Abstract
Archaeosomes (ARC), vesicles made from lipids extracted from Archaea, display strong adjuvant properties. In this study, we evaluated the ability of the highly stable ARC formulated from total polar lipids of a new Halorubrum tebenquichense strain found in Argentinean Patagonia, to act as adjuvant for soluble parasite antigens in developing prophylactic vaccine against the intracellular protozoan T. cruzi, the etiologic agent of Chagas disease. We demonstrated for the first time that C3H/HeN mice subcutaneously immunized with trypanosomal antigens entrapped in these ARC (ARC-TcAg) rapidly developed higher levels of circulating T. cruzi antibodies than those measured in the sera from animals receiving the antigen alone. Enhanced humoral responses elicited by ARC-TcAg presented a dominant IgG2a antibody isotype, usually associated with Th1-type immunity and resistance against T. cruzi. More importantly, ARC-TcAg-vaccinated mice displayed reduced parasitemia during early infection and were protected against an otherwise lethal challenge with the virulent Tulahuén strain of the parasite. Our findings suggest that, as an adjuvant, H. tebenquichense-derived ARC may hold great potential to develop a safe and helpful vaccine against this relevant human pathogen.
Chagas disease or American trypanosomiasis is a neglected tropical disease caused by the protozoan parasite Trypanosoma cruzi and has a widespread distribution in Latin America. WHO estimate that near 15 million individuals are infected worldwide and 50,000 children and adults die annually as a result of clinical complications of T. cruzi-induced heart disease and their lack of effective treatment.Citation1 The risk of transmission of the disease is high because the infection has been detected in non-endemic areas of the Americas and Europe due to large scale migrations. In light of these problems, it is essential to develop new strategies for the prevention and control of Chagas disease. At present, vaccines and immunotherapies targeted at T. cruzi infection are practically non-existent. In parallel with the efforts toward the identification of vaccine candidates, several adjuvants have been assayed to generate protective immunity to T. cruzi, but with limited success.Citation2,Citation3 In recent years, an increasing body of evidence has revealed the strong adjuvant properties of ARC.Citation4-Citation6 These vesicles enclosed by one or more bilayers prepared with total polar lipids (TPL) extracted from microorganisms belonging to the domain Archaea are more avidly internalized, both in vitro and in vivo, by macrophages and antigen presenting cells than conventional liposomes.Citation7,Citation8 They also differ from liposomes in that the inclusion of immunomodulators is not necessary to improve the adjuvancy beyond that of a simple depot effect,Citation9 favoring scale up production.
In this regard, in an earlier study we reported the ability of ARC composed of the TPL of a new H. tebenquichense strain found in Argentinean Patagonia to elicit potent antibody responses to entrapped bovine serum albumin (BSA) in mice.Citation10
ARC have demonstrated great potential as adjuvant for immunogens aimed at killing intracytoplasmic bacterial pathogens such as Listeria monocytogenes.Citation11 However, the ability of ARC-based vaccines to protect against intracellular protozoan parasites has yet to be tested.
The goal of our current study was to evaluate whether H. tebenquichense-derived ARC may serve as adjuvant for soluble parasite antigens in developing prophylactic T. cruzi vaccine.
T. cruzi protein antigens (TcAg) present in a whole homogenate (WH) of parasites were prepared from epimastigote forms disrupted by pressure-depressure as previously described.Citation12
ARC containing TcAg (ARC-TcAg) were prepared as state in Gonzalez et al.,Citation10 except that TcAg in phosphate buffered saline (PBS, 2.5 mg/ml) was used as the aqueous phase for the hydration of the thin lipidic film. Proteins were quantified by Bradford method,Citation13 and phospholipids quantified by a colorimetric method.Citation14
Female 6–8-week-old C3H/HeN mice obtained from University of Buenos Aires, Argentina, were selected for in vivo efficacy studies. Research was conducted according to the National Research Council’s guide for animal care and was approved by our internal Ethics Committee. Groups of five mice were immunized subcutaneously (sc) in the back on days 0, 14 and 21 with 12.5 μg of free TcAg in PBS or 12.5 μg of ARC-TcAg. Control mice were injected with equivalent amount of empty ARC. The injection volume was 50 μl.
To evaluate humoral response, blood was collected from the tail vein at 21 days after the last immunization and sera were analyzed by enzyme-linked immunosorbent assay (ELISA) for the presence of anti-T. cruzi antibodies as previously described.Citation15 Briefly, the antigen added to the plates was T. cruzi proteins present in a WH of parasites (200 μg/ml). The secondary antibody conjugated with peroxidase was goat anti-mouse IgG (1:5000, Pierce, Catalog # 0031430) and the substrate was 2, 2'-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid) (ABTS, Sigma-Aldrich Co). Each serum was analyzed in 2-fold serial dilutions. The optical density (OD) was measured at 405 nm using an ELISA reader (Multiskan Ex, Thermo Labsystems). End-point titers were defined as the highest serum dilution that resulted in an OD value greater than that of the mean + three standard deviations of preimmune mouse sera.
Detection of IgG subclass responses was performed as described above, except that the secondary antibodies were specific for mouse IgG1 and IgG 2a (1:1000, Santa Cruz Biotechnology, Catalog # sc-2060 and sc-2061 respectively).
Immunized animals were challenged intraperitoneally (ip) at 4 weeks postboost with 150 bloodstream trypomastigotes of Tulahuén strain of T. cruzi. Parasitemia was monitored by daily counting of number of trypomastigotes per 5 μl of fresh blood,Citation16 and mortality was recorded.
Data were analyzed using GraphPadPrism 5.0 software (GraphPad Software Inc.). The Student’s t-test, Mann-Whitney and Fisher’s exact tests were conducted to compare the possible differences between the mean values of the different groups. P values of < 0.05 were considered to be statistically significant.
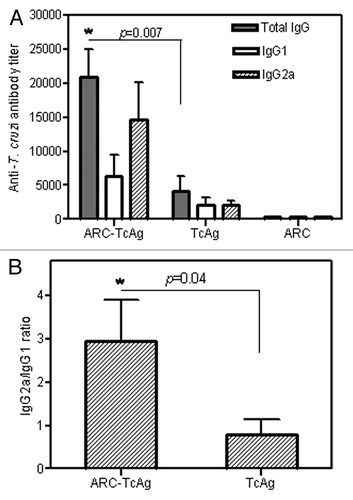
The ARC preparations were multilamellar, with a mean size of 564 ± 22 nm and Z potential of -50 mV. The amount of antigen (proteins) and phospholipids contained in ARC was 40 μg/ml and 20 mg/ml, respectively. The protein/lipid ratio was 2 μg/mg. Following sc immunization with ARC-TcAg, mice exhibited serum specific IgG antibody titers between 3 and 6-fold higher (p = 0.007) than those observed in TcAg group (). As expected, immunization with empty ARC failed to evoke any anti-T. cruzi IgG response. After vaccination, the analysis of IgG isotype profiles revealed that both TcAg-specific IgG1 and IgG2a antibodies were induced in the ARC-TcAg and free TcAg groups. However, the IgG2a/IgG1 ratio for ARC-TcAg group was significantly (p = 0.04) higher than that calculated for TcAg group (2.9 vs. 0.8, respectively, ).
Figure 1. Induction of humoral response to T. cruzi in vaccinated C3H/HeN mice. (A) ELISA analysis of antibody isotypes 3 weeks after the last immunization. (B) Ratio of IgG2a to IgG1 antibody titers. Data represent mean ± SEM of two independent experiments.
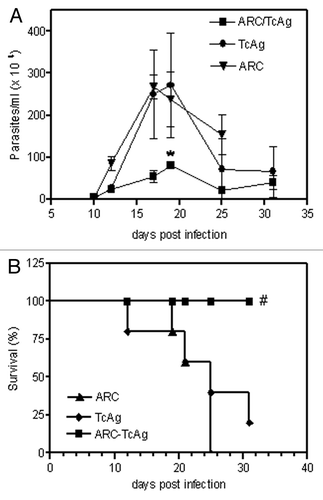
When mice vaccinated with ARC-TcAg were challenged with bloodstream Tulahuén trypomastigotes, we observed a reduction (p = 0.03) in bloodstream parasite levels at the peak of parasitemia (17–19 dpi) when compared with animals that received free TcAg (). Also, statistical analysis revealed a significant (p = 0.04) difference in mortality rates between both groups. While all animals vaccinated with ARC-TcAg survived lethal challenge, only 20% of TcAg immunized mice remained alive after 31 days of infection (). Another group of naive mice was infected with the same number of trypomastigotes and showed 100% of mortality at the peak of parasitemia. In addition, all control mice vaccinated with empty ARC developed fatal infection within 25 days post-infection.
Discussion
In recents years, an increasing body of evidence has revealed the strong adjuvant properties of archaeosomes prepared from different archaeobacteria.Citation5 Particularly, in an earlier study we demonstrated the adjuvant activity of archaeosomes formulated from total polar lipids of a new H. tebenquichense strain found in Argentinean Patagonia when they were sc administered along BSA in mice.Citation10
We herein used a murine model of acute chagasic infection to assess the potential of these new archaeosomes to act as adjuvanting vesicles with incorporated TcAg for prophylactic vaccination against T. cruzi.
We demonstrated that vaccination with ARC-TcAg induces enhanced type-1 immunity against parasite infection as measured by T. cruzi-specific IgG2a response in C3H/HeN mice. In our earlier study, upon sc immunization of this mouse strain, BSA entrapped in ARC elicited similar levels of both IgG1 and IgG2a.Citation10 Thus, we foresaw a balanced antibody isotype distribution in mice immunized with ARC-TcAg. Unexpectedly, the increased level of protection observed in these vaccinated animals was reflected by a prevalence of the anti-T. cruzi IgG2a fraction. The reason for this discrepancy is likely due to the different nature of the immunizing antigens. Previous studies have indicated that a dominant Th1 immune response is essential for the early control of Chagas disease.Citation17 It is known that circulating antibodies play a role in parasite killing and antibody titer/specificity, or a combination of these factors, are important in resistance to T. cruzi infection. Moreover, an efficient protective response against T. cruzi requires the induction of IgG2a, a Th1-type immunity-associated isotype.Citation18 Therefore, we hypothesized that the Th1-biased response elicited by ARC-TcAg in immunized mice would help confer protection against acute chagasic infection. To demonstrate this, vaccinated mice were then challenged with one of the most virulent strains of T. cruzi.Citation19 Vaccination with ARC-TcAg clearly limited the course of T. cruzi infection in mice in terms of parasitemia and mortality.
Our study focuses on the early humoral immunity after challenge that contributes to control acute T. cruzi infection. The longer-term persistence of ARC-TcAg-induced specific antibody titers is presently unknown. However, based on our previous findings, it is conceivable that the ARC-TcAg vaccine is likely to develop lasting primary IgG2a response and enhanced immunological memory.Citation10 Even though antibodies may be seen as reliable surrogate predictors of protection by vaccines, it is widely accepted that cell-mediated immune functions are critical for eradicating infections caused by intracellular pathogens, including T. cruzi. Both CD4+ and CD8+ T cell subsets appear to be important for the generation of effective immunoprotection against this protozoan infection and it is therefore desirable that the ARC-TcAg vaccine be capable of eliciting such cellular responses. Nevertheless, the lack of experimental data to clarify the ability of ARC-TcAg to raise cell-mediated protective immunity is a shortcoming of our current study. More extensive investigations on the induction of long-term memory and cellular responses upon immunization with ARC-Tc Ag, including passive transfer of antibodies and/or immune cells, will be performed in order to elucidate the protective activity of our formulation.
The mechanism responsible for adjuvancy of ARC remains elusive. ARC have been characterized as poor inducers of innate immunity via toll-like or CD1 receptors.Citation20,Citation21 However, the presence of glyco-portions of archaetydil phosphate groups glycosidically linked to short oligosaccharides,Citation22,Citation23 seems to be important to the adjuvanting process. Particularly for H. tebenquichense-derived ARC, their unique content of archaetidyl phosphatidylglycerol, phosphatidylglycerophosphate methyl ester and glycosilated sulpholipids, added to the presence of mannose-containing archaeolipids,Citation24 enabling interaction with specific receptors on APC, probably contributed to the enhanced immunogenicity of the ARC-TcAg preparation. Next steps should include the exploration of T. cruzi vaccines constituted by more defined parasite antigens formulated in ARC.
Chagas disease is increasingly understood as a problem of parasite persistence within the host, rather than primarily as a result of an inappropriate immune response driving pathology,Citation25 which has generated much interest in anti-T. cruzi vaccine development. Nonetheless, the potential harmfulness, complexity, expensiveness and difficulties to scale up some promising vaccine approaches can spoil further attempts of industrial production and acceptation by regulatory organisms. In this regard, ARC can be produced by scalable techniques and from sustainable sources. Remarkably, these lipid vesicles are derived from LPS-free archaea and have displayed low toxicity upon parenteral administration in rodents.Citation26
In conclusion, this is the first demonstration that T. cruzi antigens can be incorporated succesfully into ARC and, upon sc inoculation in mice, the resulting immunogen is capable of priming a protective response against an intracellular parasite infection. These findings indicate that ARC show promise as safe and helpful carrier-adjuvant for the design of future vaccines against this human pathogen.
| Abbreviations: | ||
| ARC | = | archaeosomes |
| ARC-TcAg | = | Trypanosoma cruzi antigens entrapped in ARC |
| TcAg | = | T. cruzi antigens |
| TPL | = | total polar lipids |
| BSA | = | bovine serum albumin |
| PBS | = | phosphate buffered saline |
| ELISA | = | enzyme-linked immunosorbent assay |
Acknowledgments
This work was supported in part by grants from the National Research Council (CONICET). P.B.P., E.L.R., R.S.C. and M.J.M. are members of the Research Career Program from CONICET. L.H.H. is a fellow from CONICET. We thank Dr. Mónica Esteva (National Institute of Parasitology Dr. Mario Fatala Chabén) for kindly providing the Tulahuén strain of T. cruzi
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- The Special Programme for Research and Training in Tropical Diseases (TDR/WHO). Reporte sobre la Enfermedad de Chagas. Buenos Aires, Argentina. Guhl F, Ladzins-Helds JK, eds, 2007: 97.
- Garg N, Bhatia V. Current status and future prospects for a vaccine against American trypanosomiasis. Expert Rev Vaccines 2005; 4:867 - 80; http://dx.doi.org/10.1586/14760584.4.6.867; PMID: 16372882
- Quijano-Hernandez I, Dumonteil E. Advances and challenges towards a vaccine against Chagas disease. Hum Vaccin 2011; 7:1184 - 91; http://dx.doi.org/10.4161/hv.7.11.17016; PMID: 22048121
- Sprott GD, Tolson DL, Patel GB. Archaeosomes as novel antigen delivery systems. FEMS Microbiol Lett 1997; 154:17 - 22; http://dx.doi.org/10.1016/S0378-1097(97)00294-2; PMID: 9297816
- Krishnan L, Dicaire CJ, Patel GB, Sprott GD. Archaeosome vaccine adjuvants induce strong humoral, cell-mediated, and memory responses: comparison to conventional liposomes and alum. Infect Immun 2000; 68:54 - 63; http://dx.doi.org/10.1128/IAI.68.1.54-63.2000; PMID: 10603368
- Li Z, Zhang L, Sun W, Ding Q, Hou Y, Xu Y. Archaeosomes with encapsulated antigens for oral vaccine delivery. Vaccine 2011; 29:5260 - 6; http://dx.doi.org/10.1016/j.vaccine.2011.05.015; PMID: 21609747
- Sprott GD, Sad S, Fleming LP, Dicaire CJ, Patel GB, Krishnan L. Archaeosomes varying in lipid composition differ in receptor-mediated endocytosis and differentially adjuvant immune responses to entrapped antigen. Archaea 2003; 1:151 - 64; http://dx.doi.org/10.1155/2003/569283; PMID: 15803661
- Krishnan L, Sad S, Patel GB, Sprott GD. The potent adjuvant activity of archaeosomes correlates to the recruitment and activation of macrophages and dendritic cells in vivo. J Immunol 2001; 166:1885 - 93; PMID: 11160236
- Nordly P, Korsholm KS, Pedersen EA, Khilji TS, Franzyk H, Jorgensen L, et al. Incorporation of a synthetic mycobacterial monomycoloyl glycerol analogue stabilizes dimethyldioctadecylammonium liposomes and potentiates their adjuvant effect in vivo. Eur J Pharm Biopharm 2011; 77:89 - 98; http://dx.doi.org/10.1016/j.ejpb.2010.10.001; PMID: 20940050
- González RO, Higa LH, Cutrullis RA, Bilen M, Morelli I, Roncaglia DI, et al. Archaeosomes made of Halorubrum tebenquichense total polar lipids: a new source of adjuvancy. BMC Biotechnol 2009; 9:71; http://dx.doi.org/10.1186/1472-6750-9-71; PMID: 19678953
- Conlan JW, Krishnan L, Willick GE, Patel GB, Sprott GD. Immunization of mice with lipopeptide antigens encapsulated in novel liposomes prepared from the polar lipids of various Archaeobacteria elicits rapid and prolonged specific protective immunity against infection with the facultative intracellular pathogen, Listeria monocytogenes.. Vaccine 2001; 19:3509 - 17; http://dx.doi.org/10.1016/S0264-410X(01)00041-X; PMID: 11348718
- Segura EL, Vazquez C, Bronzina A, Campos JM, Cerisola JA, Gonzalez Cappa SM. Antigens of the subcellular fraction of Trypanosoma cruzi. Part II: Flagellar and membrane fraction. J Protozool 1997; 24:540 - 3
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72:248 - 54; http://dx.doi.org/10.1016/0003-2697(76)90527-3; PMID: 942051
- Stewart JCM. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal Biochem 1980; 104:10 - 4; http://dx.doi.org/10.1016/0003-2697(80)90269-9; PMID: 6892980
- Corral RS, Petray PB. CpG DNA as a Th1-promoting adjuvant in immunization against Trypanosoma cruzi.. Vaccine 2000; 19:234 - 42; http://dx.doi.org/10.1016/S0264-410X(00)00172-9; PMID: 10930678
- Pizzi T. Prensa Médica Universitaria. Santiago de Chile, 1957, 38.
- Hoft DF, Eickhoff CS. Type 1 immunity provides optimal protection against both mucosal and systemic Trypanosoma cruzi challenges. Infect Immun 2002; 70:6715 - 25; http://dx.doi.org/10.1128/IAI.70.12.6715-6725.2002; PMID: 12438346
- Powell MR, Wassom DL. Host genetics and resistance to acute Trypanosoma cruzi infection in mice. I. Antibody isotype profiles. Parasite Immunol 1993; 15:215 - 21; http://dx.doi.org/10.1111/j.1365-3024.1993.tb00603.x; PMID: 8506117
- Taliaferro WH, Pizzi T. Connective tissue reactions in normal and immunized mice to a reticulotropic strain of Trypanosoma cruzi.. J Infect Dis 1955; 96:199 - 226; http://dx.doi.org/10.1093/infdis/96.3.199; PMID: 14392366
- Krishnan L, Gurnani K, Dicaire CJ, van Faassen H, Zafer A, Kirschning CJ, et al. Rapid clonal expansion and prolonged maintenance of memory CD8+ T cells of the effector (CD44highCD62Llow) and central (CD44highCD62Lhigh) phenotype by an archaeosome adjuvant independent of TLR2. J Immunol 2007; 178:2396 - 406; PMID: 17277146
- de la Salle H, Mariotti S, Angenieux C, Gilleron M, Garcia-Alles L-F, Malm D, et al. Assistance of microbial glycolipid antigen processing by CD1e. Science 2005; 310:1321 - 4; http://dx.doi.org/10.1126/science.1115301; PMID: 16311334
- Krishnan L, Sad S, Patel GB, Sprott GD. Archaeosomes induce long-term CD8+ cytotoxic T cell response to entrapped soluble protein by the exogenous cytosolic pathway, in the absence of CD4+ T cell help. J Immunol 2000; 165:5177 - 85; PMID: 11046050
- Sprott GD, Dicaire CJ, Côté J-P, Whitfield DM. Adjuvant potential of archaeal synthetic glycolipid mimetics critically depends on the glyco head group structure. Glycobiology 2008; 18:559 - 65; http://dx.doi.org/10.1093/glycob/cwn038; PMID: 18450974
- Higa LH, Schilrreff P, Perez AP, Iriarte MA, Roncaglia DI, Morilla MJ, et al. Ultradeformable archaeosomes as new topical adjuvants. Nanomedicine 2012; 8:1319 - 28; PMID: 22366598
- Tarleton RL. Chagas disease: a role for autoimmunity?. Trends Parasitol 2003; 19:447 - 51; http://dx.doi.org/10.1016/j.pt.2003.08.008; PMID: 14519582
- Esko JD, Doering TL, Raetz CRH. Eubacteria and Archaea. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, eds. Essentials of Glycobiology. 2nd edition. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press, 2009, Chapter 20.