Abstract
Results: HSP65 + IL-12 DNA vaccine showed higher protective efficacy compared with BCG in both mouse and monkey models of TB. It induced the TB-specific CTL in the mouse model of TB, while little level of activity was observed after the injection of BCG. It also showed strong therapeutic efficacy against MDR-TB. In the monkey model, the vaccine augmented the production of IFN-γ and IL-2 from PBL and the therapeutic effect was correlated with the level of IL-2. We next evaluated the potential of DNA vaccine encoding a granulysin, which is an important defensive molecule expressed by human T cells. We found that granulysin-encoding vaccine induced the differentiation of the CTL in vitro and in vivo. It also showed therapeutic efficacy against TB in the monkey as well as the mouse model. The DNA vaccine encoding a Ksp37 also induced the TB-specific CTL in vitro and in vivo in the mouse model. It augmented the production of IL-2, IFN-γ and IL-6 from T cells and spleen cells. A synergistic effect on the activation of the TB-specific CTL was observed by the combination of Ksp37 DNA vaccine with granulysin DNA vaccine.
Purpose and Methods: Emergence of the multi-drug resistant (MDR) Mycobacterium tuberculosis (TB) is a big problem in the world. We have developed novel TB vaccines [DNA vaccines encoding HSP65 + IL-12, granulysin or killer-specific secretory protein of 37kDa (Ksp37)] using Hemagglutinating virus of Japan -envelope (HVJ-E). It is suggested that the activity of the TB-specific CTL is one of the most important factor for the resistance to TB and immunity for TB in chronic human TB disease. Therefore, we examined the level of activation of the TB-specific CTL after the administration of these vaccines.
Conclusion: These data indicate that our novel vaccines (HSP65 + IL-12 DNA, granulysin and Ksp37) have a capability to activate the TB-specific CTL and will be very strong protective and therapeutic vaccines against TB.
Introduction
Tuberculosis is a major global threat to human health, with about 2 million people dying every year from Mycobacterium tuberculosis (TB) infection. The only tuberculosis vaccine currently available is an attenuated strain of Mycobacterium bovis BCG (BCG), although its efficacy against adult TB disease remains controversial. Furthermore, multi-drug resistant tuberculosis (MDR-TB) and extremely drug resistant TB (XDR-TB) are becoming big problems in the world. It has been reported that a cytotoxic T-lymphocyte (CTL) is activated during the induction of host protective immune responses to TB.Citation1-Citation4 In such circumstances, the development of therapeutic vaccine against TB as well as prophylactic vaccine against TB is expected. Therefore, we have recently developed a novel TB vaccine, a DNA vaccine expressing mycobacterial heat shock protein 65 (HSP65) and interleukin-12 (IL-12) delivered by the hemagglutinating virus of Japan (HVJ)-liposome (HSP65 + IL-12/HVJ). This vaccine was 100 fold more efficient than BCG in the murine model on the basis of the elimination of M. tuberculosis, which is mediated by the induction of CTL.Citation1,Citation5,Citation6 Furthermore, the HSP65 + IL-12 vaccine delivered by HVJ-envelope was 10,000 fold more efficient than BCG in the murine TB-prophylactic model. This vaccine induced a strong activity of CTL against TB, while BCG vaccine induced little activity of CTL in the same model. It is considered that CTL is most important lymphoid cells for the immunity to TB in chronic human TB diseases. In the present study, we analyzed CTL activity and IFN-γ production after the vaccination with our vaccines. We also evaluated the prophylactic effect in the cynomolgus monkey and mouse models of TB. A nonhuman primate model of TB is an excellent model of human tuberculosis and provides a lot of information for vaccine development. In fact, we previously evaluated the protective effects of HSP65 + IL-12/HVJ vaccine in the cynomolgus monkey model and obtained a data indicating the synergistic effect of the HSP65 + IL-12/HVJ and BCG injected by a prime-boost method.Citation5-Citation7 The combination of the two vaccines showed a strong prophylactic efficacy in the monkey model infected with M. tuberculosis (100% survival). We have previously obtained a similar data in the monkey model of TB.Citation5,Citation6,Citation8 In the present study, we examined the production of cytokines (IFN-γ and IL-2) from PBL and revealed the correlation of cytokine levels and efficacy in the monkey model of TB. We also compared the production levels of these cytokines between the combinatorial vaccination (BCG prime and HSP65+IL12/HVJ vaccine boost) group and BCG vaccination group. We also evaluated the potential of other novel vaccines (DNA vaccines encoding granulysin or Ksp37), which were expected to induce the differentiation of CTL against TB. Granulysin is a protein secreted from T cells and NK cells and has an antibacterial effect on TB. Killer-specific secretory protein of 37 kDa (Ksp37) vaccine also showed anti-TB efficacy mediated by the induction of CTL. Synergistic effect on the activation of CTL in vitro was observed by the simultaneous administration of Ksp37- and granulysin-based vaccines. In the present study, we further demonstrated the correlation of the activation of CTL and the efficacy of these novel vaccines (HSP65 + IL-12/HVJ-E DNA vaccine, granulysin vaccine and Ksp37 vaccine) in the mouse and monkey models.
Results
Induction of CTL by HSP 65+IL-12/HVJ DNA vaccine in the mouse model of TB
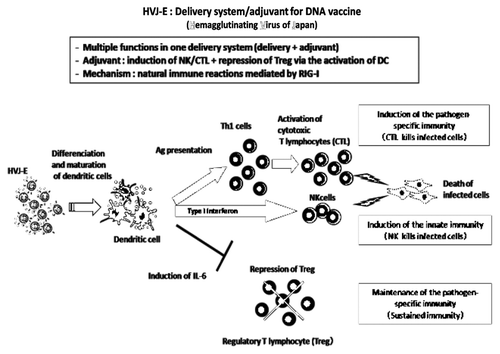
The advantage of HVJ-Envelope vector is shown in . (1) HVJ-Envelope is efficient delivery system and functions as an adjuvant for DNA vaccine, (2) It induces CTL and NK cell, (3) It induces a production of IL-6 which suppresses the regulatory T cell (T reg) and (4) It activates the innate immunity by the stimulation of RIG-I signaling pathway.
Figure 1. HVJ-Envelope exerts strong adjuvant activity by the induction of CTL/NK and repression of regulatory T cells via the activation of dendritic cells.
Mice were immunized three times with the DNA vaccine using HVJ-Envelope every three weeks. Four weeks after last immunization, H37Rv M.tuberculosis (M.tb) was challenged. Five weeks after the challenge of M.tb, mice were sacrificed and C.F.U. of M.tb in lung, liver and spleen were accessed as reported previously.Citation2,Citation9-Citation11 The C.F.U. of lungs in BCG vaccine alone group was decreased in 1 log compared with non-vaccinated mice group. The combination of the DNA vaccine (HSP65 DNA + IL-12) and BCG using prime-boost method enhanced the prophylactic efficacy (more than ten thousand fold) in the mouse model of TB (BCG prime-DNA vaccine boost). This regimen (BCG-prime then DNA vaccine boost) strongly increased the number of IFN-γ producing cells as compared with BCG vaccine alone ().
Table 1. ELISPOT assay for IFN-γ antigen-specific responses in the spleens of vaccinated mice following stimulation with HSP65 protein
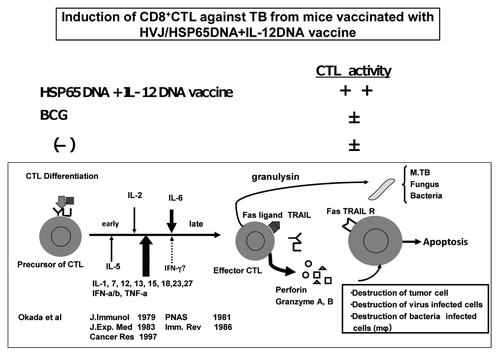
CD8+ CTLs have been considered as critical effectors of protective immunity to M. tuberculosis. This vaccine induced CD8+ CTL against TB, whereas a little or no CTL response was observed in either the naive or BCG-vaccinated mice ( and ). We used HSP65 DNA-transfected syngenic tumor cells as target cells for CTL. This vaccine augmented the induction of CD8+ CD4- CTL against target cells in vivo. On the other hand, a little or no CTL response was observed in BCG-vaccinated mice ( and ).
Figure 2. Induction of CD8+ CTL specific for HSP65 protein and M. tuberculosis by vaccination with HVJ-Envelope/HSP65 DNA + IL-12 DNA. Spleen cells from the naïve, BCG-, and HVJ-Envelope/HSP65 DNA + IL-12 DNA vaccinated mice were obtained eight weeks after the final vaccination. Cytotoxicity was assayed as release of radioactivity from 51Cr-labeled P815 target that had been transfected with HSP65 DNA using conventional 51Cr release assay as described in Materials and Methods.
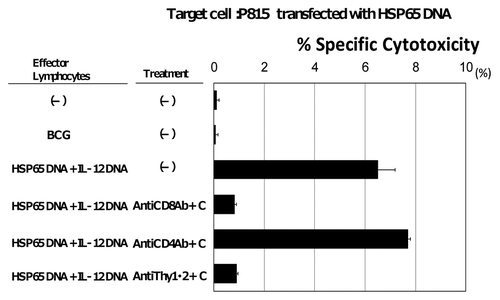
Figure 3. Induction of CD8 positive CTL against TB by HVJ-Envelope/HSP65 DNA + IL-12 DNA vaccine, and differentiation of CTL.
Furthermore, we revealed that CD8+ T cells as well as CD4+ T cells were necessary for the prophylactic effficacy of this vaccine. The administration of anti-CD8 antibody or anti-CD4 antibody during the whole immunization period decreased the antibacterial immunity against TB and increased the number of C.F.U in lungs (). Simultaneous administration of anti-CD8 and anti-CD4 antibodies resulted in the further increase of the number of C.F.U in lungs ().
Table 2. The in vivo necessity CD8 positive T cells and CD4 positive T cells for prophylactic efficacy of the HVJ-Envelope/HSP65 DNA + IL-12 DNA vaccine
T cell activation by HSP 65+IL-12/HVJ DNA vaccine in the monkey model of TB
We used cynomolgus monkeys and prime-boost methods for the evaluation of our vaccines. We immunized monkeys with BCG Tokyo as a prime vaccine, and then immunize them with this vaccine as a boost vaccine. Survival rate of monkeys vaccinated with BCG prime-HSP65+IL-12 DNA boost was 100%. In contrast, survival rate of monkeys vaccinated with BCG alone was 33%. Thus, prime-boost method showed stronger efficacy than the method vaccinated with BCG alone (data not shown).
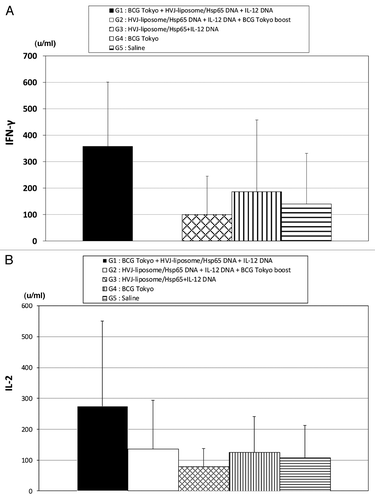
IFN-γ production from PBL was the highest level in the monkey group immunized with BCG prime-HSP65+IL-12/HVJ DNA vaccine boost (). The strongest production of IL-2 from PBL was also observed in same group (). Thus, in monkey as well as in mouse, we demonstrated that the combination of BCG Tokyo as a prime vaccine and the DNA vaccine as a boost vaccine is suitable method to get the prophylactic efficacies against TB. The prime-boost method efficiently induced the TB-specific CTL and also induced the production of IFN-γ and IL-2.
Figure 4. IFN-γ and IL-2 production efficacy of HSP65+IL-12/HVJ and BCG using prime-boost method against TB challenged cynomologus monkeys. Group of animals were vaccinated three times (every three weeks) with (1○) BCG Tokyo, (2○) HSP65 +IL-12/HVJ, (3○) HSP65+IL-12/HVJ = G1(■) BCG prime-HVJ/DNA boost group; (1○) HSP65+IL-12/HVJ, (2○) HSP65 +IL-12/HVJ, (3○)BCG = G2(□) HVJ/DNA prime-BCG boost group; (1○) HSP65+IL-12/HVJ, (2○) HSP65 +IL-12/HVJ, (3○) HSP65+IL-12/HVJ = G3(▩); (1) BCG, (2○)saline, (3○) saline = G4(▥) G4 group animals were vaccinated with BCG once.; (1○) saline, (2○)saline, (3○) saline = G5 (▤). (A) PBL from vaccinated monkeys on 56 d or 112 d after 1st immunization, were cultured for three days. IFN-γ activity on 56 d and IL-2 activity (B) on 112days were assessed by ELISA.
In Japan, BCG Tokyo vaccine is immunized in all infants. Thus it is expected to function as a prime vaccine. Therefore, we need the administration of novel vaccines (boost vaccines) for adults in Japan. We plan to use similar prime-boost method (BCG prime-DNA vaccine boost) in future clinical trial.
Correlation of IL-2 production and the therapeutic efficacy of HSP65+IL-12/HVJ DNA vaccine in the monkey model of TB
This vaccine showed therapeutic efficacy against MDR-TB and XDR-TB as well as drug-sensitive TB in mice.Citation9,Citation11,Citation12 Therefore, we confirmed the therapeutic efficacy of the DNA vaccine and T cell responses induced by the DNA vaccine in the monkey model of TB. To establish the model of TB, human TB (5 × 102 CFU) was intratracheally instillated in monkeys. After the TB infection, DNA vaccine was injected intramuscularly 9 times, three times a week. Therapeutic effect was evaluated on the basis of survival, ESR, body weight, immune responses, chest X-ray and PPD skin test.
Injection of the therapeutic vaccine improved the survival of monkeys, as compared with the saline (control) group. No death was observed in monkey group treated with HSP65 +IL-12/HVJ DNA vaccine. In contrast, the survival rate of control saline group was 60% (data not shown). The proliferation of PBL after the stimulation with HSP65 was measured to evaluate the immune response. The proliferation of PBL from the monkeys treated with the DNA vaccine was more augmented than that from the monkeys treated with saline (data not shown). IL-2 production from PBL after the stimulation with killed TB H37Ra antigens was also examined. The level of IL-2 was higher in the DNA vaccine-treated group than that in saline control group (). In addition, the IL-2 production from PBL after the stimulation with PPD was seemed to correlate with the survival after TB challenge. The level of IL-2 production of died monkeys was significantly lower than that of survived monkeys in saline group at 53days after TB challenge (). IL-2 production by the stimulation with HSP65 protein was also extremely low level in died monkeys (data not shown). These data suggested that therapeutic efficacy of this vaccine correlate with the level of IL-2 production.
Table 3. IL-2 production from PBL in the cynomolgus monkeys
Induction of CTL by 15K granulysin vaccine
Granulysin is protein especially abundant in CTL and NK cells. It is be classified into two major protein products [15kDa (15K) granulysin and 9kDa (9K) granulysin]. Latter granulysin (9K) exhibits potent cytotoxic activity against a broad panel of microbial targets, including bacteria, fungi and parasites. Granulysin is present in human CD8+ (and some CD4+) CTLs, NK cells, NKT cells and γ/δ T cells. However, the precise function of 15K granulysin has not yet been elucidated. We found that 15K granulysin was secreted from CD8 positive CTL, could enter into human macrophages and killed M. tuberculosis in the cytoplasm of macrophages. Expression of 15K granulysin protein and mRNA in CD8 positive T cells in the patients with Tuberculosis was significantly lower than those in the healthy volunteers. Moreover, the induction of 15K granulysin production after the stimulation with PPD antigen was suppressed in the supernatants of PBL from TB patients (data not shown).
Recombinant 15K granulysin protein enhanced the in vitro induction of human CTL in the MLC culture (). In vivo induction of CTL in the spleen was augmented by the administration of recombinant 15K granulyin into C57BL/6 mice (). The administration of recombinant 15K granulysin also augmented in vivo induction of CTL in the lymph node and PEC (peritoneal exudate cells) ().
Table 4. In vitro and in vivo CTL induction by 15K granulysin
Synergistic effect was observed by the combination of recombinant 15K granulysin and IL-6-related DNA vaccine (IL-6+ IL-6 receptor + gp130 DNA vaccine). In vivo induction of CTL specific for HSP65 TB antigen was augmented by the combination of two vaccines (data not shown). Two types of granulysin vaccines (recombinant 15K granulysin and 15K granulysin DNA vaccine) showed strong therapeutic efficacy in the mice infected with TB by aerosol challenge, resulted in the decrease of the number of C.F.U. in the lungs, liver and spleen (data not shown).
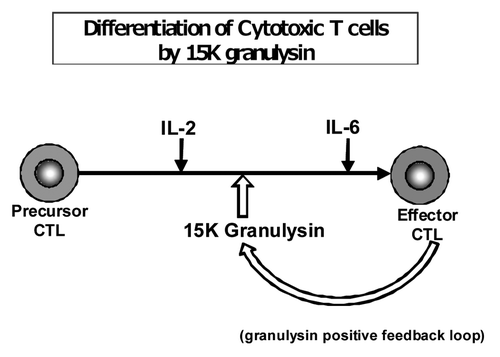
The granulysin (15K) has function as a CTL differentiation factor (). It augmented the differentiation of CTL from precursor CTL into effecter CTL. Thus, this is a first report that reveals the novel function of 15K granulysin (inducer of CTL differentiation). Effector CTLs produce 15K granulysin which induces the differentiation of CTL. Thus, there is a positive feedback loop (or autocrine system) in the regulation of the production of 15K granulysin. Granulysin (15K) might efficiently induce TB-specific CTL and enhance its therapeutic effect against TB infection by means of such a positive feedback loop system ().
Figure 5. Differentiation of CTL by 15K granulysin and positive feedback loop of 15K granulysin in CTL induction. Precursor CTL are activated by IL-2 in the early stage of CTL induction of five day MLC. On the other hand, IL-6 acts, as a cytotoxic T cell differentiation factor, on the late stage of CTL induction as shown by Okada, et al. (J.I. 1988). 15K granulysin is produced from effector CTL, and induced the differentiation of CTL as a CTL differentiation factor. (Positive feedback loop by 15K granulysin.)
CTL induction by Ksp37 vaccine
To investigate the immune function of Ksp37 protein, in vitro CTL induction by the addition of recombinant Ksp37 protein was analyzed in murine system. Splenic T cells from C57BL/6 were cultured with alloantigen (BALB/c spleen cells) in the presence or absence of recombinant Ksp37 for five days.
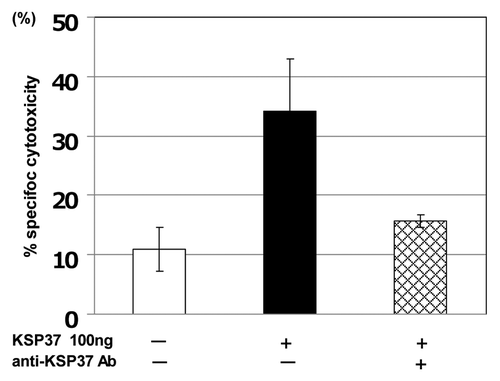
Induction of in vitro CTL differentiation was observed by the addition of recombinant Ksp37 (), suggesting that Ksp37 has function as a CTL differentiation factor.
Figure 6. Induction of CTL differentiation by killer-specific secretory protein of 37Kd (Ksp37). Splenic T cell from C57BL/6 mice (H-2b) were obtained as described in (PNAS Okada et al. 1981), and cultured with uv-treated BALB/c (H-2d) spleen cells (Mitomycin C treated) in the presence of 100ng/ml rKsp37 and/or an anti-Ksp37 antibody for 5 d. CTL activity against P815 tumor cells (H-2d) was assessed by using 51Cr assay.
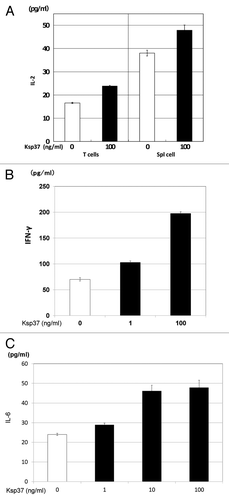
IL-2 production from T cells and spleen cells was augmented by the addition of Ksp37 protein (). Ksp37 also augmented the production of IFN-γ and IL-6 from murine spleen cells in vitro ( and C). Thus, Ksp37 is an inducer of multiple types of cytokines (IFN-γ, IL-2 and IL-6).
Figure 7. Augmentation of cytokines (IL-2, IFN-γ and IL-6) production by Ksp37 was also observed. (A) Augmentation of IL-2 production from T cells or spleen cells by Ksp37. An amount of 5 × 106 splenic T cells or spleen cells from C57BL/6 mice were cultured with 5 × 105 BALB/c spleen cells (Mitomycin-C treated) in the presence of rKsp37 for two days. IL-2, IFN-γ and IL-6 activities in the supernatants were assessed by ELISA. (B) Augmentation of IFN-γ production from spleen cells by Ksp37. (C) Augmentation of IL-6 production from spleen cells by Ksp37.
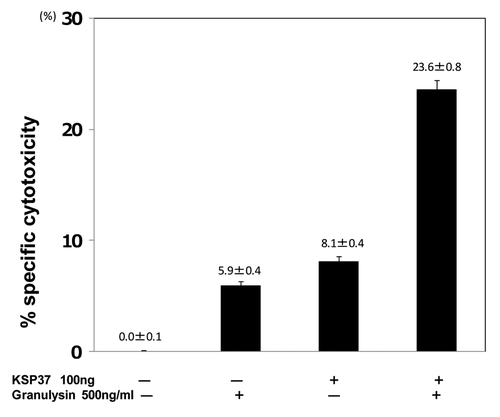
In order to study the immune function of Ksp37 in vivo, induction of CTL by the administration of Ksp37 DNA vaccine was investigated in murine system. Augmentation of CTL differentiation in vivo in the PEC was observed by the treatment with Ksp37 DNA vaccine (). Thus, Ksp37 DNA vaccine functioned as an inducer of CTL in vivo as well as in vitro. Furthermore, synergistic effect on the in vitro CTL induction was observed by the simultaneous treatment of Ksp37 and granulysin vaccines ().
Figure 8. Augmentation of CTL differentiation in vivo by the treatment with Ksp37 DNA was demonstrated. C57BL/six mice were challenged with killed H37Ra antigen (1,000 µg/mouse) in vivo (i.p.) and then treated with Ksp37 DNA (100 µg/mouse, six times) i.m. Twenty-one days after challenge of killed TB antigen, and PEC (peritoneal exudate cells) from these mice were harvested. CTL activity againt TB antigen (HSP65 antigen) was assessed by using 51Cr EL-4 which had been transfected with HSP65 DNA.
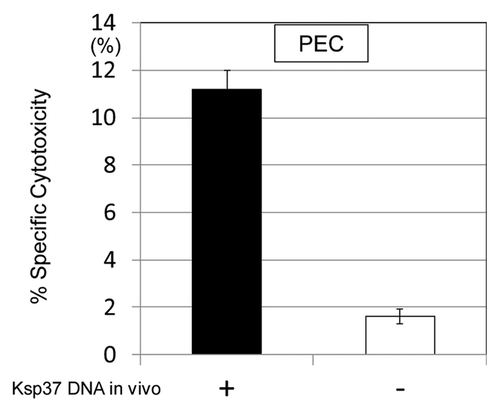
Figure 9. Synergistic efficacy of Ksp37 and granulysin on the in vitro CTL induction. Splenic T cells were cultured with uv-treated BALB/c spleen cells (Mytomycin C treated) in the presence or absence of 100ng/ml Ksp37 protein and 500ng/ml 15K granulysin protein for 5 d. Five days after culture, CTL activity against P815 tumor cells was assessed by using 51Cr release assay.
Taken together, we established three kinds of novel vaccines and examined their potential in the mouse and monkey models in vitro and in vivo.
We demonstrated that: (1) HSP65+IL-12/HVJ-E DNA vaccine (HSP65 vaccine) had a prophylactic effect against TB. We revealed the induction of CD8+ CTL and CD4+ T cell were required for the protective efficacy of HSP65 vaccine. We also confirmed currently available vaccine (BCG vaccine) induced a little or no TB-specific CTL; (2) We also revealed the 15K granulysin functioned as a cytotoxic T cell differentiation factor (CTL differentiation factor). The production of granulysin is regulated by the positive feedback system. Thus, granulysin-based vaccine might have a strong therapeutic potency against TB, since it is expected to effectively induce TB-specific CTL; (3) We also revealed that Ksp37 acts as a CTL differentiation factor in human and mouse. Ksp37 vaccine augmented the production of IL-2, IFN-γ, and IL-6 from murine T cells and spleen cells; (4) We also demonstrated the synergy of the vaccines, e.g., synergistic effect on the in vitro CTL induction was shown by the combination of Ksp37 and granulysin DNA vaccines.
These data indicate that all of our novel vaccines are able to induce the TB-specific CTL effectively and the combination regimen of those vaccines might provide the new strategy to get very strong protective and therapeutic efficacy against TB.
Discussion
In the present study, we studied the CTL differentiation against TB induced by the administration of novel vaccines (HSP65+IL-12/HVJ-E DNA vaccine, granulysin vaccine and Ksp37 vaccine). We found (1) 15K granulysin functions as a cytotoxic T cell differentiation factor (CTL differentiation factor). The therapeutic efficacy of 15K granulysin vaccine might be mediated by the induction CTL; (2) Ksp37 also acts as a CTL differentiation factor in mice; (3) Ksp37 vaccine induces the production of IL-2, IFN-γ and IL-6 from murine T cells and spleen cells; (4) Synergistic effect on the in vitro CTL induction is expected by the combination of Ksp37 vaccine and granulysin vaccine.
On the other hand, it was demonstrated that HSP65 +IL-12/HVJ-E DNA vaccine (HSP65 vaccine) has a prophylactic efficacy against TB and that the induction of CTL specific for TB is important for the efficacy of the vaccine. By using CD8 antibody and CD4 antibody we found CD8 positive CTL and CD4 positive T cell were required for the protective efficacy of HSP65 vaccine in vitro and in vivo. In contrast, BCG vaccine induced a little or no CTL against TB.
There are increasing evidences which indicate the importance of cytotoxic cells.Citation3,Citation4 Their role in immunity to TB has been revealed by using knockout mice lacking a gene or genes related to major histocompatibility complex (MHC) class I (e.g., transporter associated with antigen processing-1, CD8, 2m, and MHC class I heavy chain). These genes are involved in the antigen presentation via MHC class I, by cell-transfer experiments and by depletion of CD8+ CTLs with antibodies. CD8+ CTLs play a major role in the control of the latent TB.Citation1,Citation4,Citation6
The CTLs other than CD8+ cells might be even more important in humans. It has been reported that there are additional effector modalities, such as granulysin, that are able to kill M. tuberculosis.Citation12 Lymphocyte subsets that recognize antigens presented by molecules other than MHC class 1 were also reported to be involved in the immunity against TB. In addition to MHC class 1 molecule, these lymphocytes are able to utilize HLA-E or group1 CD1 molecules (CD1a, CD1b, etc.) for the recognition of antigens.Citation6,Citation13,Citation14
The precise function of 15K granulysin has not yet been elucidated.Citation15 We found that 15K granulysin was secreted from CD8 positive CTL and entered into human macrophages followed by the killing of M. tuberculosis in the cytoplasm.Citation10,Citation11 The expression levels of 15K granulysin protein and mRNA in CD8 positive T cells of the patients with drug sensitive TB were lower than that of the healthy volunteers. Among the TB patients, the expression level of 15K granulysin protein in CD8 positive T cells of the patients with multi-drug resistant tuberculosis (MDR-TB) was significantly lower than that in the patients with drug-sensitive TB. The expressions of 15K granulysin after the stimulation with PHA-P, ConA, alloantigens or PPD antigens were significantly suppressed in the supernatants of PBL from MDR-TB patients.Citation10,Citation11 We have currently established 15K granulysin transgenic mice and 9K granulysin transgenic mice.Citation11 It was demonstrated that 15K granulysin transgenic mice as well as 9K granulysin transgenic mice were resistant to TB infection. The number of TB (C.F.U. in tissues) was decreased in those transgenic mice. As to the induction of anti-TB immunity, differentiation and proliferation of TB-specific CTL was augmented in those transgenic mice. In addition, enhanced production of cytokines was observed.
In the present study, we demonstrated that 15K granulysin has function as a CTL differentiation factor. Granulysin (15K) augmented the differentiation of CTL from precursor CTL into effecter CTL. It has been reported that 15K granulysin is produced from effector CTL. Thus, it is suggested that the production of 15K granulysin is regulated by positive feedback loop system. Large amount of granulysin might enhance the induction of CTL resulting in the increase of the therapeutic efficacy. Recently, it was reported that 15K granulysin activated antigen-presenting cells (APC, dendritic cells or macrophages) and augmented the production of several cytokines.Citation16,Citation17 Therefore, 15K granulysin is able to activate the immune system effectively, since the target cell of it is both APC and CTL.
Vaccination with BCG prime-HSP65 + IL-12/HVJ boost showed better protective efficacy than BCG alone on the basis of the ESR, chest X-ray findings and immune responses. Importantly, treatment with HSP65 + IL-12/HVJ resulted in an increased survival for over a year.Citation5
Significantly higher levels of cytokine production from PBL (IFN-γ and IL-2) were observed in prime (BCG) - boost (HSP65 vaccine) group than those in BCG vaccine group. Prime-boost method was reported in the study of MVA85A vaccine, which is a modified vaccine virus Ankara (MVA) strain expressing antigen 85A. In phase I clinical studies, this vaccine has induced high immune responses in previously BCG-vaccinated individuals.Citation18 Boosting of BCG vaccination with MVA85A reduced the expression of immunoregulatory cytokine TGF−β.Citation19 Aeras-402 DNA (DNA that expressed 85A, 85B and TB10.4) vaccine is a recombinant adenovirus vector-based vaccine and expected as a boosting vaccine for BCG-primed individuals.Citation6 Several other vaccines use a prime-boost strategy to enhance the immune responses.Citation20 Recently, Rahman et al. established a plasmid-based vaccine (rBCG/rAd35 vaccine) which enhanced the activation of MHC class I-restricted CD8+ cytotoxic T cell. It is a recombinant BCG (rBCG) expressing a pore-forming toxin and TB antigens (Ag85A, 85B and TB10.4). A non-replicating adenovirus35 encoding the same TB antigens (rAd35) is used as a boost vaccine. The prime-boost method using rBCG and rAd35 vaccines were evaluated in nonhuman primate model.Citation21 Similar to our BCG prime- HSP65 + IL-12 DNA boost method, the results suggested that activation of CD8+ effector CTL followed by the production of granulysin at the local site were involved in the protective effects of this vaccine (prime-boost). Thus, induction of TB-specific CTL and the augmentation of the cytokine production (IFN-γ, IL-2) might be a critical factor to obtain the prophylactic efficacy.
Furthermore, we established other vaccines (granulysin vaccine and Ksp37 vaccine) which induced the differentiation of CTL against TB. The granulysin secreted from T cells and NK cells has a therapeutic effect against TB.
On the other hand, Ksp37 is expressed selectively in the effector subset of CD8+T cells, CD16+ NK cells and γ/δ T cells.Citation22 Expression of Ksp37 mRNA was closely correlated with good prognosis of ovarian cancer and gliomas.Citation23 However, immunological function(s) of Ksp37 is still unclear. Ksp37 showed anti-TB effects by the induction of CTL in the present study. We demonstrated that Ksp37 vaccine also induced murine CTL both in vitro and in vivo. Ksp37 vaccine augmented the production of IL-2, IFN-γ and IL-6 from murine T cells and spleen cells. Synergistic effect on the in vitro CTL induction was observed by the combination of Ksp37 and granulysin vaccines. We also demonstrated the correlation between the activity of CTL induction/cytokine production and the efficacy of these vaccines in the mouse and monkey models.
In conclusion, our data might provide novel strategy to obtain strong protective and therapeutic efficacy. The important factors are the induction of CTL and production of several kinds of cytokines. In addition, the combination of several kinds of vaccines will enhance the efficacy of vaccines, which will be necessary for the vaccine for the severe TBs such as MDR-TB and XDR-TB. Similar to the treatment of cancers or infectious diseases, the regimen of combination therapy will provide useful rationale that is necessary to develop more effective vaccines against TB.
Materials and Methods
Bacteria
M. tuberculosis strains H37Rv and M. bovis BCG Tokyo, were kindly provided by Dr. I. Sugawara (JATA, Tokyo, Japan). M. bovis BCG Tokyo was maintained in synthetic Sauton’s medium (Wako Chemicals, Osaka, Japan).Citation1
Animals
Inbred and specific pathogen-free female BALB/c mice and DBA/1 mice were purchased from Japan SLC (Shizuoka, Japan). Mice were maintained in isolator cages, manipulated in laminar flow hoods, and used between 8 and 10 weeks of age as described previously.Citation1
Plasmid construction
The HSP65 gene was amplified from M. tuberculosis H37Rv genomic DNA, and cloned into pcDNA3.1 (+) (Invitrogen, San Diego, CA) to generate pcDNA-hsp65 (designated as HSP65 DNA) as described previously.Citation1 The HSP65 gene was fused with mouse Igκ secretion signal sequence, and pcDNA-Ighsp65 was generated. For construction of the mouse IL-12 (mIL-12) p40 and p35 single-chain genes, mIL12p35 and mIL12p40 genes were cloned from pcDNA-p40p35,Citation1 fused and cloned into pcDNA3.1 (+) to generate pcDNA-mIL12p40p35-F (designated as mIL-12 DNA).
HVJ-E vaccination
HVJ-E was prepared as described previously.Citation1 The HVJ-E complex was aliquoted and stored at -70°C until use. DNA vaccines encoding M tuberculosis HSP65 and IL-12 were encapsulated into HVJ-Envelope or HVJ-liposomes.Citation24 HVJ-liposomes and HVJ-Envelope were prepared as described previously.Citation25-Citation29 Groups of BALB/c mice were vaccinated 3 times at 3-week intervals with 100 µL of HVJ-E solution containing 50 µg of pcDNA-IgHSP65 and 50 µg of mIL12 DNA.
Challenge infection of vaccinated animals and bacterial load determination
Mice were challenged by the intravenous route with 5 × 105 CFU of M. tuberculosis H37Rv 4 weeks after the third vaccination as described previously.Citation1
Methods for the evaluation of the prophylactic efficacy of the vaccine on the TB infection of the monkeys
Cynomolgus monkeys were housed in a BSL 3 animal facility of the Leonard Wood Memorial Research Center. The animals were vaccinated three times with the HVJ-envelope with expression plasmid of both HSP65 and human IL-12 (HSP65 + hIL-12/HVJ: 400 µg i.m.), and then challenged with the M. tuberculosis Erdman strain (5 × 102) by intratracheal instillation. Survival, immune responses (proliferation of PBL and cytokines production), body weight, ESR, PPD skin test and chest X-p findings were examined as described in our previous studies.Citation5,Citation6,Citation8,Citation9 All animal experiments were approved by the Leonard Wood Memorial Animal Care and Use Committee and the National Hospital Organization Kinki-chuo Chest Medical Center Animal Care and Use Committee.
Methods for the evaluation of the therapeutic efficacy of the vaccine on the M. tuberculosis-infected monkeys
Cynomolgus monkeys were vaccinated nine times with the HVJ-envelope with expression plasmid of both HSP65 and human IL-12 (HSP65 + hIL-12/HVJ: 400 µg i.m.), one week after the challenge with the M. tuberculosis Erdman strain (5 × 102) by intratracheal instillation. Immune responses and survival were examined as described in our previous studies.Citation5,Citation8,Citation9
Reagents and antibodies
Fetal calf serum (FCS: lot AGC6341) was obtained from Hyclone (Logan, UT). Anti-L3T4, anti-Lyt2.2 monoclonal antibodies and anti-Thy1.2 antibody were provided.Citation1,Citation2
Cell lines
A mouse mastocytoma cell line (P815: DBA/2 origin) was kindly provided by Dr. C. S. Henney (Fred Hatchinson Cancer Res. Center, Seattle).Citation1 The P815 cells were maintained in RPMI 1640 medium (Flow Laboratories, Inc. Mclean, VA) supplemented with 10% FCS, penicillin (100 U/ml), streptomycin (100 µg/ml) and 5 × 10−5 M 2-mercaptoethanol.Citation1,Citation2
Tuberculosis–specific cytotoxic test using 51Cr release
Eight weeks after the final vaccination, CTL activity of spleen cells and mesenteric lymph node cells from vaccinated mice was assessed by using the 51Cr-release assay. P815 mastocytoma cells, which have the same major histocompatibility complex (MHC) (H-2d) as BALB/c mice, were transfected with pcDNA-hsp65 and used as HSP65 protein-expressing target cells. A total of 2 × 106 cells/ml effector splenic cells were treated with anti-CD8 antibody, anti-CD4 antibody or anti-Thy1.2 antibody followed by complement.Citation1 51Cr release was assessed using the 51Cr-release assayCitation10,Citation30-Citation34 at the Effector: Target (E: T) ratio of 50:1. Spontaneous lysis (with medium alone) and maximum lysis (51Cr release after three cycles of freeze-thaw) were set up for background and targets. Percent specific lysis was determined as: [(experimental release – spontaneous release)/(maximum release – spontaneous release)] × 100.Citation30-Citation34
Production of cytokines (IL-2 and IFN-γ)
Mouse cytokines were measured in quantitative ELISAs for IL-2 and IFN-γ as described previously.Citation1,Citation2
ELISPOT assay
The spleens were removed aseptically from vaccinated mice 3 weeks after the third vaccination. Antigen-specific IFN-γ-producing cells were determined by enzyme-linked immunosorbent spot (ELISPOT) as described previously.Citation1,Citation2
Statistical analysis
Tukey-Kramer’s HSD tests were used to compare log10 value of CFU between groups following challenge and T cell responses between groups in ELISPOT assay. Student’s t-tests were performed to compare T cell responses between groups in T cell proliferation assay and granuloma formation between groups following challenge. A P-value of < 0.05 was considered significant.
| Abbreviations: | ||
| CTL | = | cytotoxic T cell |
| Ksp37 | = | Killer-specific secretory protein of 37kDa |
| 15K granulysin | = | 15 kilodalton granulysin |
| HVJ | = | hemagglutinating virus of Japan |
| MDR-TB | = | multi-drug resistant tuberculosis |
| TB | = | Mycobacterium Tuberculosis |
Acknowledgements
This study was supported by a Health and Labour Science Research Grant from MHLW (H17-shinko-5, H20-Shinko-14, H23-Shinko-2), grants from Osaka Tuberculosis research foundation and Grant-in-Aid for Scientific Research (B) from the Ministry of Education, Culture, Sports, Science and Technology Japan.
Submitted
10/10/12
Accepted
10/24/12
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Yoshida S, Tanaka T, Kita Y, Kuwayama S, Kanamaru N, Muraki Y, et al. DNA vaccine using hemagglutinating virus of Japan-liposome encapsulating combination encoding mycobacterial heat shock protein 65 and interleukin-12 confers protection against Mycobacterium tuberculosis by T cell activation. Vaccine 2006; 24:1191 - 204; http://dx.doi.org/10.1016/j.vaccine.2005.08.103; PMID: 16216394
- Okada M, Kita Y, Nakajima T, Kanamaru N, Hashimoto S, Nagasawa T, et al. Novel prophylactic vaccine using a prime-boost method and hemagglutinating virus of Japan-envelope against tuberculosis. Clin Dev Immunol 2011; 2011:549281; http://dx.doi.org/10.1155/2011/549281; PMID: 21437226
- Boom WH. New TB vaccines: is there a requirement for CD8 T cells?. J Clin Invest 2007; 117:2092 - 4; http://dx.doi.org/10.1172/JCI32933; PMID: 17671648
- van Pinxteren LA, Cassidy JP, Smedegaard BH, Agger EM, Andersen P. Control of latent Mycobacterium tuberculosis infection is dependent on CD8 T cells. Eur J Immunol 2000; 30:3689 - 98; http://dx.doi.org/10.1002/1521-4141(200012)30:12<3689::AID-IMMU3689>3.0.CO;2-4; PMID: 11169412
- Okada M, Kita Y, Nakajima T, Kanamaru N, Hashimoto S, Nagasawa T, et al. Evaluation of a novel vaccine (HVJ-liposome/HSP65 DNA+IL-12 DNA) against tuberculosis using the cynomolgus monkey model of TB. Vaccine 2007; 25:2990 - 3; http://dx.doi.org/10.1016/j.vaccine.2007.01.014; PMID: 17280753
- Okada M, Kita Y. Tuberculosis vaccine development: The development of novel (preclinical) DNA vaccine. Hum Vaccin 2010; 6:297 - 308; http://dx.doi.org/10.4161/hv.6.4.10172; PMID: 20372079
- Walsh GP, Tan EV, dela Cruz EC, Abalos RM, Villahermosa LG, Young LJ, et al. The Philippine cynomolgus monkey (Macaca fasicularis) provides a new nonhuman primate model of tuberculosis that resembles human disease. Nat Med 1996; 2:430 - 6; http://dx.doi.org/10.1038/nm0496-430; PMID: 8597953
- Kita Y, Tanaka T, Yoshida S, Ohara N, Kaneda Y, Kuwayama S, et al. Novel recombinant BCG and DNA-vaccination against tuberculosis in a cynomolgus monkey model. Vaccine 2005; 23:2132 - 5; http://dx.doi.org/10.1016/j.vaccine.2005.01.057; PMID: 15755583
- Okada M, Kita Y, Nakajima T, Kanamaru N, Hashimoto S, Nagasawa T, et al. Novel prophylactic and therapeutic vaccine against tuberculosis. Vaccine 2009; 27:3267 - 70; http://dx.doi.org/10.1016/j.vaccine.2009.01.064; PMID: 19200841
- Okada M, Kita Y, Nakajima T, Kanamaru N, Hashimoto S, Nagasawa T, et al. Novel therapeutic vaccine: granulysin and new DNA vaccine against Tuberculosis. Hum Vaccin 2011; 7:Suppl 60 - 7; http://dx.doi.org/10.4161/hv.7.0.14563; PMID: 21546794
- Kita Y, Okada M, Nakajima T, Kanamaru N, Hashimoto S, Nagasawa T, et al. Development of therapeutic and prophylactic vaccine against Tuberculosis using monkey and transgenic mice models. Hum Vaccin 2011; 7:Suppl 108 - 14; http://dx.doi.org/10.4161/hv.7.0.14571; PMID: 21263229
- Stegelmann F, Bastian M, Swoboda K, Bhat R, Kiessler V, Krensky AM, et al. Coordinate expression of CC chemokine ligand 5, granulysin, and perforin in CD8+ T cells provides a host defense mechanism against Mycobacterium tuberculosis. J Immunol 2005; 175:7474 - 83; PMID: 16301655
- Woodworth JS, Behar SM. Mycobacterium tuberculosis-specific CD8+ T cells and their role in immunity. Crit Rev Immunol 2006; 26:317 - 52; http://dx.doi.org/10.1615/CritRevImmunol.v26.i4.30; PMID: 17073557
- Lewinsohn DM, Zhu L, Madison VJ, Dillon DC, Fling SP, Reed SG, et al. Classically restricted human CD8+ T lymphocytes derived from Mycobacterium tuberculosis-infected cells: definition of antigenic specificity. J Immunol 2001; 166:439 - 46; PMID: 11123322
- Stenger S, Hanson DA, Teitelbaum R, Dewan P, Niazi KR, Froelich CJ, et al. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science 1998; 282:121 - 5; http://dx.doi.org/10.1126/science.282.5386.121; PMID: 9756476
- Castiello L, Stroncek DF, Finn MW, Wang E, Marincola FM, Clayberger C, et al. 15 kDa Granulysin versus GM-CSF for monocytes differentiation: analogies and differences at the transcriptome level. J Transl Med 2011; 9:41; http://dx.doi.org/10.1186/1479-5876-9-41; PMID: 21501511
- Clayberger C, Finn MW, Wang T, Saini R, Wilson C, Barr VA, et al. 15 kDa granulysin causes differentiation of monocytes to dendritic cells but lacks cytotoxic activity. J Immunol 2012; 188:6119 - 26; http://dx.doi.org/10.4049/jimmunol.1200570; PMID: 22586033
- McShane H, Pathan AA, Sander CR, Keating SM, Gilbert SC, Huygen K, et al. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat Med 2004; 10:1240 - 4; http://dx.doi.org/10.1038/nm1128; PMID: 15502839
- Fletcher HA, Pathan AA, Berthoud TK, Dunachie SJ, Whelan KT, Alder NC, et al. Boosting BCG vaccination with MVA85A down-regulates the immunoregulatory cytokine TGF-beta1. Vaccine 2008; 26:5269 - 75; http://dx.doi.org/10.1016/j.vaccine.2008.07.040; PMID: 18682270
- Kaufmann SH, Hussey G, Lambert PH. New vaccines for tuberculosis. Lancet 2010; 375:2110 - 9; http://dx.doi.org/10.1016/S0140-6736(10)60393-5; PMID: 20488515
- Rahman S, Magalhaes I, Rahman J, Ahmed RK, Sizemore DR, Scanga CA, et al. Prime-boost vaccination with rBCG/rAd35 enhances CD8⁺ cytolytic T-cell responses in lesions from Mycobacterium tuberculosis-infected primates. Mol Med 2012; 18:647 - 58; http://dx.doi.org/10.2119/molmed.2011.00222; PMID: 22396020
- Ogawa K, Tanaka K, Ishii A, Nakamura Y, Kondo S, Sugamura K, et al. A novel serum protein that is selectively produced by cytotoxic lymphocytes. J Immunol 2001; 166:6404 - 12; PMID: 11342666
- Elgaaen BV, Haug KB, Wang J, Olstad OK, Fortunati D, Onsrud M, et al. POLD2 and KSP37 (FGFBP2) correlate strongly with histology, stage and outcome in ovarian carcinomas. PLoS One 2010; 5:e13837; http://dx.doi.org/10.1371/journal.pone.0013837; PMID: 21079801
- Saeki Y, Matsumoto N, Nakano Y, Mori M, Awai K, Kaneda Y. Development and characterization of cationic liposomes conjugated with HVJ (Sendai virus): reciprocal effect of cationic lipid for in vitro and in vivo gene transfer. Hum Gene Ther 1997; 8:2133 - 41; http://dx.doi.org/10.1089/hum.1997.8.17-2133; PMID: 9414261
- Kaneda Y, Nakajima T, Nishikawa T, Yamamoto S, Ikegami H, Suzuki N, et al. Hemagglutinating virus of Japan (HVJ) envelope vector as a versatile gene delivery system. Mol Ther 2002; 6:219 - 26; http://dx.doi.org/10.1006/mthe.2002.0647; PMID: 12161188
- Kaneda Y. New vector innovation for drug delivery: development of fusigenic non-viral particles. Curr Drug Targets 2003; 4:599 - 602; http://dx.doi.org/10.2174/1389450033490740; PMID: 14577648
- Kaneda Y, Yamamoto S, Nakajima T. Development of HVJ envelope vector and its application to gene therapy. Adv Genet 2005; 53:307 - 32; http://dx.doi.org/10.1016/S0065-2660(05)53012-8; PMID: 16240999
- Ito M, Yamamoto S, Nimura K, Hiraoka K, Tamai K, Kaneda Y. Rad51 siRNA delivered by HVJ envelope vector enhances the anti-cancer effect of cisplatin. J Gene Med 2005; 7:1044 - 52; http://dx.doi.org/10.1002/jgm.753; PMID: 15756713
- Mima H, Yamamoto S, Ito M, Tomoshige R, Tabata Y, Tamai K, et al. Targeted chemotherapy against intraperitoneally disseminated colon carcinoma using a cationized gelatin-conjugated HVJ envelope vector. Mol Cancer Ther 2006; 5:1021 - 8; http://dx.doi.org/10.1158/1535-7163.MCT-05-0352; PMID: 16648574
- Okada M, Yoshimura N, Kaieda T, Yamamura Y, Kishimoto T. Establishment and characterization of human T hybrid cells secreting immunoregulatory molecules. Proc Natl Acad Sci U S A 1981; 78:7717 - 21; http://dx.doi.org/10.1073/pnas.78.12.7717; PMID: 6801660
- Tanaka F, Abe M, Akiyoshi T, Nomura T, Sugimachi K, Kishimoto T, et al. The anti-human tumor effect and generation of human cytotoxic T cells in SCID mice given human peripheral blood lymphocytes by the in vivo transfer of the Interleukin-6 gene using adenovirus vector. Cancer Res 1997; 57:1335 - 43; PMID: 9102222
- Okada M, Sakaguchi N, Yoshimura N, Hara H, Shimizu K, Yoshida N, et al. B cell growth factors and B cell differentiation factor from human T hybridomas. Two distinct kinds of B cell growth factor and their synergism in B cell proliferation. J Exp Med 1983; 157:583 - 90; http://dx.doi.org/10.1084/jem.157.2.583; PMID: 6600487
- Okada M, Klimpel GR, Kuppers RC, Henney CS. The differentiation of cytotoxic T cells in vitro. I. Amplifying factor(s) in the primary response is Lyt 1 + cell dependent. J Immunol 1979; 122:2527 - 33; PMID: 156228
- Okada M, Sakaguchi N, Yoshimura N, Hara H, Shimizu K, Yoshida N, et al. B cell growth factors and B cell differentiation factor from human T hybridomas. Two distinct kinds of B cell growth factor and their synergism in B cell proliferation. J Exp Med 1983; 157:583 - 90; http://dx.doi.org/10.1084/jem.157.2.583; PMID: 6600487