Abstract
Objectives: The objectives of this study were to compare the serologic responses at week 48 to primary vaccination with 23-valent pneumococcal polysaccharide vaccine (PPV) vs. 7-valent pneumococcal conjugate vaccine (PCV); and to identify factors associated with serologic response in HIV-infected adult patients with access to combination antiretroviral therapy (cART).
Methods: One hundred and four CD4-matched pairs of HIV-infected patients who underwent primary pneumococcal vaccination with 23-valent PPV or 7-valent PCV were enrolled for determinations of anti-capsular antibody responses against four serotypes (6B, 14, 19F and 23F) at baseline, 24 weeks and 48 weeks following vaccination. Significant antibody responses were defined as 2-fold or greater increase of antibody levels at week 48 compared with baseline. The logistic regression model was used to determine the factors associated with serologic response to at least one and two serotypes.
Results: At week 48, patients who received PCV demonstrated a statistically significantly higher response rate to at least 2 serotypes than those who received PPV (37.5% vs. 20.2%, p = 0.006). In multivariate analysis, factors associated with significant antibody responses to at least one or two serotypes included receipt of PCV (adjusted odds ratio [AOR], 2.42 [95% CI, 1.23–4.78] and 3.58 [95% CI. 1.76–7.28], respectively), and undetectable plasma HIV RNA load (< 400 copies/ml) at vaccination (AOR, 1.47 [95% CI, 0.60–3.64] and 3.62 [95% CI, 1.11–11.81], respectively).
Conclusions: Primary vaccination with 7-valent PCV achieved a significantly better serologic responses to one or two out of the four serotypes studied at week 48 than with 23-valent PPV in HIV-infected patients in the cART era. Suppression of HIV replication when primary vaccination was administered was associated with better serologic responses.
Introduction
HIV-infected patients are at higher risk for invasive pneumococcal disease than HIV-uninfected adults both before and after the introduction of combination antiretroviral therapy (cART).Citation1-Citation3 To prevent pneumococcal diseases among HIV-infected patients, vaccination with 23-valent pneumococcal polysaccharide vaccine (PPV) has been recommended for HIV-infected patients by the Department of Health and Human Services (DHHS) Guidelines.Citation4 However, several studies have shown that the immunogenicity and durability of 23-valent PPV are poor in HIV-infected patients, especially in those patients with low CD4 counts.Citation5-Citation7 Moreover, the only randomized clinical trial of vaccination with 23-valent PPV among HIV-infected Ugandan adults without receiving cART did not demonstrate clinical benefits in subjects who received 23-valent PPV compared with those who received placebo.Citation8
The widespread use of pneumococcal conjugate vaccine (PCV) since its licensure has significantly reduced the incidence of invasive pneumococcal disease both in HIV-uninfected and HIV-infected children.Citation9 In HIV-infected children, several studies have shown that vaccination with PCV was immunogenic and safe among the children.Citation10 Studies to investigate the serologic responses and clinical outcome among the immunosuppressed adults who receive PCV, however, are limited. A recent randomized clinical trial in Malawi estimated an efficacy of 74% in reduction of recurrent invasive pneumococcal disease due to vaccine serotypes in HIV-infected African adults who received two doses of 7-valent PCV compared with those who received placebo.Citation11 In persons aged over 40 y who had chronic obstructive pulmonary disease, Dransfield et al. have shown superiority of PCV to PPV in serologic response rate in the short term as well as long-term follow-up.Citation12 In allogeneic stem-cell transplant recipients, Kumar et al. have demonstrated that the response rate to at least one serotype was greater in patients receiving PCV.Citation13 In HIV-infected adults, previous studies have used prime-boost approach to compare the serologic responses to vaccination with PCV followed by PPV or PCV at a 4- or 8-week interval.Citation14,Citation15 Studies designed to compare the immunogenicity of one dose of PCV and PPV in HIV-infected adult patients were rarely performed. The objectives of this study were to compare the serologic responses at week 48 to primary vaccination with 23-valent PPV vs. 7-valent PCV; and to identify the associated factors with serologic response in HIV-infected adult patients with access to cART.
Results
Characteristics of the study population
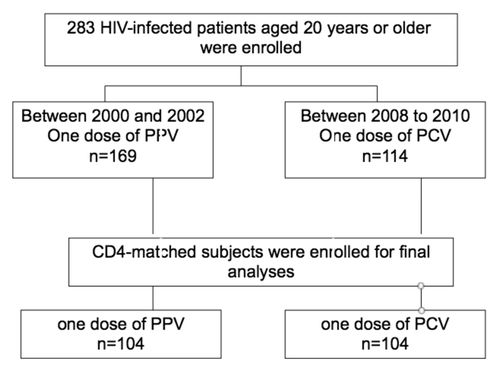
The study flow of pneumococcal vaccination is shown in . During the two study periods, a total of 283 HIV-infected patients were enrolled: 169 patients received one dose of 23-valent PPV between 2000 and 2002,Citation6 and 114 received one dose of 7-valent PCV between 2008 and 2010. One hundred and four pairs that were matched for CD4 count and plasma HIV viral load at vaccination were selected for final analysis.
Figure 1. Study flow of vaccination with 23-valent pneumococcal polysaccharide vaccine (PPV) and 7-valent pneumococcal conjugate vaccine (PCV) in HIV-infected patients
summarizes the baseline characteristics of the study subjects. Patients receiving PPV were enrolled in earlier years of the pneumococcal vaccination study when PCV was not available in Taiwan until 2005. Compared with patients who were enrolled in the latter study period (2008 to 2010) and received one dose of PCV, patients who received PPV were more likely to present for HIV care at a more immunosuppressed status with a lower mean nadir CD4 count (183.1 vs. 268.0 cells/μl, p = 0.01) and were more likely to have received cART when vaccination was administered (100% vs. 72.1%, p < 0.001) ().
Table 1. Baseline characteristics of patients receiving 23-valent pneumococcal polysaccharide vaccine (PPV) or 7-valent pneumococcal conjugate vaccine (PCV)
Serologic responses to pneumococcal vaccination
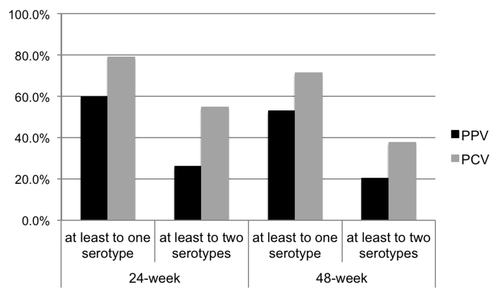
summarizes the antibody titers to the four serotypes studied following PPV or PCV vaccination. Serologic response rates to at least one and two of the four serotypes studied (serotypes 6B, 14, 19F and 23F) 24 weeks and 48 weeks following vaccination are shown in . Patients who received one dose of PCV maintained significantly higher response rates than those who received PPV at both 24 and 48 weeks of follow-up. Twenty-four weeks following vaccination, 62 patients (59.6%) who received one dose of PPV and 82 (78.9%) who received PCV had serologic response to at least to one serotype (p = 0.003), and 27 patients (26.0%) who received PPV and 57 (54.8%) who received PCV had serologic response to at least to two serotypes (p < 0.001). Forty-eight weeks following vaccination, 55 patients (52.9%) who received PPV and 74 (71.2%) who received PCV had serologic response to at least to one serotype (p = 0.007) and 21 patients (20.2%) who received PPV and 39 (37.5%) who received PCV had serologic response to at least to two serotypes (p = 0.006).
Table 2. Median antibody titers (interquartile range, ng/ml) of the four serotypes studied for subjects receiving 23-valent pneumococcal polysaccharide vaccine and those receiving 7-valent pneumococcal conjugate vaccine
Predictive factors of serologic responses
summarizes the results of linear regression to identify the independent factors that were associated with significant antibody responses to at least one and two serotypes in intenton-to-treat (ITT) analysis. At week 48, vaccination with PCV was more likely to achieve significant serologic response to either one or two serotypes compared with vaccination with PPV, with an adjusted odds ratio (AOR) of 2.42 (95% CI, 1.23–4.78) and 3.58 (95% CI, 1.76–7.28), respectively. An undetectable plasma HIV RNA load (< 400 copies/ml) at vaccination was associated with a higher response rate to at least two serotypes at week 48 with AOR of 3.62 (95% CI, 1.11–11.81) and at least one serotype with AOR of 1.47 (95% CI, 0.60–3.64).
Table 3. Factors associated with a significant antibody response in multivariate analysis
Discussion
In the comparisons made between the two groups of subjects who were enrolled in two respective 48-week longitudinal follow-up studies, we demonstrated that vaccination with 7-valent PCV resulted in a higher serologic response rate to at least one and two serotypes compared with vaccination with 23-valent PPV in HIV-infected adult patients with access to cART. The response rates to either vaccine could be improved in patients who had achieved suppression of HIV replication.
The Food and Drug Administration (FDA) has approved 13-valent PCV for children, including HIV-infected, and adults aged over 50 y. This approval was based on serologic and clinical studies that showed adequate immunogenicity and safety in these populations.Citation10,Citation16 Recently, the Advisory Committee on Immunization Practices (ACIP) also recommended its use for adults aged 19 y older with functional or anatomic asplenia, cerebrospinal fluid leaks, cochlear implants and immunocompromising conditions such as congenital or acquired immunodeficiencies, HIV infection, chronic renal failure or nephrotic syndrome, malignancies, solid-organ transplantation and disease requiring immunosuppressives. However, more clinical and serologic studies are warranted to investigate the appropriate doses and dosing schedule and to demonstrate the benefit of using PCV as primary vaccination in the patients with chronic disease or immunocompromising conditions.
Over the past 10 y, several studies have investigated the serologic response to PCV in the immunocompromised hosts or subjects with chronic lung disease. The study design and results of these studies are summarized in . In patients with chronic obstructive pulmonary diseases (COPD), Dransfield et al. have found that PCV vaccination induced a superior immune response at one month post vaccination compared with PPV; and elderly and previous vaccination with PPV were associated with a lower serologic response rate.Citation12 In another study with a 2-y follow-up duration, vaccination with PCV induced a significantly greater functional antibody response than PPV in patients with COPD.Citation17 In renal transplant recipients, Kumar and his colleagues found that vaccination with PCV vaccination generated a higher antibody response to serotypes 6B and 23F than with PPV;Citation18 however, the observed differences waned off three years after primary vaccination.Citation19 In contrast, by using prime-booster strategy, priming with PCV was not demonstrated to increase immunogenicity in the liver transplant recipients.Citation20
Table 4. Summary of studies of vaccination with pneumococcal conjugate vaccine in immunocompromised adult patients
In HIV-infected adult patients, we have demonstrated that vaccination with 7-valent PCV did not increase plasma HIV RNA load or decrease CD4 count in those who received cART when vaccination was administered.Citation21 In terms of serologic responses, previous studies using prime-booster strategies yielded inconsistent results (). Lesprit et al. found that serologic response rate 24 weeks following vaccination was higher in patients who were primed by PCV.Citation15 However, Penaranda et al. showed there was no difference in the response rate eight weeks following vaccination.Citation22 Feikin et al. showed that priming with PCV yielded higher antibody levels but the antibody levels remained similar after boosted vaccination with PCV eight weeks later.Citation14 Unlike previous studies that reported occurrence of hyporesponsiveness after booster vaccination with PPV,Citation23 no hyporesponseiveness was noted after booster vaccination with PPV in patients receiving priming vaccination with PCV.Citation14,Citation15 Our study was the first study to compare the immunogenicity after vaccination with one dose of PCV and PPV in HIV-infected patients who did not receive booster vaccination within the 48-week follow-up period. In our study, we demonstrated that PCV vaccination yielded statistically significantly higher serologic response rates to at least one and two of the four serotypes studied than PPV vaccination during the 48 weeks of follow up. In addition, we enrolled patients with CD4 counts < 200 cells/µl into the trial. However, CD4 counts < 200 cells/µl at vaccination was not associated with serologic response in the multivariate analysis, probably due to the small number of patients with CD4 counts < 200 cells/µl. Only suppression of HIV replication at vaccination was associated with a higher response rate to at least one or two serotypes. Consistent with the finding in HIV-infected children,Citation24 our findings also support the recommendation that pneumococcal vaccination should be given in HIV-infected patients who have achieved undetectable plasma HIV RNA load.
There are several limitations in this study. First, this is not a double-blind, randomized controlled trial and the subjects were enrolled in two different study periods, although we used an objective end point to compare the immunogenicity between PPV and PCV in the subjects that were matched for CD4 count at vaccination. Second, we did not enroll subjects who did not receive pneumococcal vaccine because of ethical concerns. Third, significant differences were observed in the proportion of patients receiving cART between the two groups. Subjects who received PPV vaccination were enrolled in earlier days prior to introduction of PCV into Taiwan and were more likely to be late presenters than those receiving PCV. However, the two groups of patients were matched for CD4 counts at vaccination and multivariate analysis did not reveal statistically significant impact of use of cART. Fourth, we did not use clinical end point to examine the effectiveness of vaccination in preventing invasive pneumococcal diseases, and therefore, population-based follow-up study or randomized clinical trials of a large sample size is warranted. Finally, appropriate doses of PCV in HIV-infected adults remain to be studied, although our previous study has shown that two doses of PCV achieved a better serologic response to at least one serotype than one dose in HIV-infected patients during the 48 weeks of follow-up.Citation25
In conclusion, primary vaccination with one dose of 7-valent PCV achieved a significantly better serologic responses to one or two out of the four serotypes studied at week 48 than with 23-valent PPV in HIV-infected patients in the cART era. Suppression of HIV replication was associated with better serologic responses.
Patients and Methods
Study population
HIV-infected patients who were aged 20 y or greater and sought HIV care at the National Taiwan University Hospital were approached to participate in primary vaccination with one dose of 23-valent PPV (Pneumovax® 23, Merck and Co., Inc.) between 2000 and 2002; or vaccination with one dose of 7-valent PCV (Prevenar®, Wyeth) between 2008 and 2010 when PCV became available in Taiwan in 2005.Citation25,Citation26 After receipt of pneumococcal vaccine, patients continued their routine follow-up at the outpatient clinics for antiretroviral therapy and related HIV care. Sequential blood specimens were collected at baseline, and every 12 weeks afterwards for 48 weeks. Plasma HIV RNA load and CD4 lymphocyte count were determined every four to six months. The blood specimens were stored at -70°C until determinations of anti-capsular antibody titers. Subjects in the two groups selected for final analysis were matched for CD4 count and plasma HIV RNA load at vaccination. The two studies were approved by the Research Ethics Committee of the hospital in 2000 and 2008, respectively, and every patient who agreed to participate in the vaccination study gave written informed consent. The PCV study was registered in ClinicalTrial.gov (identifier, NCT00885628).
Plasma HIV RNA load was quantified using Cobas Amplicor HIV-1 Monitor test (Cobas Amplicor version 1.5, Roche Diagnostics Corporation), for which two different manipulating methods were used in the two study periods: the standard method with a detection range of 400 to 750,000 copies/mL from 2000 and 2006 and ultrasensitive method with a detection range of 40 to 1,000,000 copies/mL after 2006. Therefore, we decided to use 400 copies/ml as a cut-off to define undetectable plasma HIV RNA load. CD4 lymphocyte count was determined using FACFlow (BD FACS Calibur, Becton Dickinson). CART was defined as the combination of at least three antiretroviral agents containing two nucleoside reverse transcriptase inhibitors (NRTIs) plus protease inhibitors or one non-NRTIs; or triple NRTIs.
Determinations of anti-capsular antibody
Serum samples were separated from clotted blood samples by centrifugation and stored at –70°C. The determinations of anti-capsular antibody concentrations to four most prevalent pneumococcal serotypes (serotypes 6B, 14, 19F and 23F) in TaiwanCitation27 in serially collected blood specimens were performed with the use of ELISA by following the methods described previously with minor modifications.Citation28 Briefly, 1 mL of serum was mixed with 10 μg of cell-wall polysaccharide (CWPS) and incubated at room temperature on a rocking platform for 30 min. Sera from patients with acquired immunodeficiency syndrome (AIDS) who have no antibody to S. pneumoniae serotypes 6B, 14, 19F and 23F capsules and < 1 μg of antibody to CWPS per mL was used as negative controls. Capsular polysaccharides from S. pneumoniae serotypes 6B, 14, 19F, or 23F, were obtained from the American Type Culture Collection (ATCC). These capsular polysaccharides were suspended in phosphate-buffered saline (PBS, pH7.4) at concentration of 10 μg/mL and used directly to coat wells by incubation at 4°C overnight. After washing, blocking was done with PBS containing 1% of bovine serum albumin at 4°C overnight. Duplicate serum samples were studied in 2-fold serial dilutions, a laboratory reference standard for each serotype that contained known amount of IgG reactive with specific capsular polysaccharide was included in each plate as a positive control. Following washing, this first antibody incubation was performed at 37°C for 2h. After thorough washing of unbounded antibodies to wells, Horseradish Peroxidase (HRP)—conjugated goat antibody to human IgG (ZYMED LABORATORIES INC.) at 1: 2,000 dilution was used to detect IgG, and the reaction is developed 10 min at dark by addition of K-blue substrate (Neogen Corporation), followed by adding 1N sulfuric acid to stop the reaction. All washings between each incubation were done with PBS buffer containing 0.05% Tween 20. Optical density was read in an ELISA reader (SpectraMAX 340, Molecular Devices) at a wavelength of 450 nm, with subtraction of optical density of the appropriate blank. The concentration of IgG was calculated against a reference standard curve generated with the use of the WHO approved reference standard 89F.
Study end points
The primary endpoint of the study was achievement of a significant antibody response that was defined as ≥ 2-fold increase in the IgG level. Response rate was estimated by intention-to-treat (ITT) analysis, in which patients with missing data were considered non-responders. The laboratory staff who performed the determinations of antibody responses was blinded to the identity and clinical characteristics of the patients, vaccination status, or whether cART was initiated.
Statistical analyses
All statistical analyses are performed using Stata software, version 10 (StataCorp). Categorical variables were compared using χ2 or Fisher’s exact test whereas non-categorical variables were compared using Student’s t-test. To reduce the selection bias for vaccine response caused by the differences in the baseline characteristics between the PCV and PPV groups, propensity score match was conducted. First, the probability for receiving PCV rather than PPV was estimated considering baseline CD4 and plasma HIV RNA load using a multivariable logistic regression model. Each subject in the PCV group was 1:1 matched with the nearest neighbor in the PPV group by the logit of the estimated propensity score. The sample variance of the logit (estimated propensity score) was 0.1362, and the chosen size of the caliper was 0.03405. All relevant clinical and laboratory variables such as age, sex, CD4 count and plasma HIV RNA load at vaccination were tested by univariate analysis first. The variables with a P value less than 0.25 and biological significance were then put into the multivariate analysis. A stepwise model comparison and selection were used to determine the final model of multiple variable analysis.Citation29 Odds ratio for each associated factor and 95% confidence intervals (CI) were also calculated. A P value < 0.05 was considered as statistically significant.
Acknowledgments
Preliminary analyses of these data were presented as abstract no. G-867 at the 52nd “Interscience Congress of Antimicrobial Agents and Chemotherapy,” San Francisco, 9–12 September, 2012.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding Statement
The study was sponsored by the National Science Council, Taiwan (NSC-96-2314-B-002-048-MY3). The funding source had no role in the study design, conduct, data collection and analysis, preparation of manuscript, or decision of submission.
References
- Klugman KP, Madhi SA, Feldman C. HIV and pneumococcal disease. Curr Opin Infect Dis 2007; 20:11 - 5; http://dx.doi.org/10.1097/QCO.0b013e328012c5f1; PMID: 17197876
- Jordano Q, Falcó V, Almirante B, Planes AM, del Valle O, Ribera E, et al. Invasive pneumococcal disease in patients infected with HIV: still a threat in the era of highly active antiretroviral therapy. Clin Infect Dis 2004; 38:1623 - 8; http://dx.doi.org/10.1086/420933; PMID: 15156452
- Nunes MC, von Gottberg A, de Gouveia L, Cohen C, Kuwanda L, Karstaedt AS, et al. Persistent high burden of invasive pneumococcal disease in South African HIV-infected adults in the era of an antiretroviral treatment program. PLoS ONE 2011; 6:e27929; http://dx.doi.org/10.1371/journal.pone.0027929; PMID: 22140487
- Kaplan JE, Benson C, Holmes KH, Brooks JT, Pau A, Masur H, Centers for Disease Control and Prevention (CDC), National Institutes of Health, HIV Medicine Association of the Infectious Diseases Society of America. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. [quiz CE1-4] MMWR Recomm Rep 2009; 58:RR-4 1 - 207, quiz CE1-4; PMID: 19357635
- Kroon FP, van Dissel JT, Ravensbergen E, Nibbering PH, van Furth R. Enhanced antibody response to pneumococcal polysaccharide vaccine after prior immunization with conjugate pneumococcal vaccine in HIV-infected adults. Vaccine 2000; 19:886 - 94; http://dx.doi.org/10.1016/S0264-410X(00)00232-2; PMID: 11115712
- Hung CC, Chang SY, Su CT, Chen YY, Chang SF, Yang CY, et al. A 5-year longitudinal follow-up study of serological responses to 23-valent pneumococcal polysaccharide vaccination among patients with HIV infection who received highly active antiretroviral therapy. HIV Med 2010; 11:54 - 63; http://dx.doi.org/10.1111/j.1468-1293.2009.00744.x; PMID: 19659943
- French N, Gilks CF, Mujugira A, Fasching C, O’Brien J, Janoff EN. Pneumococcal vaccination in HIV-1-infected adults in Uganda: humoral response and two vaccine failures. AIDS 1998; 12:1683 - 9; http://dx.doi.org/10.1097/00002030-199813000-00017; PMID: 9764789
- French N, Nakiyingi J, Carpenter LM, Lugada E, Watera C, Moi K, et al. 23-valent pneumococcal polysaccharide vaccine in HIV-1-infected Ugandan adults: double-blind, randomised and placebo controlled trial. Lancet 2000; 355:2106 - 11; http://dx.doi.org/10.1016/S0140-6736(00)02377-1; PMID: 10902624
- Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, Lynfield R, et al, Active Bacterial Core Surveillance of the Emerging Infections Program Network. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med 2003; 348:1737 - 46; http://dx.doi.org/10.1056/NEJMoa022823; PMID: 12724479
- Bliss SJ, O’Brien KL, Janoff EN, Cotton MF, Musoke P, Coovadia H, et al. The evidence for using conjugate vaccines to protect HIV-infected children against pneumococcal disease. Lancet Infect Dis 2008; 8:67 - 80; http://dx.doi.org/10.1016/S1473-3099(07)70242-6; PMID: 17974480
- French N, Gordon SB, Mwalukomo T, White SA, Mwafulirwa G, Longwe H, et al. A trial of a 7-valent pneumococcal conjugate vaccine in HIV-infected adults. N Engl J Med 2010; 362:812 - 22; http://dx.doi.org/10.1056/NEJMoa0903029; PMID: 20200385
- Dransfield MT, Nahm MH, Han MK, Harnden S, Criner GJ, Martinez FJ, et al, COPD Clinical Research Network. Superior immune response to protein-conjugate versus free pneumococcal polysaccharide vaccine in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2009; 180:499 - 505; http://dx.doi.org/10.1164/rccm.200903-0488OC; PMID: 19556517
- Kumar D, Chen MH, Welsh B, Siegal D, Cobos I, Messner HA, et al. A randomized, double-blind trial of pneumococcal vaccination in adult allogeneic stem cell transplant donors and recipients. Clin Infect Dis 2007; 45:1576 - 82; http://dx.doi.org/10.1086/523583; PMID: 18190318
- Feikin DR, Elie CM, Goetz MB, Lennox JL, Carlone GM, Romero-Steiner S, et al. Randomized trial of the quantitative and functional antibody responses to a 7-valent pneumococcal conjugate vaccine and/or 23-valent polysaccharide vaccine among HIV-infected adults. Vaccine 2001; 20:545 - 53; http://dx.doi.org/10.1016/S0264-410X(01)00347-4; PMID: 11672921
- Lesprit P, Pédrono G, Molina JM, Goujard C, Girard PM, Sarrazin N, et al, ANRS 114-Pneumovac Study Group. Immunological efficacy of a prime-boost pneumococcal vaccination in HIV-infected adults. AIDS 2007; 21:2425 - 34; http://dx.doi.org/10.1097/QAD.0b013e3282887e91; PMID: 18025879
- Musher DM, Sampath R, Rodriguez-Barradas MC. The potential role for protein-conjugate pneumococcal vaccine in adults: what is the supporting evidence?. Clin Infect Dis 2011; 52:633 - 40; http://dx.doi.org/10.1093/cid/ciq207; PMID: 21292668
- Dransfield MT, Harnden S, Burton RL, Albert RK, Bailey WC, Casaburi R, et al, NIH COPD Clinical Research Network. Long-term comparative immunogenicity of protein conjugate and free polysaccharide pneumococcal vaccines in chronic obstructive pulmonary disease. Clin Infect Dis 2012; 55:e35 - 44; http://dx.doi.org/10.1093/cid/cis513; PMID: 22652582
- Kumar D, Rotstein C, Miyata G, Arlen D, Humar A. Randomized, double-blind, controlled trial of pneumococcal vaccination in renal transplant recipients. J Infect Dis 2003; 187:1639 - 45; http://dx.doi.org/10.1086/374784; PMID: 12721944
- Kumar D, Welsh B, Siegal D, Chen MH, Humar A. Immunogenicity of pneumococcal vaccine in renal transplant recipients--three year follow-up of a randomized trial. Am J Transplant 2007; 7:633 - 8; http://dx.doi.org/10.1111/j.1600-6143.2007.01668.x; PMID: 17217436
- Kumar D, Chen MH, Wong G, Cobos I, Welsh B, Siegal D, et al. A randomized, double-blind, placebo-controlled trial to evaluate the prime-boost strategy for pneumococcal vaccination in adult liver transplant recipients. Clin Infect Dis 2008; 47:885 - 92; http://dx.doi.org/10.1086/591537; PMID: 18715160
- Lu CL, Chang SY, Sun HY, Liu WC, Tseng YT, Hsieh CY, et al. Impact of vaccination with seven-valent pneumococcal conjugate vaccine on virologic and immunologic outcomes among HIV-infected adult patients in the era of highly active antiretroviral therapy. J Formos Med Assoc 2012; 111:445 - 51; http://dx.doi.org/10.1016/j.jfma.2011.06.027; PMID: 22939663
- Peñaranda M, Payeras A, Cambra A, Mila J, Riera M, Majorcan Pneumococcal Study Group. Conjugate and polysaccharide pneumococcal vaccines do not improve initial response of the polysaccharide vaccine in HIV-infected adults. AIDS 2010; 24:1226 - 8; http://dx.doi.org/10.1097/QAD.0b013e3283389de5; PMID: 20299956
- Tasker SA, Wallace MR, Rubins JB, Paxton WB, O’Brien J, Janoff EN. Reimmunization with 23-valent pneumococcal vaccine for patients infected with human immunodeficiency virus type 1: clinical, immunologic, and virologic responses. Clin Infect Dis 2002; 34:813 - 21; http://dx.doi.org/10.1086/339044; PMID: 11850863
- Abzug MJ, Pelton SI, Song LY, Fenton T, Levin MJ, Nachman SA, et al, Pediatric AIDS Clinical Trials Group P1024 Protocol Team. Immunogenicity, safety, and predictors of response after a pneumococcal conjugate and pneumococcal polysaccharide vaccine series in human immunodeficiency virus-infected children receiving highly active antiretroviral therapy. Pediatr Infect Dis J 2006; 25:920 - 9; http://dx.doi.org/10.1097/01.inf.0000237830.33228.c3; PMID: 17006288
- Lu CL, Hung CC, Chuang YC, Liu WC, Su CT, Hsiao CF, et al. Comparison of serologic responses to vaccination with one dose or two doses of 7-valent pneumococcal conjugate vaccine in HIV-infected adult patients. Vaccine 2012; 30:3526 - 33; http://dx.doi.org/10.1016/j.vaccine.2012.03.070; PMID: 22484349
- Hung CC, Chen MY, Hsieh SM, Hsiao CF, Sheng WH, Chang SC. Clinical experience of the 23-valent capsular polysaccharide pneumococcal vaccination in HIV-1-infected patients receiving highly active antiretroviral therapy: a prospective observational study. Vaccine 2004; 22:2006 - 12; http://dx.doi.org/10.1016/j.vaccine.2003.10.030; PMID: 15121313
- Hsueh PR, Teng LJ, Lee LN, Yang PC, Ho SW, Lue HC, et al. Increased prevalence of erythromycin resistance in streptococci: substantial upsurge in erythromycin-resistant M phenotype in Streptococcus pyogenes (1979-1998) but not in Streptococcus pneumoniae (1985-1999) in Taiwan. Microb Drug Resist 2002; 8:27 - 33; http://dx.doi.org/10.1089/10766290252913728; PMID: 12002646
- Concepcion NF, Frasch CE. Pneumococcal type 22f polysaccharide absorption improves the specificity of a pneumococcal-polysaccharide enzyme-linked immunosorbent assay. Clin Diagn Lab Immunol 2001; 8:266 - 72; PMID: 11238206
- Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 1986; 42:121 - 30; http://dx.doi.org/10.2307/2531248; PMID: 3719049