socioeconomic status were compiled into a subset database designed by the Mexican National Institute of Public Health based on unique identification numbers of those subjects selected for the purpose of this study. No personally identifiable information was retrieved from the database.
Subjects were excluded if informed consent had not been previously obtained, if information required for the study was unavailable or incomplete, or if the serum sample volume was insufficient or wrongly identified.
Laboratory analysis
Blood samples were collected, processed, stored in 2.5 ml aliquots and maintained in freezers at -150°C as part of ENSANUT 2006. For the current study, they were processed at the Mexican National Institute of Public Health to measure antibodies against HAV using a chemiluminescent immunoassay, ARCHITECT HAVAb® (Abbott Laboratories).
Statistical Analyses
The analysis was performed on the ATP cohort. Subjects with anti-HAV antibody concentrations ≥ 1 mIU/ml were considered to be seropositive (seropositivity for HAV was defined as non-reactive for values < 1 mIU/ml and reactive for values > 1 mIU/ml).
Overall HAV seroprevalence and HAV seroprevalence stratified by gender, age group [children (aged 1–9 y), adolescents (aged 10–19 y) and adults (aged ≥ 20 y)], region (Central, Mexico City, Southern and Northern Mexico) and by socioeconomic status (low, medium, high) were reported with 95% confidence interval (CI). In order to estimate the national seroprevalence in Mexico, a post-stratification weight was obtained for each subject taking into consideration non-responsiveness, probability of selection and knowledge of population parameters in addition to the above-mentioned factors. The calculation of the weighted number of subjects (in thousands) was based on the use of the weighting method and the sample selection procedures. Comparison of differences in HAV seroprevalence categorized by gender, age groups, region and socioeconomic status was performed using the chi-square test. All statistical analyses were performed using Statistical Analysis Software (SAS) version 9.1.
| Abbreviations: | ||
| ATP | = | according-to-protocol |
| CI | = | confidence interval |
| GCP | = | good clinical practice |
| HAV | = | Hepatitis A Virus |
| IEC | = | Independent Ethics Committee |
| IRB | = | Institutional Review Board |
| ENSANUT | = | National Health and Nutrition Survey |
| UMV | = | Universal Mass Vaccination |
Acknowledgments
This study was sponsored and funded by the GlaxoSmithKline Biologicals SA., Rixensart, Belgium who was involved in all stages of the study conduct and analysis. GlaxoSmithKline Biologicals SA also took charge of all costs associated with the development and the publishing of the present manuscript.
The authors would like to thank the following from Instituto Nacional de Salud Pública: Manuel Velázquez-Meza and Víctor Guerrero-Lemus for their valuable support in running laboratory testing and preparing database results for hepatitis A virus; Jacqueline Vargas and Claudia R. Rodriguez for contributing and supporting the coordination and monitoring activities for this study; and the following from GlaxoSmithKline Biologicals: Ashmita Ravishankar for medical writing; Camilo Moreno and Jessica Mattos for editorial assistance and coordination in the development of this manuscript.
Submitted
08/20/2012
Revised
10/26/2012
Accepted
11/04/2012
Disclosure of Potential Conflicts of Interest
All investigators at the study clinical site were funded though their institution to do the study. Rodrigo DeAntonio, Luis Romano-Mazzotti, Yolanda Cervantes and Eduardo Ortega-Barria are employees of GlaxoSmithKline group of companies. Rodrigo DeAntonio, Yolanda Cervantes and Luis Romano-Mazzotti have stock options. Eduardo Ortega-Barria has stock ownership.
Trademark
ARCHITECT is a registered trademark of Abbott Laboratories.
References
- Hepatitis A vaccines. Wkly Epidemiol Rec 2000; 75:38 - 44; PMID: 10693358
- WHO. The immunological basis for immunization series: module 18: hepatitis A. 2011. Available at: http://whqlibdoc.who.int/publications/2011/9789241501422_eng.pdf. Accessed: February 10th, 2012.
- Chodick G, Heymann AD, Ashkenazi S, Kokia E, Shalev V. Long-term trends in hepatitis A incidence following the inclusion of Hepatitis A vaccine in the routine nationwide immunization program. J Viral Hepat 2008; 15:Suppl 2 62 - 5; http://dx.doi.org/10.1111/j.1365-2893.2008.01032.x; PMID: 18837837
- FitzSimons D, Hendrickx G, Vorsters A, Van Damme P. Hepatitis A and E: update on prevention and epidemiology. Vaccine 2010; 28:583 - 8; http://dx.doi.org/10.1016/j.vaccine.2009.10.136; PMID: 19925903
- Bell BP, Feinstone SM. Hepatitis A Vaccine. In: Plotkin SA, Oreinstein WA, eds. Vaccines. 4th ed. Philadelphia, PA: Saunders, 2004:269-97.
- Franco E, Meleleo C, Serino L, Sorbara D, Zaratti L, Hepatitis A. Hepatitis A: Epidemiology and prevention in developing countries. World J Hepatol 2012; 4:68 - 73; http://dx.doi.org/10.4254/wjh.v4.i3.68; PMID: 22489258
- Cilla G, Pérez-Trallero E, Artieda J, Serrano-Bengoechea E, Montes M, Vicente D. Marked decrease in the incidence and prevalence of hepatitis A in the Basque Country, Spain, 1986-2004. Epidemiol Infect 2007; 135:402 - 8; http://dx.doi.org/10.1017/S0950268806006959; PMID: 16848926
- Almuneef MA, Memish ZA, Balkhy HH, Qahtani M, Alotaibi B, Hajeer A, et al. Epidemiologic shift in the prevalence of Hepatitis A virus in Saudi Arabia: a case for routine Hepatitis A vaccination. Vaccine 2006; 24:5599 - 603; http://dx.doi.org/10.1016/j.vaccine.2006.04.038; PMID: 16757065
- Sacy RG, Haddad M, Baasiri G, Khoriati A, Gerbaka BJ, Abu-Elyazeed R. Hepatitis a in Lebanon: a changing epidemiological pattern. Am J Trop Med Hyg 2005; 73:453 - 6; PMID: 16103621
- Tosun S, Ertan P, Kasirga E, Atman U. Changes in seroprevalence of hepatitis A in children and adolescents in Manisa, Turkey. Pediatr Int 2004; 46:669 - 72; http://dx.doi.org/10.1111/j.1442-200x.2004.01969.x; PMID: 15660865
- Jacobsen KH, Wiersma ST. Hepatitis A virus seroprevalence by age and world region, 1990 and 2005. Vaccine 2010; 28:6653 - 7; http://dx.doi.org/10.1016/j.vaccine.2010.08.037; PMID: 20723630
- Ciocca M, Moreira-Silva SF, Alegría S, Galoppo MC, Ruttiman R, Porta G, et al. Hepatitis A as an etiologic agent of acute liver failure in Latin America. Pediatr Infect Dis J 2007; 26:711 - 5; http://dx.doi.org/10.1097/INF.0b013e3180f60bed; PMID: 17848883
- Tapia-Conyer R, Santos JI, Cavalcanti AM, Urdaneta E, Rivera L, Manterola A, et al. Hepatitis A in Latin America: a changing epidemiologic pattern. Am J Trop Med Hyg 1999; 61:825 - 9; PMID: 10586919
- Tanaka J. Hepatitis A shifting epidemiology in Latin America. Vaccine 2000; 18:Suppl 1 S57 - 60; http://dx.doi.org/10.1016/S0264-410X(99)00466-1; PMID: 10683550
- García-Juárez I, Solórzano Santos F, Alvarez-y-Muñoz MT, Vázquez-Rosales JG. [Is there a shift in the epidemiology of hepatitis A in Mexican children?]. Rev Invest Clin 2008; 60:292 - 6; PMID: 18956550
- Valdespino JL, Ruiz-Gomez J, Olaiz-Fernandez G, et al. Seroepidemiology of hepatitis A in Mexico. A detector of social inequity and monitor of immunization policies. Salud Publica Mex 2007; 49:Suppl. 3 S377 - 85; http://dx.doi.org/10.1590/S0036-36342007000900009
- Vacchino MN. Incidence of Hepatitis A in Argentina after vaccination. J Viral Hepat 2008; 15:Suppl 2 47 - 50; http://dx.doi.org/10.1111/j.1365-2893.2008.01029.x; PMID: 18837834
- Dora E, DeAntonio-Suarez R, Rodolfo C, et al. Impact of hepatitis A vaccination in Panama following universal immunization, 2000-2009. Sociedad Latinoamericana de Infectologia Pediatrica, May, 2011. Available: http://www.slipe.org/. Accessed: October 19th, 2011.
- Van Herck K, Jacquet JM, Van Damme P. Antibody persistence and immune memory in healthy adults following vaccination with a two-dose inactivated hepatitis A vaccine: long-term follow-up at 15 years. J Med Virol 2011; 83:1885 - 91; http://dx.doi.org/10.1002/jmv.22200; PMID: 21915861
- Hendrickx G, Van Herck K, Vorsters A, Wiersma S, Shapiro C, Andrus JK, et al. Has the time come to control hepatitis A globally? Matching prevention to the changing epidemiology. J Viral Hepat 2008; 15:Suppl 2 1 - 15; http://dx.doi.org/10.1111/j.1365-2893.2008.01022.x; PMID: 18837827
- Advisory Committee on Immunization Practices (ACIP). Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55:1 - 23
- Krebs LS, Ranieri TM, Kieling CO, Ferreira CT, Silveira TR. Shifting susceptibility to hepatitis A among children and adolescents over the past decade. J Pediatr (Rio J) 2011; 87:213 - 8; PMID: 21468471
- Moisseeva AV, Marichev IL, Biloschitchkay NA, Pavlenko KI, Novik LV, Kovinko LV, et al. Hepatitis A seroprevalence in children and adults in Kiev City, Ukraine. J Viral Hepat 2008; 15:Suppl 2 43 - 6; http://dx.doi.org/10.1111/j.1365-2893.2008.01028.x; PMID: 18837833
- World Health Organization Media Center. Hepatitis A. Available: http://www.who.int/mediacentre/factsheets/fs328/en/index.html. Accessed: October 4th, 2012.
- Bell BP. Global epidemiology of hepatitis A: Implications for control strategies. In: Margolis HS, Alter MJ, Liang TJ, Dienstag JL, editors. Viral hepatitis and liver disease. London: International Medical Press; 2002:9-14.
- Markus JR, Cruz CR, Maluf EMCP, Tahan TT, Hoffmann MM. Seroprevalence of hepatitis A in children and adolescents. J Pediatr (Rio J) 2011; 87:419 - 24; PMID: 21842115
- Los Pueblos Indígenas en Áreas Urbanas y la Migración. Retos y Oportunidades. United Nations. Available: http://www.un.org/esa/socdev/unpfii/documents/6_session_factsheet2_es.pdf. Accessed: October 8th, 2012.
- Olaiz-Fernández G, Rivera-Dommarco J, Shamah-Levy T, et al. Encuesta Nacional de Salud y Nutrición 2006. Cuernavaca, México: Instituto Nacional de Salud Pública, 2006. Available: http://www.insp.mx/ensanut/ensanut2006.pdf. Accessed: October 8th, 2012.
- McMahon BJ, Beller M, Williams J, Schloss M, Tanttila H, Bulkow L. A program to control an outbreak of hepatitis A in Alaska by using an inactivated hepatitis A vaccine. Arch Pediatr Adolesc Med 1996; 150:733 - 9; http://dx.doi.org/10.1001/archpedi.1996.02170320079014; PMID: 8673200
- Príkazský V, Oleár V, Cernoch A, Safary A, André FE. Interruption of an outbreak of hepatitis A in two villages by vaccination. J Med Virol 1994; 44:457 - 9; http://dx.doi.org/10.1002/jmv.1890440427; PMID: 7897381
- Craig AS, Watson B, Zink TK, Davis JP, Yu C, Schaffner W. Hepatitis A outbreak activity in the United States: responding to a vaccine-preventable disease. Am J Med Sci 2007; 334:180 - 3; http://dx.doi.org/10.1097/MAJ.0b013e3181425411; PMID: 17873531
- Consejo Nacional De Vacunacion. Manual de vacunacion 2008-2009. Available: http://www.scribd.com/doc/16701329/Manual-Vacunacion-Mexico-20082009. Accessed: October 8th, 2012.
- Ellis A, Rüttimann RW, Jacobs RJ, Meyerhoff AS, Innis BL. Cost-effectiveness of childhood hepatitis A vaccination in Argentina: a second dose is warranted. Rev Panam Salud Publica 2007; 21:345 - 56; http://dx.doi.org/10.1590/S1020-49892007000500002; PMID: 17761046
- Rein DB, Hicks KA, Wirth KE, Billah K, Finelli L, Fiore AE, et al. Cost-effectiveness of routine childhood vaccination for hepatitis A in the United States. Pediatrics 2007; 119:e12 - 21; http://dx.doi.org/10.1542/peds.2006-1573; PMID: 17200237
- Wasley A, Fiore A, Bell BP. Hepatitis A in the era of vaccination. Epidemiol Rev 2006; 28:101 - 11; http://dx.doi.org/10.1093/epirev/mxj012; PMID: 16775039
- Armstrong GL, Billah K, Rein DB, Hicks KA, Wirth KE, Bell BP. The economics of routine childhood hepatitis A immunization in the United States: the impact of herd immunity. Pediatrics 2007; 119:e22 - 9; http://dx.doi.org/10.1542/peds.2006-1572; PMID: 17200247
- Dagan R, Leventhal A, Anis E, Slater P, Ashur Y, Shouval D. Incidence of hepatitis A in Israel following universal immunization of toddlers. JAMA 2005; 294:202 - 10; http://dx.doi.org/10.1001/jama.294.2.202; PMID: 16014594
- Domínguez A, Oviedo M, Carmona G, Batalla J, Bruguera M, Salleras L, et al. Impact and effectiveness of a mass hepatitis A vaccination programme of preadolescents seven years after introduction. Vaccine 2008; 26:1737 - 41; http://dx.doi.org/10.1016/j.vaccine.2008.01.048; PMID: 18325642
- Effelterre TV, De Antonio-Suarez R. Cassidy Adrian, Romano-Mazzotti L, Marano C. Model-based projections of the population-level impact of hepatitis A vaccination in Mexico. Hum Vaccin Immunother 2012; 8:1099 - 108
- Santos JI. El Programa Nacional de Vacunacion: orgullo de Mexico. Rev Fac Med UNAM 2002; 45:142 - 53
- Mesa redonda XXVI Vacunas para la edad adolescente. Salud Publica Mex 2007; 49:suppl 1 322 - 4