Abstract
Pseudomonas aeruginosa is an important prognostic determinant in cystic fibrosis (CF). Little is known however, about P. aeruginosa induced local mucosal and systemic immune responses. Twenty CF children were categorized according to their P. aeruginosa status: (1) chronic lower respiratory tract infection (LRTI), (2) prior successfully treated initial LRTI, (3) isolated upper respiratory tract (URT) colonization, and (4) no known URT colonization or previous LRTI. Their antibody responses, and those of six non-CF disease controls, in serum and bronchoalveolar lavage (BAL) fluid to potential P. aeruginosa vaccine antigens outer membrane protein F (OprF), outer membrane protein H (OprH), catalase A (KatA) and a whole killed cell (WKC) extract were evaluated. Outer membrane protein G (OprG) responses were also measured in blood. Natural exposure, colonization and infection resulted in detectable antibody levels in BAL and serum in all CF groups. Both chronically infected and URT colonized CF children had substantially elevated immunoglobulin A antibody levels in the BAL fluid and sera toward the WKC extract and OprF antigen compared with the other groups of CF children and non-CF controls. The serum levels of specific P. aeruginosa antibodies involving immunoglobulin G and M isotypes increased with chronic LRTI, especially antibody levels to KatA, OprH and WKC extract, which were substantially greater in chronically infected children compared with all other groups. In conclusion, natural exposure, URT colonization and LRTI with P. aeruginosa all induce substantial mucosal and systemic antibody responses to potential vaccine antigens with chronically infected CF children having the highest levels.
Introduction
Cystic fibrosis (CF) is a life-limiting autosomal recessive disorder caused by mutations in the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR) protein. The mutations result in a defective anion channel and altered composition and quantity of fluid on the surface of epithelial cells.Citation1 As a result, the pulmonary environment is markedly altered making CF patients more susceptible to Pseudomonas aeruginosa infections, which, once established are difficult to eradicate despite a strong antibody response in serum, saliva and pulmonary secretions.Citation2-Citation5 Presently, chronic infection of the respiratory tract with mucoid strains of P. aeruginosa is the leading cause of morbidity and mortality in CF patients.Citation6-Citation8 Previous studies from our group have demonstrated in animal models that protection against both acute and chronic P. aeruginosa respiratory infection can be achieved through immunization with whole killed cell (WKC) and purified protein antigens.Citation9-Citation13 Furthermore, oral WKC immunization of healthy adults was found to be safe and immunogenic, while WKC immunization of patients with bronchiectasis showed a significant decrease in the total bacterial sputum count.Citation14 It is also well documented that outer membrane proteins (Oprs), F (OprF) and I (OprI), are lead vaccine candidate antigens.Citation15-Citation17 Preventing P. aeruginosa infection by vaccinating CF patients has been a goal for many years, but despite numerous animal studies and several human trials, an efficacious vaccine for P. aeruginosa remains elusive.Citation18-Citation20
Several P. aeruginosa antigens invoke the characteristic rise in antibody titers as the disease state progresses and can be detected in the sera, sputa, saliva, tears and bronchoalveolar lavage (BAL) fluid from CF patients.Citation21-Citation27 Specific antibody responses to various P. aeruginosa antigens have been studied in the sera of adult patients, however, the characterization of antibody responses in children who differ in their pulmonary clinical status during the early years of life and initial stages of infection has not been conducted.Citation28-Citation30 A study investigating serum antibodies against alkaline phosphatase, elastase and exotoxin A in 183 CF patients (mean age 16.7 y) indicated that regular determination of serum antibody may be a useful indicative measure of probable infection for CF patients with negative or intermittent P. aeruginosa cultures.Citation31
As P. aeruginosa infects the mucosal surfaces of the respiratory tract, examining the mucosal immune response of young CF children could provide important complementary knowledge to concurrent systemic serology studies. Also, there is little information on the antibody response in bronchial secretions to natural exposure, colonization and infection of the respiratory tract with P. aeruginosa. The aim of this study was to quantitate the mucosal and systemic antibody responses toward specific P. aeruginosa proteins that are potential vaccine candidates. Antibodies to OprF, OprH, OprG, the enzyme catalase A (KatA) and a WKC extract were measured in young CF children to assess responses as a result of colonization, initial and chronic P. aeruginosa lower respiratory tract infection (LRTI). In addition, OprG antibody was also measured in serum.
KatA is one of two heme-containing catalases that detoxifies hydrogen peroxide during aerobic metabolism and enables P. aeruginosa to neutralize potentially hazardous oxygen reduction products. KatA is located in both the cytoplasm and periplasm, but is also located on the bacterial surface.Citation12 Animal studies have shown that KatA is an efficacious vaccine antigen in a rodent model of acute respiratory infection.Citation12 However, its protective ability has not been evaluated in microaerophilic environments such as biofilms. OprF and OprH are well characterized Oprs. OprF is an outer membrane porin and an important virulence factor.Citation32 OprH provides stability to the outer membrane through interaction with lipopolysaccharide,Citation33 while OprG has potential porin function.Citation34 A literature search did not reveal any vaccine studies on OprH or OprG. However, given their structure and function, they are likely to be conserved and therefore may be worthwhile pursuing as potential vaccine antigens.
Results
BAL immunoglobin G (IgG)
Despite P. aeruginosa being undetectable by culture, both non-CF controls (Group 5) and CF children without a history of this organism being present in either upper respiratory tract (URT) or lower respiratory tract (LRT) secretions (Group 4) had similar and substantial detectable levels of IgG antibody to P. aeruginosa antigens in their BAL fluid.
P. aeruginosa is found ubiquitously in the environment and these responses most likely represent environmental exposure.
Children with a prior acute P. aeruginosa LRTI treated successfully with an eradication antibiotic protocol (Group 2) tended to have lower antibody levels than those observed in either non-CF controls (Group 5) or CF children who were P. aeruginosa negative (Group 4). IgG levels to the WKC extract, OprF and OprH were increased in children with URT colonization (Group 3) or chronic LRTI (Group 1). The order of the antigen specific IgG antibody response was clearly WKC extract > OprF > KatA > OprH once a colonization or infection event occurred ().
Table 1. Bronchoalveolar lavage fluid antibody responses to P. aeruginosa antigens.
BAL immunoglobin A (IgA)
Negligible levels of IgA specific antibodies were detected against all P. aeruginosa antigens in non-CF children (Group 5). In contrast, all the CF groups had substantially detectable levels of antibody for each of the antigens. The levels of IgA specific antibodies in children who had only been colonized in the URT (Group 3) were higher than those who had previously experienced an acute LRTI (Group 2). Once the CF children developed a chronic LRTI (Group 1), a substantial specific IgA antibody response was observed across all antigens. The patterns of specific antibody responses to OprF was similar to that observed for the WKC extract and KatA for all CF groups. The lowest IgA response was seen for OprH. However, within Group 4 (i.e. CF children without a history of URT colonization or LRTI), the response to OprH was the second highest response following OprF ().
BAL immunoglobin M (IgM)
All five groups of children had detectable levels of IgM antibody against all antigens tested in BAL fluid. The levels were quantitatively similar for most antigens across all groups ().
Serum IgG
Across all antigens, there was a pattern where the levels of antibodies increased with progression of disease, i.e. CF chronic P. aeruginosa LRTI (Group 1) > CF acute LRTI (Group 2) > CF URT colonization (Group 3) > CF no LRTI or URT colonization (Group 2) ≥ non-CF children (Group 5). Generally, the lowest responses were observed for OprH and OprG ().
Table 2. Serum antibody responses to P. aeruginosa antigens.
Serum IgA
Interestingly, children who presented with URT colonization (Group 3) had similar serum specific antibody levels to WKC extract, OprF and KatA to those with chronic LRTI (Group 1). The only substantial responses to OprH and OprG were in children with chronic LRTI (Group 1). Negligible responses were observed in both non-CF (Group 5) and CF children without a history of URT or LRTI colonization with P. aeruginosa (Group 4, ).
Serum IgM
Specific IgM antibodies were present in the sera of most children with CF. Highest levels were observed for all antigens in the CF children with chronic LRTI (Group 1). Negligible responses were observed in non-CF children (Group 5, ).
Longitudinal analysis of individual patients
While there was considerable variation in the clinical progression of each child over time and the individual antibody responses for each child also varied, some interesting observations can be made from the data: (1) When a child became culture positive (at any level) in either the URT or LRT, an increase in antibody levels to one or more antigens, involving one or more isotypes was observed (example Patient 1: ) and (2) In two CF children who remained culture negative over the observation period, fluctuations in individual antigen-specific antibody profiles may have been due to infrequent sampling in a setting of intermittent colonization or infection of the airways by P. aeruginosa or, alternatively, small numbers of the organism being present but falling below the level of detection for routine bacterial cultures (example Patient 4: ).
Figure 1. Patient 1. A child whose matching bronchoalveolar lavage (BAL) samples, throat swabs and sera were available annually for three of four years of monitoring. This child had no prior history of P. aeruginosa and the BAL and throat swabs remained P. aeruginosa culture negative for samples obtained in year one and two of the observation period. However, in year four the child developed a persistent cough and a throat swab was culture positive for P. aeruginosa, while a simultaneously collected BAL sample grew low numbers of P. aeruginosa (102 colony forming units/ml) and demonstrated signs of increased airway inflammation.
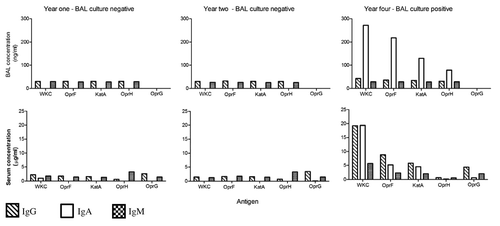
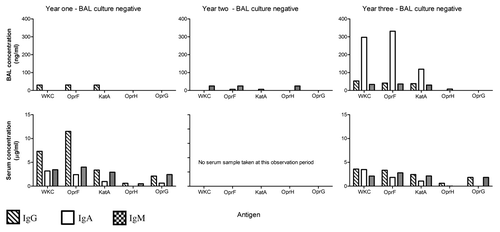
Figure 2. Patient 4. This child had a history of successfully treated initial P. aeruginosa lower respiratory tract infection and was monitored for the next three years during which time they remained stable. Bronchoalveolar lavage and throat swab samples were available for years one, two and three. Matching sera samples were only available for year one and three. Over the three-year observation period, P. aeruginosa was not cultured in any throat swab or BAL samples and the latter showed minimal signs of airway inflammation.
Discussion
This limited, exploratory study was conducted in order to characterize the humoral and mucosal immune responses generated toward potential P. aeruginosa vaccine antigens in infants and young CF children and to determine if these responses change as a result of P. aeruginosa colonization and infection. Following colonization or infection, antibodies were detected in the BAL and serum of all CF children. This varied with antibody isotype and antigen. We used a small group of non-CF children without P. aeruginosa LRTI as a comparator group. These, however, had a secondary role and with the exception of specific IgA levels in BAL fluid provided reassurance that the results seen in the non-colonized and uninfected CF group were comparable to what might be found in children without CF. The observation that non-CF disease controls and CF children who were culture negative in both the URT and LRT had detectible antibodies to several antigens, albeit at low levels, suggests that environmental exposure to P. aeruginosa is able to stimulate both mucosal and systemic antibody responses.
CF children with chronic LRTI and those who were colonized in the URT had the highest levels of specific IgA antibody in both BAL and serum. This was particularly so for the WKC extract, OprF and KatA. Children with more advanced CF lung disease, characterized by chronic LRTI, had the highest level of antibodies for all antibody isotypes and antigens in serum. It was also apparent from the study that CF children develop antibodies to P. aeruginosa, particularly BAL IgA, to a greater extent than non-CF children. While this may simply be a function of age, given that the comparator group was on average much younger than the CF participants, it could also result from a greater baseline level exposure in CF children. The IgA response also suggests that this may have been local (aerosol) exposure to the LRT.Citation35 It is possible that this came from attending a CF clinic where at the time those harboring P. aeruginosa mixed freely with other patients.Citation36 Alternatively, frequent environmental exposureCitation37 in young CF patients may have led to intermittent or very low levels of bacterial colonization in the airways undetectable by culture.
Previous studies have demonstrated that OprH is one of the earliest proteins to elicit an antibody response following colonization or infection.Citation28,Citation30,Citation38 In this study, IgA specific OprH antibody in BAL was higher in CF children without a history of positive P. aeruginosa airway cultures compared with the other CF groups and non-CF children. In contrast, BAL IgG antibody levels toward OprH tended to be lower in CF children with chronic LRTI. While the significance of this finding is unclear and is limited by the small sample size, the decline in antibody titer toward OprH in comparison with responses being developed to the other P. aeruginosa proteins during chronic infection requires further examination.
A sustained immune response to OprF is the predominant finding in the sera of chronically infected adult patients and has stimulated much research into using this protein as a potential vaccine candidate.Citation29,Citation30,Citation39,Citation40 In this study, a strong response was also observed to OprF for all immunoglobulin isotypes in serum, which increased with disease progression and culture positive status. In BAL, the OprF specific antibody response was most prominent in the IgA isotype.
KatA consistently exhibited both a strong systemic and mucosal immune response that mirrored that observed for OprF, but at approximately half the concentration. This protein is located both in the cytoplasm and periplasm of the bacterial cell and breaks down hazardous hydrogen perioxide molecules.Citation41 Previous studies have demonstrated that the immunoglobulin response to this protein enhances bacterial clearance in a rat model of acute respiratory infection.Citation12
This is the first clinical study demonstrating a mucosal IgA antibody response toward Oprs of P. aeruginosa in the BAL fluid of young CF children. Chronically infected CF children had significantly elevated IgA titers in their BAL fluid and sera toward crude extract, OprF and KatA, compared with other clinical groups. This observation correlates with adult findings.Citation42 Despite high levels of mucosal IgA antibodies directed toward these proteins during colonization and chronic infection, these children failed to clear the bacteria, suggesting that immunization with these proteins may invoke an ineffective antibody response. It is well established that mucoid exopolysaccharide (MEP) expression by the P. aeruginosa is more common in chronically infected patients and while highly immunogenic, the protective abilities of the antibody response is reduced by the effects of this MEP.Citation24,Citation43 However, other factors must also be operating as only two of the five children with chronic P. aeruginosa infection cultured colonies with the typical mucoid phenotype (data not shown). Levels of specific IgG and IgM antibodies directed against the Oprs of P. aeruginosa were also detectable in the BAL fluid, although, at much lower concentrations than those observed for IgA.
The systemic immune response differed from the mucosal immune response both across the clinical groups and also in response to specific proteins. A strong IgG response predominates with a several fold increase in concentration in the chronically infected group compared with all other CF and non-CF groups. Interestingly, the CF children with a previous history of a successfully treated acute P. aeruginosa infection demonstrated an increased mean IgG titer compared with those with isolated URT colonization by the organism. The elevated systemic IgG titer may be reflective of the previous LRTI these patients experienced.
This study has provided evidence of the immunogenic properties of selected P. aeruginosa Oprs in eliciting both systemic and mucosal Opr-specific antibody titers following exposure to P. aeruginosa through colonization, infection and probably, natural environmental exposure. However, despite these substantial responses, infection in subjects with CF ultimately occurs. While in this study the functional activity and protective ability of the stimulated antibodies was not assessed in these children, animal models of acute lung infection have found that immunization with WKC, OprF and KatA all induce enhanced clearance of the P. aeruginosa from the lungs.Citation9-Citation11 In these models, the experimental animals were immunologically naïve for P. aeruginosa. In subjects with CF, even from a very young age, any vaccination intervention is most likely to occur in the presence of natural antibodies acquired through exposure, colonization or infection with P. aeruginosa. In addition to these naturally acquired antibodies being ineffective in preventing progression from initial intermittent colonization to chronic infection, they may also interfere with the protective activities of vaccine-induced antibody responses. Skurnik and coworkersCitation44 recently demonstrated that both vaccine-induced and natural antibodies to Staphylococcus aureus capsular polysaccharides were able to interfere with the functional activities of vaccine induced antibodies to these antigens. The least naturally immunogenic Oprs observed in this study were OprH and particularly, OprG. Given the porin nature of these Oprs, vaccine studies with these antigens would be worth considering. Natural antibody interference and the possibility of impaired immune responses against P. aeruginosa in subjects with CFCitation45,Citation46 may explain the relatively low efficacy of those P. aeruginosa vaccines that have been trialled to date. In a recent review by Gerd Doring,Citation19 he suggests that hygiene interventions and early antibiotic eradication therapy will remain the primary strategies to prevent chronic lung infection in CF patients for the time being. However, the pursuit for a vaccine against P. aeruginosa in patients who are prone to life-threatening infections caused by this pathogen must remain a priority for researchers and the pharmaceutical industry.Citation47 Antigens that are conserved on the outer membrane and to which there is little antibody response following natural exposure, should be prioritized.
Materials and Methods
Study design
A nested exploratory study was undertaken using material (BAL and sera) obtained as part of a prospective, longitudinal study of early CF lung disease in children in the state of Victoria, Australia.Citation27,Citation42,Citation48 The Royal Children’s Hospital Ethics Committee approved this study and written informed consent was obtained from the parents or guardians of each child before undergoing flexible bronchoscopy and BAL.
Participants
The samples analyzed were from 20 infants identified by a state-based CF newborn screening program and whose diagnosis had been confirmed by CF gene mutation analysis and a sweat chloride concentration ≥ 60 mmol/L. Each were participants in the major birth cohort study.Citation27,Citation42,Citation48 Using methods described previously, paired serum and BAL fluid samples were obtained when the child was in a stable clinical state shortly after diagnosis and then annually until at least two to three years of age.Citation49,Citation50 Throat swabs were also obtained for culture on each of these occasions and at the time of their regular three monthly clinic visits. Patients hospitalized due to respiratory exacerbations at any time during the study submitted additional samples for analysis. Conventional laboratory bacteriology was used to identify P. aeruginosa from all clinical samples.Citation51 A group of otherwise healthy infants and young children who had not experienced an acute respiratory infection or received antibiotics in the previous two weeks and were undergoing bronchoscopy for congenital stridor served as a non-CF disease control group. None of the control children grew P. aeruginosa from their BAL or throat swab cultures.
The CF patients were categorized into one of four microbiologically defined groups (), each comprising of five individuals with BAL and sera samples, plus an additional group of six non-CF controls. Samples were analyzed in a blinded manner with each of the five groups being identified by an alphanumerical code. This code was broken upon completion of the study, revealing both the nature of the groups and the sequence in which the samples were taken. Those with chronic (≥ 6 mo) P. aeruginosa LRTI (BAL culture ≥ 105 colony-forming units (CFU)/mL)Citation49,Citation50 were maintained on inhaled tobramycin therapy. Six children with CF were followed longitudinally for up to five years after the cross-sectional study. Three were from Group 4 who had not previously grown P. aeruginosa from either throat swab or BAL fluid cultures. Three were from Group 2 who had prior evidence of an initial P. aeruginosa LRTI, but after anti-pseudomonal eradication therapy,Citation48 had a subsequent negative BAL culture, suggesting that the infection was acute and had been treated successfully. The clinical and culture status of these children progressed over time and matching BAL and sera were collected semi-annually from these children.
Table 3. Study participants
Procedures for protein preparations
A mucoid P. aeruginosa strain 385, serotype 2, phage type 21/44/109/119X/1214, obtained previously from a chronically infected CF patientCitation13 and confirmed by the Central Public Health Laboratory London, United Kingdom, was used for purification of the proteins and preparation of the whole cell extract. Bacterial stocks were stored at -85°C in nutrient broth (Oxoid; Thermo Fisher Scientific, Australia) supplemented with 10% glycerol (v/v) and revived on nutrient agar (Oxoid) at 37°C in a 5% C02 atmosphere overnight.
Crude protein was extracted using the Zwittergent® (EMD Millipore, Australia) based extraction method.Citation52 To facilitate removal of the Zwittergent® detergent from the crude protein extract, the supernatant was dialyzed against 4 L of nanopure water overnight at 4°C and frozen to -85°C for one hour before lyophilization. This extract was used as the WKC antigen in the antibody assays and as the first step in the purification of the Oprs.
Oprs were separated from the lyophilized crude protein extract using anion exchange chromatography as described by Thomas et al.Citation12 and a Bio-scale Q2 anion exchange column (Bio-Rad, Australia). The lyophilized crude Oprs were resuspended in low ionic strength 20 mM tris(hydroxymethylaminomethane), pH 8.5, to a concentration of ~20 mg/mL and eluted from the column using the gradual introduction of 20 mM tris-HCl, 500 mM sodium chloride pH 8.5 at a flow rate of 1 mL/min. The bound proteins eluted in this method were frozen to -85°C, lyophylized and analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Unbound proteins from the second elution peak were further separated. Pooled unbound fractions were exchanged into 2.4 M ammonium sulfate, 100 mM sodium phosphate, pH 6.8, using PD10 columns (GE Healthcare Life Sciences, Australia). The eluted fraction was applied to a hydrophobic interaction column (BioRad) and the proteins were eluted by the gradual introduction of 100 mM sodium phosphate, pH 6.8, at a flow rate of 1mL/min over 30 min. The eluted fractions were frozen to -85°C, lyophilized and analyzed by SDS-PAGE. Fractions containing semi-pure Oprs were subjected to preparative gel electrophoresis (Model 491 Prep Cell; Bio-Rad). The fractions were reconstituted in 1.0 mL nanopure water and diluted 4-fold in SDS reducing buffer containing 500 mM Tris-HCI, pH 6.8, 10% (w/v) glycerol, 0.05% (w/v) SDS, 0.05% (w/v) bromophenol blue, 0.01% (v/v) β-mercaptoethanol and incubated at 37°C for 30 min. The suspension was loaded onto a 20 mL 9% resolving gel and 10 mL 4% stacking gel, cast in a 28 mm internal diameter column, with running conditions set at constant 40 mÅ and 10 W for 14 h. A flow rate of 1 mL/min of 25 mM Tris/HCI, pH 8.3, was used to collect the fractions. The fractions containing purified proteins were identified from SDS-PAGE analysis, pooled, and the residual SDS in these fractions removed according to the method described by Suzuki and Terada.Citation53 All analytical SDS-PAGE was performed using the MinPROTEAN® II Electrophoresis Unit (BioRad) according to the manufacturer’s instructions. The gels were stained with Coomassie brilliant blue R-250 (Thermo Fisher Scientific) and following initial assessment, those that appeared to contain purified proteins were silver stained to increase the sensitivity of protein detection and to ensure purity.
Enzyme linked immunosorbent assay (ELISA)
Oprs were suspended in sodium carbonate/bicarbonate coating buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.6) to a final concentration of l μg/mL and incubated overnight at 4°C. ELISA plate (Nunc; Thermo Scientific, USA) wells were coated in duplicate with 100 μL of coating buffer containing purified proteins, human antibody standards (IgG 0.5 μg/mL, IgA 0.25 μg/mL, IgM 0.l μg/mL) or coating buffer alone (Invitrogen; Life Technologies, USA). For BAL samples, all antibody classes were blocked at a final concentration of 2% (w/v) skim milk suspended in phosphate buffered saline (PBS)/Tween 20 solution. In the sera, blocking was increased to 5% (w/v) skim milk for the IgG and IgM antibody classes. Plates were washed [PBS containing 0.05% (v/v) Tween 20] and patient samples were applied and incubated at room temperature for 90 min. BAL samples were diluted to a final concentration of 1:5 or 1:10, whereas, sera dilutions ranged from 1:250 to 1:1,000, dependent on clinical group. Peroxidase conjugated anti-rat immunoglobulins (Invitrogen; Life Technologies) were diluted to the appropriate concentration for each antibody class (IgG 1/10,000, IgA 1/8,000 and IgM 1/1,000) and 100 μL applied to all wells. After incubation at room temperature for 90 min, the plates were washed and developed with 100 μL of substrate tetramethylbenzidine [TMB; 1% (w/v) TMB in demethylsulfoxide; Fluka, Sigma-Aldrich, USA] diluted 1:100 in a phosphate citrate buffer (0.1 M Na2HPO4, 0.05 M citric acid, pH 5.0) containing 0.05% (v/v) H2O2. The reaction was allowed to proceed for 15 min before stopping by adding 100 μl of 0.5 M H2SO4. The color development of each sample was measured at 450nm using an automatic microplate reader (Model 3550, BioRad). Logarithmic conversion of patient samples, using the immunoglobulin standard curves, converted the absorbance values into μg/mL (serum) or ng/mL (BAL). The between plate co-efficient of variation was 8.30% for IgG, 8.9% for IgA and 5.6% for IgM.
Statistical methods
As this was an exploratory study involving residual BAL fluid and serum collected from CF and non-CF infants and young children undergoing general anaesthesia, the numbers of biological specimens available for analysis were limited. Therefore, only descriptive statistics are presented for both serum and BAL results, including the mean and 95% confidence interval (CI) of the antibody measurements. In some instances, the lower CI limits were negative, which while being statistically correct, were meaningless. Thus, any negative lower CI was reset to zero. For CF children who were not colonized or infected with P. aeruginosa and healthy non-CF disease controls, the samples were pooled and analyzed and only the means of the assays are presented. The longitudinal data are described qualitatively.
| Abbreviations: | ||
| BAL | = | bronchoalveolar lavage |
| CF | = | cystic fibrosis |
| CFU | = | colony forming units |
| CI | = | confidence interval |
| ELISA | = | enzyme linked immunosorbent assay |
| KatA | = | catalase A |
| IgA | = | immunoglobulin A |
| IgG | = | immunoglobulin G |
| IgM | = | immunoglobulin M |
| LRT | = | lower respiratory tract |
| LRTI | = | lower respiratory tract infection |
| MEP | = | mucoid exopolysaccharide |
| OprF | = | outer membrane protein F |
| OprG | = | outer membrane protein G |
| OprH | = | outer membrane protein H |
| Oprs | = | outer membrane proteins |
| PBS | = | phosphate buffered saline |
| SDS-PAGE | = | sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| TMB | = | tetramethylbenzidine |
| TrisHCl | = | tris(hydroxymethylaminomethane) |
| URT | = | upper respiratory tract |
| WKC | = | whole killed cell |
Acknowledgments
This study was supported by grants from the Royal Children’s Hospital Research Foundation. The participation of the children and their families in this study is gratefully acknowledged. We thank the Department of Anaesthesia, Royal Children’s Hospital, Melbourne, Australia, for their assistance with obtaining BAL samples and Penny Chapman for her editorial assistance.
Submitted
10/10/12
Accepted
10/23/12
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- McCullin M, Fick RBJ. Pathogenesis of Pseudomonal lung disease in cystic fibrosis. Boca Raton, Fla: CRC Press, 1993.
- Parsons YN, Panagea S, Smart CH, Walshaw MJ, Hart CA, Winstanley C. Use of subtractive hybridization to identify a diagnostic probe for a cystic fibrosis epidemic strain of Pseudomonas aeruginosa. J Clin Microbiol 2002; 40:4607 - 11; http://dx.doi.org/10.1128/JCM.40.12.4607-4611.2002; PMID: 12454160
- Levitan IB. The basic defect in cystic fibrosis. Science 1989; 244:1423; http://dx.doi.org/10.1126/science.2544028; PMID: 2544028
- Speert DP. Prevention of severe lower respiratory infections in patients with cystic fibrosis. Semin Respir Infect 1989; 4:266 - 71; PMID: 2516638
- Piedra P, Ogra PL. Immunologic aspects of surface infections in the lung. J Pediatr 1986; 108:817 - 23; http://dx.doi.org/10.1016/S0022-3476(86)80751-X; PMID: 3084749
- Welsh MJ, Smith AE. Cystic fibrosis. Sci Am 1995; 273:52 - 9; http://dx.doi.org/10.1038/scientificamerican1295-52; PMID: 8525348
- Buret A, Cripps AW. The immunoevasive activities of Pseudomonas aeruginosa. Relevance for cystic fibrosis. Am Rev Respir Dis 1993; 148:793 - 805; http://dx.doi.org/10.1164/ajrccm/148.3.793; PMID: 8368651
- Wood RE, Boat TF, Doershuk CF. Cystic fibrosis. Am Rev Respir Dis 1976; 113:833 - 78; PMID: 779549
- Cripps AW, Dunkley ML, Taylor DC, Cousins S, Clancy RL. Immunity to Pseudomonas aeruginosa induced by OprF following intestinal immunization. Adv Exp Med Biol 1995; 371B:761 - 3; PMID: 7502893
- Thomas LD, Kyd JM, Bastin DA, Dunkley ML, Cripps AW. Immunisation with non-integral OMPs promotes pulmonary clearance of Pseudomonas aeruginosa. FEMS Immunol Med Microbiol 2003; 37:155 - 60; http://dx.doi.org/10.1016/S0928-8244(03)00073-7; PMID: 12832119
- Thomas LD, Cripps AW, Kyd JM. Immune response mechanisms against Pseudomonas aeruginosa associated with mucosal immunization with protein antigens in a rat model of acute lung infection. Vaccine 2009; 27:3324 - 30; http://dx.doi.org/10.1016/j.vaccine.2009.01.085; PMID: 19200832
- Thomas LD, Dunkley ML, Moore R, Reynolds S, Bastin DA, Kyd JM, et al. Catalase immunization from Pseudomonas aeruginosa enhances bacterial clearance in the rat lung. Vaccine 2000; 19:348 - 57; http://dx.doi.org/10.1016/S0264-410X(00)00146-8; PMID: 10930690
- Cripps AW, Dunkley ML, Clancy RL. Mucosal and systemic immunizations with killed Pseudomonas aeruginosa protect against acute respiratory infection in rats. Infect Immun 1994; 62:1427 - 36; PMID: 8132349
- Cripps AW, Peek K, Dunkley M, Vento K, Marjason JK, McIntyre ME, et al. Safety and immunogenicity of an oral inactivated whole-cell pseudomonas aeruginosa vaccine administered to healthy human subjects. Infect Immun 2006; 74:968 - 74; http://dx.doi.org/10.1128/IAI.74.2.968-974.2006; PMID: 16428742
- Döring G, Pier GB. Vaccines and immunotherapy against Pseudomonas aeruginosa. Vaccine 2008; 26:1011 - 24; http://dx.doi.org/10.1016/j.vaccine.2007.12.007; PMID: 18242792
- Bumann D, Behre C, Behre K, Herz S, Gewecke B, Gessner JE, et al. Systemic, nasal and oral live vaccines against Pseudomonas aeruginosa: a clinical trial of immunogenicity in lower airways of human volunteers. Vaccine 2010; 28:707 - 13; http://dx.doi.org/10.1016/j.vaccine.2009.10.080; PMID: 19887136
- Sharma A, Krause A, Worgall S. Recent developments for Pseudomonas vaccines. Hum Vaccin 2011; 7:999 - 1011; http://dx.doi.org/10.4161/hv.7.10.16369; PMID: 21941090
- Sorichter S, Baumann U, Baumgart A, Walterspacher S, von Specht BU. Immune responses in the airways by nasal vaccination with systemic boosting against Pseudomonas aeruginosa in chronic lung disease. Vaccine 2009; 27:2755 - 9; http://dx.doi.org/10.1016/j.vaccine.2009.03.010; PMID: 19366571
- Döring G. Prevention of Pseudomonas aeruginosa infection in cystic fibrosis patients. Int J Med Microbiol 2010; 300:573 - 7; http://dx.doi.org/10.1016/j.ijmm.2010.08.010; PMID: 20940108
- Döring G. Vaccine development for patients with cystic fibrosis. Expert Rev Vaccines 2012; 11:259 - 61; http://dx.doi.org/10.1586/erv.12.1; PMID: 22380816
- Beckmann C, Brittnacher M, Ernst R, Mayer-Hamblett N, Miller SI, Burns JL. Use of phage display to identify potential Pseudomonas aeruginosa gene products relevant to early cystic fibrosis airway infections. Infect Immun 2005; 73:444 - 52; http://dx.doi.org/10.1128/IAI.73.1.444-452.2005; PMID: 15618183
- Rao AR, Splaingard MS, Gershan WM, Havens PL, Thill A, Barbieri JT. Detection of Pseudomonas aeruginosa type III antibodies in children with tracheostomies. Pediatr Pulmonol 2005; 39:402 - 7; http://dx.doi.org/10.1002/ppul.20194; PMID: 15666370
- Jaffar-Bandjee MC, Carrère J, Bally M, Guy-Crotte O, Galabert C. Immunoenzymometric assays for alkaline protease and exotoxin A from Pseudomonas aeruginosa: development and use in detecting exoproteins in clinical isolates from patients with cystic fibrosis. Eur J Clin Chem Clin Biochem 1994; 32:893 - 9; PMID: 7696436
- Pedersen SS, Møller H, Espersen F, Sørensen CH, Jensen T, Høiby N. Mucosal immunity to Pseudomonas aeruginosa alginate in cystic fibrosis. APMIS 1992; 100:326 - 34; http://dx.doi.org/10.1111/j.1699-0463.1992.tb00879.x; PMID: 1581041
- Pressler T, Pedersen SS, Espersen F, Høiby N, Koch C. IgG subclass antibodies to Pseudomonas aeruginosa in sera from patients with chronic Ps. aeruginosa infection investigated by ELISA. Clin Exp Immunol 1990; 81:428 - 34; http://dx.doi.org/10.1111/j.1365-2249.1990.tb05351.x; PMID: 2118845
- Fomsgaard A, Dinesen B, Shand GH, Pressler T, Høiby N. Antilipopolysaccharide antibodies and differential diagnosis of chronic Pseudomonas aeruginosa lung infection in cystic fibrosis. J Clin Microbiol 1989; 27:1222 - 9; PMID: 2502558
- Baltimore RS, Fick RB Jr., Fino L. Antibody to multiple mucoid strains of Pseudomonas aeruginosa in patients with cystic fibrosis, measured by an enzyme-linked immunosorbent assay. Pediatr Res 1986; 20:1085 - 90; http://dx.doi.org/10.1203/00006450-198611000-00005; PMID: 3099248
- Shand GH, Pedersen SS, Brown MR, Høiby N. Serum antibodies to Pseudomonas aeruginosa outer-membrane proteins and iron-regulated membrane proteins at different stages of chronic cystic fibrosis lung infection. J Med Microbiol 1991; 34:203 - 12; http://dx.doi.org/10.1099/00222615-34-4-203; PMID: 1902261
- Likavcanova E, Lagacé J. Quantitative analysis of immunoglobulin G subclass responses to Pseudomonas aeruginosa antigens in cystic fibrosis. J Med Microbiol 1992; 36:437 - 44; http://dx.doi.org/10.1099/00222615-36-6-437; PMID: 1613784
- Kubesch P, von Specht BU, Tümmler B. Immune response in cystic fibrosis to outer membrane proteins of Pseudomonas aeruginosa. Zentralbl Bakteriol Mikrobiol Hyg A 1988; 269:395 - 410; http://dx.doi.org/10.1016/S0176-6724(88)80183-4; PMID: 3146171
- Kappler M, Kraxner A, Reinhardt D, Ganster B, Griese M, Lang T. Diagnostic and prognostic value of serum antibodies against Pseudomonas aeruginosa in cystic fibrosis. Thorax 2006; 61:684 - 8; http://dx.doi.org/10.1136/thx.2005.049536; PMID: 16449259
- Fito-Boncompte L, Chapalain A, Bouffartigues E, Chaker H, Lesouhaitier O, Gicquel G, et al. Full virulence of Pseudomonas aeruginosa requires OprF. Infect Immun 2011; 79:1176 - 86; http://dx.doi.org/10.1128/IAI.00850-10; PMID: 21189321
- Edrington TC, Kintz E, Goldberg JB, Tamm LK. Structural basis for the interaction of lipopolysaccharide with outer membrane protein H (OprH) from Pseudomonas aeruginosa. J Biol Chem 2011; 286:39211 - 23; http://dx.doi.org/10.1074/jbc.M111.280933; PMID: 21865172
- McPhee JB, Tamber S, Bains M, Maier E, Gellatly S, Lo A, et al. The major outer membrane protein OprG of Pseudomonas aeruginosa contributes to cytotoxicity and forms an anaerobically regulated, cation-selective channel. FEMS Microbiol Lett 2009; 296:241 - 7; http://dx.doi.org/10.1111/j.1574-6968.2009.01651.x; PMID: 19486157
- Wainwright CE, France MW, O’Rourke P, Anuj S, Kidd TJ, Nissen MD, et al. Cough-generated aerosols of Pseudomonas aeruginosa and other Gram-negative bacteria from patients with cystic fibrosis. Thorax 2009; 64:926 - 31; http://dx.doi.org/10.1136/thx.2008.112466; PMID: 19574243
- Griffiths AL, Jamsen K, Carlin JB, Grimwood K, Carzino R, Robinson PJ, et al. Effects of segregation on an epidemic Pseudomonas aeruginosa strain in a cystic fibrosis clinic. Am J Respir Crit Care Med 2005; 171:1020 - 5; http://dx.doi.org/10.1164/rccm.200409-1194OC; PMID: 15709051
- Kidd TJ, Ritchie SR, Ramsay KA, Grimwood K, Bell SC, Rainey PB. Pseudomonas aeruginosa exhibits frequent recombination, but only a limited association between genotype and ecological setting. PLoS One 2012; 7:e44199; http://dx.doi.org/10.1371/journal.pone.0044199; PMID: 22970178
- Kubesch P, Lingner M, Grothues D, Wehsling M, Tümmler B. Strategies of Pseudomonas aeruginosa to colonize and to persist in the cystic fibrosis lung. Scand J Gastroenterol Suppl 1988; 143:77 - 80; http://dx.doi.org/10.3109/00365528809090222; PMID: 3133755
- von Specht B, Knapp B, Hungerer K, Lücking C, Schmitt A, Domdey H. Outer membrane proteins of Pseudomonas aeruginosa as vaccine candidates. J Biotechnol 1996; 44:145 - 53; http://dx.doi.org/10.1016/0168-1656(95)00161-1; PMID: 8717398
- Pressler T, Kronborg G, Shand GH, Mansa B, Høiby N. Determination of IgG subclass antibodies to Pseudomonas aeruginosa outer membrane proteins in cystic fibrosis lung infection using immunoblotting and enzyme-linked immunosorbent assay. Med Microbiol Immunol 1992; 181:339 - 49; http://dx.doi.org/10.1007/BF00191546; PMID: 1287420
- Brown SM, Howell ML, Vasil ML, Anderson AJ, Hassett DJ. Cloning and characterization of the katB gene of Pseudomonas aeruginosa encoding a hydrogen peroxide-inducible catalase: purification of KatB, cellular localization, and demonstration that it is essential for optimal resistance to hydrogen peroxide. J Bacteriol 1995; 177:6536 - 44; PMID: 7592431
- Brett MM, Ghoneim AT, Littlewood JM, Losowsky MS. Development of enzyme linked immunosorbent assay (ELISA) to detect antibodies to Pseudomonas aeruginosa cell surface antigens in sera of patients with cystic fibrosis. J Clin Pathol 1986; 39:1124 - 9; http://dx.doi.org/10.1136/jcp.39.10.1124; PMID: 3097080
- Pedersen SS. Lung infection with alginate-producing, mucoid Pseudomonas aeruginosa in cystic fibrosis. APMIS Suppl 1992; 28:1 - 79; PMID: 1449848
- Skurnik D, Kropec A, Roux D, Theilacker C, Huebner J, Pier GB. Natural antibodies in normal human serum inhibit Staphylococcus aureus capsular polysaccharide vaccine efficacy. Clin Infect Dis 2012; 55:1188 - 97; http://dx.doi.org/10.1093/cid/cis624; PMID: 22806596
- Hayes E, Pohl K, McElvaney NG, Reeves EP. The cystic fibrosis neutrophil: a specialized yet potentially defective cell. Arch Immunol Ther Exp (Warsz) 2011; 59:97 - 112; http://dx.doi.org/10.1007/s00005-011-0113-6; PMID: 21311988
- Ratner D, Mueller C. Immune responses in cystic fibrosis: are they intrinsically defective?. Am J Respir Cell Mol Biol 2012; 46:715 - 22; http://dx.doi.org/10.1165/rcmb.2011-0399RT; PMID: 22403802
- Sedlak-Weinstein E, Cripps AW, Kyd JM, Foxwell AR. Pseudomonas aeruginosa: the potential to immunise against infection. Expert Opin Biol Ther 2005; 5:967 - 82; http://dx.doi.org/10.1517/14712598.5.7.967; PMID: 16018741
- Nixon GM, Armstrong DS, Carzino R, Carlin JB, Olinsky A, Robertson CF, et al. Clinical outcome after early Pseudomonas aeruginosa infection in cystic fibrosis. J Pediatr 2001; 138:699 - 704; http://dx.doi.org/10.1067/mpd.2001.112897; PMID: 11343046
- Armstrong DS, Grimwood K, Carzino R, Carlin JB, Olinsky A, Phelan PD. Lower respiratory infection and inflammation in infants with newly diagnosed cystic fibrosis. BMJ 1995; 310:1571 - 2; http://dx.doi.org/10.1136/bmj.310.6994.1571; PMID: 7787647
- Armstrong DS, Grimwood K, Carlin JB, Carzino R, Olinsky A, Phelan PD. Bronchoalveolar lavage or oropharyngeal cultures to identify lower respiratory pathogens in infants with cystic fibrosis. Pediatr Pulmonol 1996; 21:267 - 75; http://dx.doi.org/10.1002/(SICI)1099-0496(199605)21:5<267::AID-PPUL1>3.0.CO;2-K; PMID: 8726151
- Kidd TJ, Ramsay KA, Hu H, Bye PTB, Elkins MR, Grimwood K, et al, ACPinCF Investigators. Low rates of Pseudomonas aeruginosa misidentification in isolates from cystic fibrosis patients. J Clin Microbiol 2009; 47:1503 - 9; http://dx.doi.org/10.1128/JCM.00014-09; PMID: 19261796
- Murphy TF, Bartos LC. Surface-exposed and antigenically conserved determinants of outer membrane proteins of Branhamella catarrhalis. Infect Immun 1989; 57:2938 - 41; PMID: 2476393
- Suzuki H, Terada T. Removal of dodecyl sulfate from protein solution. Anal Biochem 1988; 172:259 - 63; http://dx.doi.org/10.1016/0003-2697(88)90440-X; PMID: 3189769