Abstract
The potential for implementation of HIV vaccine trials in hard-to-reach female sex workers in an inner city area of Barcelona, Spain was assessed via a study of HIV risk, willingness to participate and the success of retention strategies. In 130 women, serological HIV status, behavioral risk exposures and willingness to participate in future HIV vaccine trials were recorded every six months using a confidential questionnaire. An enhanced retention (ER) strategy was compared with a control retention (CR) strategy comprising the recording of data on appointment cards. HIV seroincidence and retention rates were estimated. Retention rates after 6 and 12 mo of follow-up in the ER group were 76% and 69% respectively compared with 16% and 13% in the CR group. Among the ER group 97% were willing to participate in HIV vaccine trials at baseline and, after 12 mo of follow-up. Willingness was significantly associated with higher HIV risk exposure, and higher education level. Successfully retaining these cohorts over time in settings with a high HIV seroincidence rate is an ongoing challenge that will need to be addressed to ensure participation in future trials. Furthermore, as we have demonstrated, the fact that retaining hard-to-reach populations is difficult should not exclude this target population for HIV vaccine and prevention trials.
Introduction
According to the UNAIDS World AIDS Day Report for 2011, at the end of 2010 an estimated 34 million people were living with HIV worldwide and 2.7 million individuals became newly infected with the virus in 2010. In that year the number of people dying of AIDS-related causes totalled 1.8 million, and it is estimated that more than 16 million children have been orphaned by AIDS.Citation1 In Spain, 1,162 new diagnoses of HIV infection were recorded in 2010. The percentage of HIV infected women due to unprotected heterosexual relations rises to almost 60%. From 2001 to 2010, in Catalonia, an autonomous community of Spain (north-eastern of Spain and over 7 million inhabitants with Barcelona as its largest city), 7,136 cases of HIV infection were recorded, of which 78.5% were men and 21.5% were women.Citation2 Women’s participation in HIV vaccine trials and prevention interventions is particularly important for those with limited prevention options. Condom use, a mainstay of prevention, requires male cooperation and beside that women at highest risk for HIV infection often are impacted by multiple competing health and life challenges including poverty, unstable housing, substance abuse, illegal activities and violence.Citation3
Although enormous progress has been made in curbing the epidemic, the successes in treatment and care have not been matched by progress in prevention. New evidence of rising HIV infection rates is emerging, particularly in marginalized communities. New strategies to prevent the spread of HIV are urgently needed, such as vaccines, topical microbicides and providing antiretrovirals to people who are not infected with HIV but who are at high risk of acquiring the infection, called pre-exposure prophylaxis (PrEP). The development of an HIV preventive vaccine is critical for women with limited access to health care and prevention interventions. Phase 1 and 2 clinical studies with preventive vaccine candidates are ongoing.Citation4,Citation5 Both women and men will be needed to participate in future Phase 2 and 3 HIV vaccine efficacy trials. Biomedical HIV prevention methods, as well as behavioral prevention, will be most effective if one takes into account structural and social factors, that increase risk and vulnerability, including gender inequality, HIV stigma and discrimination, and the social marginalization of the populations most at risk of HIV exposure.
Studies assessing prevention interventions, including prophylactic vaccines, require recruitment, long-term follow-up and high retention rates of high risk volunteers who are willing to consider future participation in vaccine trials.Citation6,Citation7 Previous studies have considered willingness to participate (WTP), knowledge, barriers and motivators of enrollment in HIV vaccine trials.Citation8-Citation12 One study with high-risk groups conducted in Philadelphia indicated a declining WTP after participants learned of the hazards of HIV vaccine trials.Citation13 However, other studies have shown that the more participants know about the trials, the more willing they are to volunteer.Citation14,Citation15 Long-term follow-up and high retention rates are often particularly difficult to achieve when subjects are homeless or live in temporary housing, with no formal address or telephone and few resources to travel to health centers. In recent years strategies for retaining participants in health care research have been identified focused on participant retention in randomized controlled trial studies of health problems such as diabetes, heart disease and cancer.Citation16-Citation19 Surprisingly few reports have been published on retention strategies for HIV vaccine preparedness studies among the hard-to-reach population.Citation20,Citation21
In 2005 we embarked on a pilot study to identify both women and men in three high risk target populations: commercial sex workers (CSW), injection and non-injection drug users (IDUs and NIDUs, respectively) and men who have sex with men (MSM).Citation22 In 2008 we launched the Project “Cuenta Contigo,” a vaccine preparedness study targeting women at risk for HIV transmission through sexual contact with men. This is a collaborative study between the Hospital Clínic Barcelona, and Àmbit-Prevenció, a non-governmental organization (NGO) with extensive experience in providing psychological, social and health support services for CSW. This study was designed to focus on recruitment of volunteers, retention of the cohort and HIV seroincidence. In this paper, we describe the strategies used to recruit and retain women and compare retention outcomes between two groups in which different strategies were applied: enhanced retention (ER) and control retention (CR). We also present the socio-demographic characteristics of the women at baseline, risk behaviors over time, HIV testing histories and correlates of willingness to participate in future vaccine trials among the enhanced retention (ER) group. We also discuss implications for enrolling women in HIV vaccine efficacy trials. The results of the study should help to design targeted HIV prevention strategies among at risk populations in Spain and provide critical insights for the execution of successful future international HIV vaccine efficacy studies.
Results
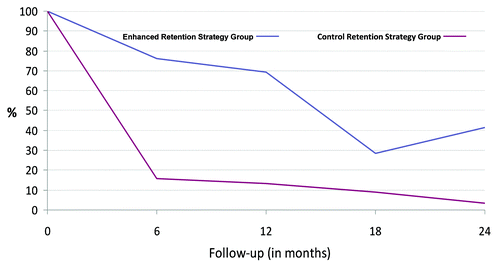
shows retention rates over time in ER and CR groups. At enrollment, 130 women/transgenders in the ER group and 121 in the CR group from the Ambit-Prevenció center were participating in the “Cuenta Contigo” project. At 6 mo, 76% of participants in the ER arm and 16% in the CR group remained in follow-up; these figures were 69% and 13% at 12 mo and 28% and 9% at 18 mo. The retention rate increased to 41% at month 24 in the ER group. Trends in retention differed significantly (p < 0.0001) between the two groups. We examined loss to follow-up by demographic and risk behavior characteristics in the ER group and found no differences between participants who completed the 6 and 12 mo follow-up visits and those who did not. No variables were associated with retention in the ER group (data not shown).
In we describe baseline demographic, risk behavior, serologic testing, vaccination and willingness to participate in CR (n = 121) and ER arms (n = 130). In both groups, participants were primarily female (over 70%) and most of them inmigrants. More than half were born in Latin American, around 30% from Africa and only 7% from Spain. Almost 80% were aged between 18–30 y and heterosexually identified. In the CR group, over half had completed secondary studies and 8% had completed university studies compared with less than half of completing secondary or university studies in the ER group. In the CR group, 46% were non-injection drug-users, compared with 31% in the ER group (p = 0.01). There were also significant differences (p = 0.03) regarding the participants who had had sex with a known HIV positive partner in the last 6 mo (4% in the CR group vs. 1.5% in the ER group). In contrast, more participants in the ER study arm had had sex with an IDU partner in the last month (3% vs. 0.8%, p < 0.01). Regarding serologic testing, more than half of the ER group had been previously tested for HIV, HCV and syphilis compared with fewer than one-third in the CR group (p < 0.01). In addition, participants from the ER study arm were more willing to participate in future vaccine trials at baseline (97%), compared with the CR group (89%) (p = 0.02).
Table 1. Baseline demographic, risk behavior, serologic testing, vaccination and willingness to participate in Control and Retaining Group
summarizes the significant bivariate and multivariate predictors of WTP in future vaccine trials for the ER group. Among the 130 participants interviewed regarding HIV vaccine trial willingness, a large majority (97%) reported that they would probably (60%) or definitely participate (37%) at baseline. We observed that willingness to participate was significantly associated with time in the study (12 mo vs. baseline, p < 0.01) and level of education (primary and secondary school, p < 0.05). Regarding HIV risk behaviors, WTP was associated with performing sex with a known HIV positive partner in the last month (p < 0.01). Importantly, the ER group presented an increase in proportion definitely willing to participate from 37.0% at baseline, 39.4% at 6-mo study visit, to 55.0% at the 12-mo study visit (p < 0.01) (data not shown).
Table 2. Bivariate and Multivariate Predictors of willingness to participate in future vaccine trials among retaining group
shows the bivariate predictors of WTP in future HIV vaccine trials among the CR and ER groups at baseline. We observed statistically significant differences between groups, with a greater WTP among transgender (p = 0.02) participants (p < 0.01) in the CR group than in the ER group. In addition we observed higher WTP in the CR arm than in the ER arm among those who had had sex with a partner using other drugs in the last 6 mo (p = 0.05) and those who had had sex with a partner having multiple sexual partners in the last month (p = 0.04).
Table 3. Bivariate predictors of willingness to participate in future vaccine trials among Control and Retaining group at Baseline
Overall, the estimated HIV-1 seroincidence rate was 0.9 /100PYO (95% CI: 0.2–3.6). Two new HIV-1 infections were found at the 12 mo study visit in the ER study arm, yielding an estimated seroincidence rate of 1.1/100 PYO (95% CI: 0.3–4.3).
Discussion
To our knowledge, this longitudinal study is the first to evaluate and monitor sexual behavioral risk, retention rates and WTP in HIV vaccine trials among female commercial sex-workers in Spain. We achieved higher levels of retention rates compared with our previous results in the same target population.Citation22 Moreover, the retention rates in our study were comparable to those in other previous studies with a similar target population.Citation20 The rate of WTP in an HIV vaccine trial found in this study was higher than those reported in other high risk groups in hypothetical HIV vaccine trials: female commercial sex workers and men attending sexually transmitted disease clinics (63%),Citation23male IDUs (77%),Citation24 female IDUs (60%), IDUs and non-IDUs (83%),Citation25 and also sex-workers and motorcycle taxi drivers (66.5%).Citation26
Analyzing both study arms of these high risk sex-workers from the inner city of Barcelona, we detected significant differences in retention rates between CR and ER groups. At the 6 and 12 mo follow-up visits the influence of implementing retention strategies and financial incentives in the ER group was clear, with high levels of retention rates compared with the CR group. Furthermore, at 18 mo of follow-up, when neither retaining strategies were applied nor financial compensation provided, the retention rate decreased to its lowest level in ER participants. Significantly, at the 24 mo follow-up, when only retaining strategies and no financial incentives were used, the retention rate increased but remained below 50%. Thus, we demonstrate the importance and influence of offering financial compensation and implementing enhanced retention strategies in retaining hard-to-reach populations. Other studies in experienced trial sites (USA, Caribbean, Southern Africa and in Latin America) have already shown how financial compensations and retention measures help with retention of marginalized populations.Citation27-Citation30
Although each group reported substantial levels of risk, the overall proportion of participants with behavioral risk was higher among CR participants. A potential explanation could be the decreased contact with education and prevention programs provided in the Ambit-Prevenció center compared with the ER arm. We observed significantly higher rates of HIV, HCV and syphilis serological testing among the ER participants than in the CR group at baseline, probably reflecting the importance of NGOs like Àmbit-Prevenció in reinforcing prevention and education programs for early HIV and other STD testing and diagnosis.
Willingness to participate was associated with several independent predictors in the ER population. In multivariate analyses, it was significantly associated with time in the study (receiving vaccine trial education) and level of education. A higher level of education promotes access to information and aids understanding of the modes of transmission, prevention and WTP in vaccine trials. It may also increase an individual's perception of risk, and may promote behavioral change and the adoption of safer sex practices. Previous studies have found a direct relationship between an individual’s level of education and knowledge of vaccines and vaccine studies.Citation31-Citation34 On the other hand, the high proportion of WTP observed in both groups (CR and ER) in individuals at greater risk for HIV may indicate their desire for protection due to a high perception of the risk combined with a lack of knowledge regarding modes of transmission and prevention. Our findings were similar to those reported in previous studies involving other populations with a high risk of HIV infection.Citation35-Citation39 It is important that these groups receive education on clinical trial procedures including randomization and blinding, as well as messaging about unknown protection against HIV infection with trial participation. Although it was a vaccine preparedness study (VPS), WTP was hypothetical, as no specific information about the trial or candidate vaccine was given. Participants may be less willing to participate once they are presented with details of an actual vaccine trial. Other study described the relationship between hypothetical and actual willingness to enroll in a preventive vaccine trial.Citation40
This study has some limitations. First, the non-randomized design and potential explanation for our results is that only those participants with high health seeking behavior may have been captured in the study, which may not reflect the true population thereby limiting the external validity of the study. The non-randomized nature of the assigning intervention may have introduced some confounding effect. For example all those who had attended the health facility more than once were assigned to ER and all first timers were assigned to CR group. The higher retention rates found in the ER group could then be due to confound. The fact that those participants had better information and knowledge about the facility programs offered by the institution and better rapport with the social workers may account for our results. Despite this limitation, our study provides substantial evidence of high WTP in future HIV vaccine trials and introduces a successful model for achieving high retention among this hard-to-reach CSW population. Second, we might have underestimated the prevalence of these behaviors in some groups using interviewer-administered questionnaires due to socially desirable reporting. In future studies we might consider using audio computer-assisted self-interviewing (ACASI) which has been shown to capture risk reporting more accuratelyCitation41,Citation42 and can be used in low literacy populations. However, programming ACASI questionnaires even in one language is time-consuming and may present barriers to enrolling people who are fluent in other languages instead of the majority language. Third, the retention rates observed in the ER group are insufficient for a vaccine trial; despite our efforts, one-third of the original sample was lost to follow-up after a year. Unfortunately, many who enrolled were reluctant to give us the name and address of two contacts, which left us with less-than-adequate locator information when participants moved. Importantly, the retention rates in our study at 12 mo of follow-up (69%) were similar to those in other previous studies with a same target population (67%).Citation20 In fact, our site can be similar to other currently successful biomedical HIV prevention trial sites, which may have had to learn to improve their retention strategies over time.Citation29,Citation30,Citation35 Finally, in this cohort only two seroconversions were detected. It may be that our sample was not large enough to measure seroincidence over the period of 24 mo. It is also possible that any seroconversions that occurred were among women who were lost to follow-up. Nevertheless, the non-detection of high seroincidence rates during the study period does not minimize the HIV risk status of this cohort.
Our results indicate that recruitment, retention and WTP are feasible in vaccine preparedness studies among hard-to-reach populations. To ensure broad participation in clinical trials and cohort studies including women at high risk for HIV infection, modifications of the retention strategies, such as building in more study visits to maintain sufficient participant contact and fully identification may also be required. Furthermore, financial compensations and outreach activities might increase the engagement to participate in future vaccine trials. On the other hand, the ability to produce reliable identification may be a reasonable requirement in Barcelona, but in many areas this could be a barrier for recruiting marginalized subjects. Our findings highlight the continued need for targeting prevention programs, harm reduction interventions and counseling as well as vaccine development and vaccine trial education. Successfully retaining these cohorts over time in settings with a high HIV seroincidence rate is an ongoing challenge that will need to be addressed to ensure participation in future trials. Furthermore, as we have demonstrated, the fact that retaining hard-to-reach populations is difficult should not exclude this target population for HIV vaccine and prevention trials.
Materials and Methods
Ethics statement
The study protocol and materials were reviewed and approved by the Ethical and Clinical Research Committee of the Hospital Clínic Barcelona.
Participants
From February to December 2008, women and transgender commercial sex workers (CSW) who attended the Àmbit-Prevenció center were invited to enroll. Àmbit-Prevenció is an NGO based in the Ciutat Vella district of Barcelona and has offered social, psychological and health assistance to CSW since 1995. The Ciutat Vella district is located in the inner city, with a population of approximately 90,000 and high rates of social marginalization and poverty. The study was coordinated by the AIDS Research Unit at the Hospital Clínic Barcelona, a referral health center for HIV/AIDS care and treatment. Women/transgenders meeting the following criteria were included: (1) 18–40 y old; (2) residence in Spain; (3) ability to provide informed consent in the participant’s preferred language; (4) HIV serostatus negative or unknown and 5) classification in one of the following risk behavior groups: (A) being at a high risk of HIV through unprotected vaginal or anal intercourse; (B) having a recent or current sex partner infected with HIV, multiple sexual partners, or one or more IDU sexual partners in the previous year; (C) having exchanged sex for money; or (D) having had a sexually transmitted infection in the last year. The exclusion criteria were: (1) documented positive HIV test; (2) inability to provide consent, reliable identification or contact information; (3) unwillingness to participate in the study; (4) current pregnancy or intention to become pregnant and (5) injection drug use in the past year, in order to limit the study population to those at risk of HIV through sexual contact. Subjects were approached by trained staff (social workers) in waiting rooms, consultation rooms and HIV care workshops at recruitment site and through outreach activities in the community.
Study design and measures
Participants were assigned sequentially to either the enhanced retention group (ER) or control retention group (CR) in a non-randomized fashion. Participants who had had previous visits (i.e.: attended during the last year) in the Àmbit-Prevenció center were assigned to the ER group, because we had contact information available and we are interested to recruit them in the study. Those attending the center for baseline visit between February and December 2008 were considered as the CR group. Each group was asked to return for study visits every 6 mo over a 24 mo period. Participants in the ER group received an enhanced retention intervention (strategies described below), and participants in the CR group were the control group.
After obtaining signed informed consent and confirming eligibility, volunteers underwent pre-test counseling by a trained HIV counselor. Counseling was client-centered and included information on HIV and other sexually transmitted infections (STI), with advice on reducing sexual exposure risk, and HIV testing information including an explanation of testing procedures and a guide to interpreting the results. Following pre-test counseling, a venous blood sample was obtained for HIV-1, hepatitis and syphilis testing. Socio-demographic characteristics, behavioral risk factors and willingness to participate were recorded using an interviewer-administered questionnaire in the participant’s primary language (Spanish, English, Romanian, German or French) by trained interviewers fluent in the respective language. Post-test HIV risk reduction counseling was performed in coordination with the disclosure of HIV, hepatitis and STI testing results, interpretation and associated referrals for comprehensive health care and treatment. WTP was measured at baseline and at each follow-up visit on a 4-point scale, ranging from: (1) Definitely not willing; (2) Probably not willing; (3) Probably willing; to (4) Definitely willing, in response to the question, “How willing would you be to join a study of a hypothetical vaccine trial to prevent HIV infection?.” Predictors of WTP were determined only in the ER group at 6 and 12 mo (shown in ) because too few of the CR participants were followed to permit analysis. Measurements at 18 and 24 mo could not be performed because we did not have enough follow-up visits to perform the analysis. Factors associated with willingness to participate at baseline in both ER and CR groups are described in .
HBV vaccine (three dose series) was offered to all susceptible participants. Information and materials for prevention of sexually and parenterally transmitted infections were supplied at all sites. Condoms were also provided and a workshop was held every three months to review HIV, STIs and sex education topics. HIV positive participants were scheduled and referred to the Hospital Clínic Barcelona for confirmatory testing, HIV care, treatment and follow-up.
Retention strategies and follow-up visits
HIV-1 seronegative women and transgenders at risk of HIV infection were invited to come back every 6 mo over a 24 mo period to repeat HIV-1 counseling, antibody testing and clinical visits. Contact tracing information was collected for each participant in the first visit and every three months. At enrollment, participants from the ER and CR groups gave their name, mail and e-mail addresses, phone number (if available) and when possible, two additional phone contacts. At the end of each interview (baseline or first follow-up), they received project appointment cards displaying the day and time scheduled for the follow-up interview. The appointment cards also included the address and phone number of the Àmbit-Prevenció center office in case participants needed to contact the research staff. Several retention strategies were implemented in the ER group: (1) Contact visits were held every 3 mo to update contact information; (2) A reminder phone call was made to the participant, or her phone contacts when she did not have a phone, the day before the visit; (3) In the event that a participant was located in the field, the outreach worker attempted to bring the participant back to the office for her appointment; (4) Sending e-mails and short message service (sms) to thank responders for their participation and to remind them of their upcoming scheduled follow-up interview appointments. In a few cases, when the women were unable to visit the study site, visits were conducted at Hospital Clinic; (5) The participants in the ER group were compensated with 15€ at the 6-mo-study visit, and 30 € at the 12-mo study visit for the time invested and travel expenses. All these retention strategies were implemented till the 12-mo follow-up visit in the ER group; between the 12 and 18 mo follow-ups, no financial compensation was provided and only an appointment card was given to remind them of their visits. Between the 18 mo and 24 mo follow-up visits, no financial compensation was provided but the retention strategies described above were implemented in order to assess the effect of the financial incentives on retention rates.
Laboratory methods
Sera were tested for HIV-1 antibody by enzyme-linked immunosorbent assay (ELISA, Sanofi Diagnostic Pasteur). Positive or indeterminate samples were confirmed by Western Blot (New LAB- Blot 1, Sanofi Diagnostic Pasteur). Syphilis screening tests were performed with a rapid test (Determine) and confirmed by Treponema pallidum hemagglutination assay (TPHA). Hepatitis B virus surface antigen (HBsAg) and core antibodies (anti-HBc) as well as antibodies to hepatitis C virus (anti-HCV) were tested by ELISA. After blood collection, all individuals were scheduled to return one week later to receive their serology results and post-test counseling.
Data analysis
At the baseline visit we calculated frequency and percentage of demographic characteristics, serologic testing and vaccination history, test results, drug and sexual risk behavior and willingness to participate in future HIV vaccine trials. Differences between the CR and ER groups were assessed using the Pearson Chi-square test and Fisher exact test for cell sizes of 5 or less. Visit retention rates were estimated as the number of participants who completed the visit within the required time window divided by the number of participants who were not HIV-infected before the visit. Study visits occurring between 120–299 d from baseline were counted as 6-mo visits, visits occurring 300–479 d from baseline were counted as 12-mo visits, visits occurring 480–659 d from baseline were counted as 18-mo visits, and visits during 660–719 d from baseline were counted as 24-mo visits. Differences in retention by demographic, drug and sexual risk behavior and willingness to participate in the ER group were assessed using the Pearson Chi-square test and Fisher exact test for cell sizes of 5 or less. Retention rates in both groups over time were assessed by the Cochran-Armitage test for trend.
A dichotomous outcome variable was created from the 4-category WTP responses comparing “Definitely willing” vs. “Probably willing,” “Probably not willing,” and “Definitely not willing.” Odds ratios and 95% confidence intervals for baseline willingness between CR and ER groups were calculated for demographic and risk characteristics using logistic regression models. Differences in baseline willingness between study groups were assessed by adding an interaction term for study group by characteristic in each model and considered significant if the p-value was < 0.05. The proportion of subjects “willing to participate” in the ER group was calculated at the 6 and 12 mo visits and bivariate and multivariate predictors of WTP were determined using a repeated-measures regression model. Bivariate risk ratios of WTP were calculated for each demographic and risk characteristic. Risk ratios with a p-value of < 0.05 were entered into a multivariate model and retained if the adjusted risk ratio had a p-value of < 0.05. Analyses were performed using STATA 11.0 (STATA).
Acknowledgments
This study was supported by HIVACAT, the Catalan AIDS Vaccine Research Project, RIS (Red Española de Investigación en SIDA) and “Obra Social Fundació La Caixa” (call 2010; Fight against poverty and social exclusion). Dr Page and Ms. Evans receive funding from National Institutes of Health R01 DA030156 and R01DA016017. We wish to thank the entire research and community team for their continuous collaboration and support and give special thanks to all individuals who participated actively in the research study. We are also grateful to the Ambit-Prevenció center in implementing this ambitious research project.
Submitted
08/29/12
Revised
10/31/12
Accepted
11/10/12
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Author contributions
Conceived and designed the study: J.J. and F.E. Performed the study: J.J., F.E., E.M. and M.M.. Analyzed the data: J.J., F.E., J.E., E.M. and J.M.G. Contributed materials/analysis tools: J.E., K.P. and E.S. Wrote the paper: J.J. and F.E.
References
- UNAIDS World AIDS Day Report 2011 (2011). Joint United Nations Programme on HIV/AIDS (UNAIDS). Available: http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2011/JC2216_WorldAIDSday_report_2011_en.pdf
- Center for Epidemiological Studies on HIV/AIDS and STIs in Catalonia (CEEISCAT). (2010). Integrated System of Epidemiological surveillance of HIV/AIDS /STIs in Catalonia (SIVES 2010): Biannual Report: Generalitat de Catalunya, Health Department: http://www.ceescat.org/Index_Ing.htm.
- Brown-Peterside P, Rivera E, Lucy D, Slaughter I, Ren L, Chiasson MA, et al. Retaining hard-to-reach women in HIV prevention and vaccine trials: Project ACHIEVE. Am J Public Health 2001; 91:1377 - 9; http://dx.doi.org/10.2105/AJPH.91.9.1377; PMID: 11527761
- Tarimo EA, Thorson A, Kohi TW, Bakari M, Sandstrom E, Mhalu F, et al. A qualitative evaluation of volunteers’ experiences in a phase I/II HIV vaccine trial in Tanzania. BMC Infect Dis 2011; 11:283; http://dx.doi.org/10.1186/1471-2334-11-283; PMID: 22023776
- Bansal GP, Malaspina A, Flores J. Future paths for HIV vaccine research: Exploiting results from recent clinical trials and current scientific advances. Curr Opin Mol Ther 2010; 12:39 - 46; PMID: 20140815
- Joseph J, Etcheverry F, Alcami J, Gatell JM. A safe, effective and affordable HIV vaccine--an urgent global need. AIDS Rev 2005; 7:131 - 8; PMID: 16302460
- Schechter M, do Lago RF, de Melo MF, Sheppard HW, Guimarães NC, Moreira RI, et al, Projeto Praço Onze Study Group. Identification of a high-risk heterosexual population for HIV prevention trials in Rio de Janeiro, Brazil. J Acquir Immune Defic Syndr 2000; 24:175 - 7; PMID: 10935694
- Dhalla S, Poole G. Motivators of enrolment in HIV vaccine trials: a review of HIV vaccine preparedness studies. AIDS Care 2011; 23:1430 - 47; http://dx.doi.org/10.1080/09540121.2011.555750; PMID: 21722022
- Dhalla S, Poole G. Barriers of enrolment in HIV vaccine trials: a review of HIV vaccine preparedness studies. Vaccine 2011; 29:5850 - 9; http://dx.doi.org/10.1016/j.vaccine.2011.06.055; PMID: 21740947
- Seage GR 3rd, Holte SE, Metzger D, Koblin BA, Gross M, Celum C, et al. Are US populations appropriate for trials of human immunodeficiency virus vaccine? The HIVNET Vaccine Preparedness Study. Am J Epidemiol 2001; 153:619 - 27; http://dx.doi.org/10.1093/aje/153.7.619; PMID: 11282787
- Markowitz LE, Sirisopana N, Charonwatanachokchai A, Julvanichpong W, Siraprapasiri T, Palanuvej T, et al. Feasibility of a preventive HIV-1 vaccine cohort among persons attending sexually transmitted disease clinics in Thailand. J Acquir Immune Defic Syndr Hum Retrovirol 1999; 20:488 - 94; http://dx.doi.org/10.1097/00042560-199904150-00012; PMID: 10225232
- Colfax G, Buchbinder S, Vamshidar G, Celum C, McKirnan D, Neidig J, et al. Motivations for participating in an HIV vaccine efficacy trial. J Acquir Immune Defic Syndr 2005; 39:359 - 64; http://dx.doi.org/10.1097/01.qai.0000152039.88422.ec; PMID: 15980699
- Halpern SD, Metzger DS, Berlin JA, Ubel PA. Who will enroll? Predicting participation in a phase II AIDS vaccine trial. J Acquir Immune Defic Syndr 2001; 27:281 - 8; PMID: 11464149
- Kiwanuka N, Robb M, Kigozi G, Birx D, Philips J, Wabwire-Mangen F, et al. Knowledge about vaccines and willingness to participate in preventive HIV vaccine trials: a population-based study, Rakai, Uganda. J Acquir Immune Defic Syndr 2004; 36:721 - 5; http://dx.doi.org/10.1097/00126334-200406010-00009; PMID: 15167291
- Smit J, Middelkoop K, Myer L, Seedat S, Wood R, Stein DJ, et al. Sexual risk factors associated with volunteering for HIV vaccine research in South Africa. AIDS Care 2006; 18:569 - 73; http://dx.doi.org/10.1080/09540120500274976; PMID: 16831784
- Robinson KA, Dennison CR, Wayman DM, Pronovost PJ, Needham DM. Systematic review identifies number of strategies important for retaining study participants. 2007; 60:757 - 65
- Froelicher ES, Miller NH, Buzaitis A, Pfenninger P, Misuraco A, Jordan S, et al. The Enhancing Recovery in Coronary Heart Disease Trial (ENRICHD): strategies and techniques for enhancing retention of patients with acute myocardial infarction and depression or social isolation. J Cardiopulm Rehabil 2003; 23:269 - 80; http://dx.doi.org/10.1097/00008483-200307000-00004; PMID: 12894001
- Parra-Medina D, D’antonio A, Smith SM, Levin S, Kirkner G, Mayer-Davis E, POWER study. Successful recruitment and retention strategies for a randomized weight management trial for people with diabetes living in rural, medically underserved counties of South Carolina: the POWER study. J Am Diet Assoc 2004; 104:70 - 5; http://dx.doi.org/10.1016/j.jada.2003.10.014; PMID: 14702587
- Bailey JM, Bieniasz ME, Kmak D, Brenner DE, Ruffin MT. Recruitment and retention of economically underserved women to a cervical cancer prevention trial. Appl Nurs Res 2004; 17:55 - 60; http://dx.doi.org/10.1016/j.apnr.2003.12.002; PMID: 14991556
- Brown-Peterside P, Chiasson MA, Ren L, Koblin BA. Involving women in HIV vaccine efficacy trials: lessons learned from a vaccine preparedness study in New York City. J Urban Health 2000; 77:425 - 37; http://dx.doi.org/10.1007/BF02386751; PMID: 10976615
- Dhalla S, Poole G, Singer J, Patrick DM, Kerr T. Cognitive factors and willingness to participate in an HIV vaccine trial among HIV-positive injection drug users. Psychol Health Med 2012; 17:223 - 34; http://dx.doi.org/10.1080/13548506.2011.608803; PMID: 22250925
- Etcheverry MF, de Lazzari E, Fuchs JD, Meroño M, Sierra E, Del Romero J, et al. Pilot study assessing HIV vaccine trial readiness among female sex workers, injection and non-injection drug users, and men who have sex with men in Spain. AIDS Behav 2010; 14:607 - 17; http://dx.doi.org/10.1007/s10461-008-9486-x; PMID: 19037720
- Celentano DD, Beyrer C, Natpratan C, Eiumtrakul S, Sussman L, Renzullo PO, et al. Willingness to participate in AIDS vaccine trials among high-risk populations in northern Thailand. AIDS 1995; 9:1079 - 83; http://dx.doi.org/10.1097/00002030-199509000-00015; PMID: 8527082
- Koblin BA, Holte S, Lenderking B, Heagerty P, The HIVNET Vaccine Preparedness Study Protocol Team. Readiness for HIV vaccine trials: changes in willingness and knowledge among high-risk populations in the HIV network for prevention trials. J Acquir Immune Defic Syndr 2000; 24:451 - 7; PMID: 11035616
- Etcheverry MF, Lum PJ, Evans JL, Sanchez E, de Lazzari E, Mendez-Arancibia E, et al. HIV vaccine trial willingness among injection and non-injection drug users in two urban centres, Barcelona and San Francisco. Vaccine 2011; 29:1991 - 6; http://dx.doi.org/10.1016/j.vaccine.2010.12.043; PMID: 21241735
- Aliyu G, Mohammad M, Saidu A, Mondal P, Charurat M, Abimiku A, et al. HIV infection awareness and willingness to participate in future HIV vaccine trials across different risk groups in Abuja, Nigeria. AIDS Care 2010; 22:1277 - 84; http://dx.doi.org/10.1080/09540121003692219; PMID: 20661789
- de Bruyn G, Hudgens MG, Sullivan PS, Duerr AC. Participant retention in clinical trials of candidate HIV vaccines. J Acquir Immune Defic Syndr 2005; 39:499 - 501; http://dx.doi.org/10.1097/01.qai.0000148532.12329.df; PMID: 16010176
- Golub ET, Purvis LA, Sapun M, Safaeian M, Beyrer C, Vlahov D, et al. Changes in willingness to participate in HIV vaccine trials among HIV-negative injection drug users. AIDS Behav 2005; 9:301 - 9; http://dx.doi.org/10.1007/s10461-005-9004-3; PMID: 16088366
- Djomand G, Metch B, Zorrilla CD, Donastorg Y, Casapia M, Villafana T, et al, 903 Protocol Team. The HVTN protocol 903 vaccine preparedness study: lessons learned in preparation for HIV vaccine efficacy trials. J Acquir Immune Defic Syndr 2008; 48:82 - 9; http://dx.doi.org/10.1097/QAI.0b013e31817236ab; PMID: 18391750
- Jaspan HB, Flisher AJ, Myer L, Mathews C, Middelkoop K, Mark D, et al. Sexual health, HIV risk, and retention in an adolescent HIV-prevention trial preparatory cohort. J Adolesc Health 2011; 49:42 - 6; http://dx.doi.org/10.1016/j.jadohealth.2010.10.009; PMID: 21700155
- Chauhan H, Lal P, Kumar V, Malhotra R, Ingle GK. Awareness status about HIV/AIDS among Indian railway’s employees and their family members. J Commun Dis 2008; 40:295 - 9; PMID: 19579724
- Sudha RT, Vijay DT, Lakshmi V. Awareness, attitudes, and beliefs of the general public towards HIV/AIDS in Hyderabad, a capital city from South India. Indian J Med Sci 2005; 59:307 - 16; http://dx.doi.org/10.4103/0019-5359.16506; PMID: 16062018
- Lindegger G, Quayle M, Ndlovu M. Local knowledge and experiences of vaccination: implications for HIV-preventive vaccine trials in South Africa. Health Educ Behav 2007; 34:108 - 23; http://dx.doi.org/10.1177/1090198105277852; PMID: 16740504
- McGrath JW, George K, Svilar G, Ihler E, Mafigiri D, Kabugo M, et al. Knowledge about vaccine trials and willingness to participate in an HIV/AIDS vaccine study in the Ugandan military. J Acquir Immune Defic Syndr 2001; 27:381 - 8; PMID: 11468427
- Koblin BA, Heagerty P, Sheon A, Buchbinder S, Celum C, Douglas JM, et al. Readiness of high-risk populations in the HIV Network for Prevention Trials to participate in HIV vaccine efficacy trials in the United States. AIDS 1998; 12:785 - 93; http://dx.doi.org/10.1097/00002030-199807000-00015; PMID: 9619811
- Suhadev M, Nyamathi AM, Swaminathan S, Venkatesan P, Raja Sakthivel M, Shenbagavalli R, et al. A pilot study on willingness to participate in future preventive HIV vaccine trials. Indian J Med Res 2006; 124:631 - 40; PMID: 17287550
- Suhadev M, Nyamathi AM, Swaminathan S, Suresh A, Venkatesan P. Factors associated with willingness to participate in HIV vaccine trials among high-risk populations in South India. AIDS Res Hum Retroviruses 2009; 25:217 - 24; http://dx.doi.org/10.1089/aid.2007.0312; PMID: 19239362
- Van de Ven P, Mao L, Crawford J, Prestage G, Grulich A, Kaldor J, et al. Willingness to participate in HIV vaccine trials among HIV-negative gay men in Sydney, Australia. Int J STD AIDS 2005; 16:314 - 7; http://dx.doi.org/10.1258/0956462053654212; PMID: 15899086
- Bartholow BN, MacQueen KM, Douglas JM Jr., Buchbinder S, McKirnan D, Judson FN. Assessment of the changing willingness to participate in phase III HIV vaccine trials among men who have sex with men. J Acquir Immune Defic Syndr Hum Retrovirol 1997; 16:108 - 15; http://dx.doi.org/10.1097/00042560-199710010-00006; PMID: 9358105
- Buchbinder SP, Metch B, Holte SE, Scheer S, Coletti A, Vittinghoff E. Determinants of enrollment in a preventive HIV vaccine trial: hypothetical versus actual willingness and barriers to participation. J Acquir Immune Defic Syndr 2004; 36:604 - 12; http://dx.doi.org/10.1097/00126334-200405010-00009; PMID: 15097304
- Pluhar E, McDonnell Holstad M, Yeager KA, Denzmore-Nwagbara P, Corkran C, Fielder B, et al. Implementation of audio computer-assisted interviewing software in HIV/AIDS research. J Assoc Nurses AIDS Care 2007; 18:51 - 63; http://dx.doi.org/10.1016/j.jana.2007.05.002; PMID: 17662924
- Simoes AA, Bastos FI, Moreira RI, Lynch KG, Metzger DS. A randomized trial of audio computer and in-person interview to assess HIV risk among drug and alcohol users in Rio De Janeiro, Brazil. J Subst Abuse Treat 2006; 30:237 - 43; http://dx.doi.org/10.1016/j.jsat.2005.12.002; PMID: 16616168